Toxicity Assessment of a Single Dose of Poly(ethylene glycol) Diglycidyl Ether (PEGDE) Administered Subcutaneously in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Substances
2.2. Experimental Animals
2.3. Experimental Design
2.3.1. General Clinical Observations
2.3.2. Absolute and Relative Organ Weights
2.3.3. Cage-Side Observations
2.3.4. Histological Analysis
2.4. Statistical Analysis
3. Results
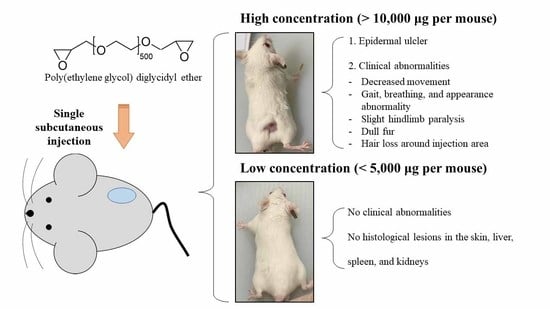
3.1. Clinical Observation and Skin Injury
3.2. Feed Intake, Body Weight, and Organ Weight
3.3. Histological Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vasylieva, N.; Barnych, B.; Meiller, A.; Maucler, C.; Pollegioni, L.; Lin, J.-S.; Barbier, D.; Marinesco, S. Covalent enzyme immobilization by Poly(ethylene glycol) diglycidyl ether (PEGDE) for microelectrode biosensor preparation. Biosens. Bioelectron. 2011, 26, 3993–4000. [Google Scholar] [CrossRef]
- Li, F.; Nie, M.; He, X.; Fei, J.; Ding, Y.; Feng, B. Direct electrochemistry and electrocatalysis of hemoglobin on a glassy carbon electrode modified with poly (ethylene glycol diglycidyl ether) and gold nanoparticles on a quaternized cellulose support. A sensor for hydrogen peroxide and nitric oxide. Microchim. Acta 2014, 181, 1541–1549. [Google Scholar] [CrossRef]
- Belfer, S.; Purinson, Y.; Fainshtein, R.; Radchenko, Y.; Kedem, O. Surface modification of commercial composite polyamide reverse osmosis membranes. J. Membr. Sci. 1998, 139, 175–181. [Google Scholar] [CrossRef]
- Chisca, S.; Marchesi, T.; Falca, G.; Musteata, V.-E.; Huang, T.; Abou-Hamad, E.; Nunes, S.P. Organic solvent and thermal resistant polytriazole membranes with enhanced mechanical properties cast from solutions in non-toxic solvents. J. Membr. Sci. 2020, 597, 117634. [Google Scholar] [CrossRef]
- Kono, H.; Nakamura, T.; Hashimoto, H.; Shimizu, Y. Characterization, molecular dynamics, and encapsulation ability of β-cyclodextrin polymers crosslinked by polyethylene glycol. Carbohydr. Polym. 2015, 128, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Tavsanli, B.; Okay, O. Preparation and fracture process of high strength hyaluronic acid hydrogels cross-linked by ethylene glycol diglycidyl ether. React. Funct. Polym. 2016, 109, 42–51. [Google Scholar] [CrossRef]
- Chisca, S.; Falca, G.; Musteata, V.E.; Boi, C.; Nunes, S.P. Crosslinked polytriazole membranes for organophilic filtration. J. Membr. Sci. 2017, 528, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Zerbinati, N.; Esposito, C.; Cipolla, G.; Calligaro, A.; Monticelli, D.; Martina, V.; Golubovic, M.; Binic, I.; Sigova, J.; Gallo, A.L. Chemical and mechanical characterization of hyaluronic acid hydrogel cross-linked with polyethylen glycol and its use in dermatology. Dermatol. Ther. 2020, 33, e13747. [Google Scholar] [CrossRef]
- Calles, J.A.; Tartara, L.I.; Lopez-García, A.; Diebold, Y.; Palma, S.D.; Valles, E.M. Novel bioadhesive hyaluronan–itaconic acid crosslinked films for ocular therapy. Int. J. Pharm. 2013, 455, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Chirico, F.; Colella, G.; Cortese, A.; Bove, P.; Fragola, R.; Rugge, L.; Audino, G.; Sgaramella, N.; Tartaro, G. Non-Surgical Touch-Up with Hyaluronic Acid Fillers Following Facial Reconstructive Surgery. Appl. Sci. 2021, 11, 7507. [Google Scholar] [CrossRef]
- Tezel, A.; Fredrickson, G.H. The science of hyaluronic acid dermal fillers. J. Cosmet. Laser Ther. 2008, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ulubayram, K.; Aksu, E.; Gurhan, S.I.D.; Serbetci, K.; Hasirci, N. Cytotoxicity evaluation of gelatin sponges prepared with different cross-linking agents. J. Biomater. Sci. Polym. Ed. 2002, 13, 1203–1219. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.C.; Chang, W.H.; Lin, K.J.; Sung, H.W. Genipin-crosslinked gelatin microspheres as a drug carrier for intramuscular administration: In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2003, 65, 271–282. [Google Scholar] [CrossRef]
- Geier, J.; Lessmann, H.; Hillen, U.; Jappe, U.; Dickel, H.; Koch, P.; Frosch, P.J.; Schnuch, A.; Uter, W. An attempt to improve diagnostics of contact allergy due to epoxy resin systems. First results of the multicentre study EPOX 2002. Contact Dermat. 2004, 51, 263–272. [Google Scholar] [CrossRef]
- Jolanki, R.; Estlander, T.; Kanerva, L. Contact allergy to an epoxy reactive diluent: 1, 4-butanediol diglycidyl ether. Contact Dermat. 1987, 16, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Yune, J.H.; Kwon, H.C.; Shin, D.-M.; Sohn, H.; Lee, K.H.; Choi, B.; Kim, E.S.; Kang, J.H.; Kim, E.K. In vitro toxicity assessment of crosslinking agents used in hyaluronic acid dermal filler. Toxicol. In Vitro 2021, 70, 105034. [Google Scholar] [CrossRef]
- Jeong, C.H.; Kwon, H.C.; Kim, D.H.; Cheng, W.N.; Kang, S.; Shin, D.-M.; Yune, J.H.; Yoon, J.E.; Chang, Y.H.; Sohn, H. Effects of aluminum on the integrity of the intestinal epithelium: An in vitro and in vivo study. Environ. Health Perspect. 2020, 128, 017013. [Google Scholar] [CrossRef] [Green Version]
- Damiano Monticelli, V.M.; Mocchi, R.; Rauso, R.; Zerbinati, U.; Cipolla, G.; Zerbinati, N. Chemical characterization of hydrogels crosslinked with polyethylene glycol for soft tissue augmentation. Open Access Maced. J. Med Sci. 2019, 7, 1077. [Google Scholar] [CrossRef] [Green Version]
- Kono, H. Characterization and properties of carboxymethyl cellulose hydrogels crosslinked by polyethylene glycol. Carbohydr. Polym. 2014, 106, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boulle, K.; Glogau, R.; Kono, T.; Nathan, M.; Tezel, A.; Roca-Martinez, J.X.; Paliwal, S.; Stroumpoulis, D. A review of the metabolism of 1, 4-Butanediol Diglycidyl ether–crosslinked hyaluronic acid dermal fillers. Dermatol. Surg. 2013, 39, 1758–1766. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.C.; Yoo, M.A.; Lee, S.Y.; Lee, H.J.; Son, D.H.; Jung, J.; Noh, I.; Kim, C.W. Modulation of biomechanical properties of hyaluronic acid hydrogels by crosslinking agents. J. Biomed. Mater. Res. Part A 2015, 103, 3072–3080. [Google Scholar] [CrossRef]
- OECD. OECD Guideline for Testing of Chemicals. Guideline 402: Acute Dermal Toxicity; OECD: Paris, France, 1987. [Google Scholar]
- Van Dyke, S.; Hays, G.P.; Caglia, A.E.; Caglia, M. Severe acute local reactions to a hyaluronic acid-derived dermal filler. J. Clin. Aesthetic Dermatol. 2010, 3, 32. [Google Scholar]
- Choi, W.I.; Hwang, Y.; Sahu, A.; Min, K.; Sung, D.; Tae, G.; Chang, J.H. An injectable and physical levan-based hydrogel as a dermal filler for soft tissue augmentation. Biomater. Sci. 2018, 6, 2627–2638. [Google Scholar] [CrossRef] [PubMed]
- Kucharzewski, M.; Rojczyk, E.; Wilemska-Kucharzewska, K.; Wilk, R.; Hudecki, J.; Los, M.J. Novel trends in application of stem cells in skin wound healing. Eur. J. Pharmacol. 2019, 843, 307–315. [Google Scholar] [CrossRef]
- Catlin, N.R.; Herbert, R.; Janardhan, K.; Hejtmancik, M.R.; Fomby, L.M.; Vallant, M.; Kissling, G.E.; DeVito, M.J. Dose-response assessment of the dermal toxicity of Virginia cedarwood oil in F344/N rats and B6C3F1/N mice. Food Chem. Toxicol. 2016, 98, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzbay, T.; Yertutanol, F.D.K.; Ahmet, M.; Cevreli, B. Subcutaneous Toxicity of Agmatine in Rats. Turk. J. Pharm. Sci. 2017, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Weil, C.; McCollister, D. Relationship between short-and long-term feeding studies in designing an effective toxicity test. J. Agric. Food Chem. 1963, 11, 486. [Google Scholar] [CrossRef]
- OECD. Environment Directorate, Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology; OECD: Paris, France, 2000. [Google Scholar]
- Yang, P.; Wang, Y.; Tang, W.; Sun, W.; Ma, Y.; Lin, S.; Jing, J.; Jiang, L.; Shi, H.; Song, Z. Western diet induces severe nonalcoholic steatohepatitis, ductular reaction, and hepatic fibrosis in liver CGI-58 knockout mice. Sci. Rep. 2020, 10, 4701. [Google Scholar] [CrossRef]
- Reza, M.S.A.; Hasan, M.M.; Kamruzzaman, M.; Hossain, M.I.; Zubair, M.A.; Bari, L.; Abedin, M.Z.; Reza, M.A.; Khalid-Bin-Ferdaus, K.M.; Haque, K.M.F. Study of a common azo food dye in mice model: Toxicity reports and its relation to carcinogenicity. Food Sci. Nutr. 2019, 7, 667–677. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr266. [Google Scholar] [CrossRef] [Green Version]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Reinke, J.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liang, X.; Chai, J.; Cui, Z.; Wang, Q.; He, W.; Liu, X.; Liu, Z.; Cui, G.; Feng, J. High Performance Solid Polymer Electrolytes for Rechargeable Batteries: A Self-Catalyzed Strategy toward Facile Synthesis. Adv. Sci. 2017, 4, 1700174. [Google Scholar] [CrossRef] [PubMed]
- Roig-Roig, F.; Solans, C.; Esquena, J.; García-Celma, M.J. Preparation, characterization, and release properties of hydrogels based on hyaluronan for pharmaceutical and biomedical use. J. Appl. Polym. Sci. 2013, 130, 1377–1382. [Google Scholar] [CrossRef]
- Nishi, C.; Nakajima, N.; Ikada, Y. In vitro evaluation of cytotoxicity of diepoxy compounds used for biomaterial modification. J. Biomed. Mater. Res. 1995, 29, 829–834. [Google Scholar] [CrossRef]
- Manson, M.M. Epoxides—Is there a human health problem? Occup. Environ. Med. 1980, 37, 317–336. [Google Scholar] [CrossRef]



| Group | Injection Dose (μg PEGDE/mouse) | PEGDE Volume (μL) | PBS Volume (μL) | Total Volume (μL/mouse) |
|---|---|---|---|---|
| PBS | 0 | 0 | 200 | 200 |
| PEGDE500 | 500 | 0.439 | 199.561 | 200 |
| PEGDE1000 | 1000 | 0.877 | 199.123 | 200 |
| PEGDE5000 | 5000 | 4.386 | 195.614 | 200 |
| PEGDE10000 | 10,000 | 8.772 | 191.228 | 200 |
| PEGDE20000 | 20,000 | 17.544 | 182.456 | 200 |
| PEGDE40000 | 40,000 | 35.088 | 164.912 | 200 |
| Cage-Side Observation | |||||||
|---|---|---|---|---|---|---|---|
| Sample | PBS | PEGDE | |||||
| PEGDE Dose (μg/mouse) | 0 | 500 | 1000 | 5000 | 10,000 | 20,000 | 40,000 |
| ppm | 0 | 14 | 29 | 143 | 286 | 571 | 1143 |
| Mortality | 0 * | 0 | 0 | 0 | 0 | 0 | 1 |
| Morbidity | 0 | 0 | 0 | 0 | 0 | 1 | 3 |
| Appearance | 0 | 0 | 0 | 0 | 1 | 1 | 3 |
| Behavior pattern | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Gait | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Condition of the fur | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| Breathing abnormalities | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Organ Weights | ||||||
|---|---|---|---|---|---|---|
| Sample | PBS | PEGDE | ||||
| PEGDE Dose (μg/mouse) | 0 | 500 | 1000 | 5000 | 10,000 | 20,000 |
| ppm | 0 | 14 | 29 | 143 | 286 | 571 |
| Absolute organ weight | ||||||
| Liver (g) | 1.41 ± 0.03 a | 1.27 ± 0.05 a | 1.25 ± 0.01 a | 1.38 ± 0.06 a | 1.33 ± 0.07 a | 1.25 ± 0.05 a |
| Kidney (g) | 0.46 ± 0.07 a | 0.42 ± 0.01 a | 0.43 ± 0.02 a | 0.45 ± 0.01 a | 0.47 ± 0.01 a | 0.45 ±0.02 a |
| Spleen (g) | 0.13 ± 0.01 a | 0.13 ± 0.01 a | 0.13 ± 0.01 a | 0.14 ± 0.01 a | 0.13 ± 0.01 a | 0.13 ± 0.01 a |
| Relative organ weight | ||||||
| Liver (% of body weight) | 5.67 ± 0.14 a | 5.21 ± 0.25 a | 5.01 ± 0.1 a | 5.46 ± 0.23 a | 5.26 ± 0.19 a | 5.14 ± 0.15 a |
| Kidney (% of body weight) | 1.86 ± 0.28 a | 1.74 ± 0.04 a | 1.72 ± 0.07 a | 1.79 ± 0.03 a | 1.86 ± 0.02 a | 1.84 ± 0.05 a |
| Spleen (% of body weight) | 0.51 ± 0.03 a | 0.52 ± 0.03 a | 0.51 ± 0.03 a | 0.6 ± 0.02 a | 0.53 ± 0.02 a | 0.53 ± 0.02 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Han, J.-H.; Kwon, H.-C.; Lim, S.-J.; Han, S.-G.; Jung, H.-S.; Lee, K.-H.; Kang, J.-H.; Han, S.-G. Toxicity Assessment of a Single Dose of Poly(ethylene glycol) Diglycidyl Ether (PEGDE) Administered Subcutaneously in Mice. Toxics 2021, 9, 354. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9120354
Kim D-H, Han J-H, Kwon H-C, Lim S-J, Han S-G, Jung H-S, Lee K-H, Kang J-H, Han S-G. Toxicity Assessment of a Single Dose of Poly(ethylene glycol) Diglycidyl Ether (PEGDE) Administered Subcutaneously in Mice. Toxics. 2021; 9(12):354. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9120354
Chicago/Turabian StyleKim, Do-Hyun, Jong-Hyeon Han, Hyuk-Cheol Kwon, Su-Jin Lim, Seo-Gu Han, Hyun-Su Jung, Keyong-Ho Lee, Ju-Hee Kang, and Sung-Gu Han. 2021. "Toxicity Assessment of a Single Dose of Poly(ethylene glycol) Diglycidyl Ether (PEGDE) Administered Subcutaneously in Mice" Toxics 9, no. 12: 354. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9120354