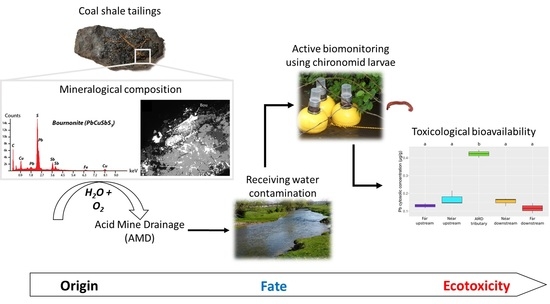
A Multidisciplinary Approach for the Assessment of Origin, Fate and Ecotoxicity of Metal(loid)s from Legacy Coal Mine Tailings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling Strategy
2.2.1. Coal Tailing Sampling from the Heap
2.2.2. Water and Suspended Particles Sampling
2.3. Analyses of Materials from the Coal Waste Dump
2.3.1. Mineralogical Characterization
2.3.2. Elemental Composition
2.4. Water and Suspended Particle Analyses
2.4.1. Physico-chemical Analyses of Water
2.4.2. Suspended Particle Contamination
2.5. Ecotoxicological Assessment
2.5.1. Obtaining Chironomus Rirarius Larvae
2.5.2. Active Biomonitoring Campaign
2.5.3. Tissue Fractionation and Metal Analyses
2.6. Statistical Analyses
3. Results
3.1. State of Contamination of the Beuveroux and AMD Identification
3.2. Composition of Materials from the Heap
3.3. Biological Responses of Chironomus Riparius Larvae
3.3.1. Mortality and Growth of Individuals
3.3.2. Emergence Kinetics
3.3.3. Bioaccumulation of Metals
4. Discussion
4.1. From the Elementary and Mineralogical Composition of Materials to Aquatic Compartment Contamination
4.1.1. Origin of the AMD Contamination
4.1.2. Contamination of the AMD Receiving Waters
4.2. Water Quality and Its Implications for the Biotic Compartment
4.2.1. Ecotoxicity of AMD and the Beuveroux
4.2.2. Internal Distribution and Toxicological Bioavailability of Metal(loid)s to C. Riparius
4.3. Mitigation and Remediation Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nriagu, J.O. A History of Global Metal Pollution. Science 1996, 272, 223. [Google Scholar] [CrossRef]
- Tylecotte, R.F. The Early History of Metallurgy in Europe; Longman, Ed.; Cambridge University Press: London, UK; New York, NY, USA, 1987. [Google Scholar]
- Gałuszka, A.; Migaszewski, Z.; Namiesnik, J. The role of analytical chemistry in the study of the Anthropocene. TrAC Trends Anal. Chem. 2017, 97, 146–152. [Google Scholar] [CrossRef]
- Beane, S.J.; Comber, S.D.; Rieuwerts, J.; Long, P. Abandoned metal mines and their impact on receiving waters: A case study from Southwest England. Chemosphere 2016, 153, 294–306. [Google Scholar] [CrossRef]
- Jain, R.K.; Cui, Z.C.; Domen, J.K. Environmental Impacts of Mining. In Environmental Impact of Mining and Mineral Processing; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 53–157. [Google Scholar]
- Chudy, K.; Marszałek, H.; Kierczak, J. Impact of hard-coal waste dump on water quality — A case study of Ludwikowice Kłodzkie (Nowa Ruda Coalfield, SW Poland). J. Geochem. Explor. 2014, 146, 127–135. [Google Scholar] [CrossRef]
- Peijnenburg, W.J.G.M. Implementation of Bioavailability in Prospective and Retrospective Risk Assessment of Chemicals in Soils and Sediments; Barceló, D., Kostianoy, A.G., Eds.; The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–32. [Google Scholar]
- ISO 19204. Soil Quality. Procedure for Site-Specific Ecological Risk Assessment of Soil Contamination; (Soil Quality TRIAD Approach); International Standardization Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Mariet, A.-L.; Gauthier-Manuel, H.; Lagiewski, T.; Bégeot, C.; Walter-Simonnet, A.-V.; Gimbert, F. Impact assessment of legacy wastes from ancient mining activities on current earthworm community. J. Hazard. Mater. 2020, 393, 122369. [Google Scholar] [CrossRef]
- Pauwels, M.; Frérot, H.; Souleman, D.; Vandenbulcke, F. Using biomarkers in an evolutionary context: Lessons from the analysis of biological responses of oligochaete annelids to metal exposure. Environ. Pollut. 2013, 179, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Holt, E.A.; Miller, S.W. Bioindicators: Using Organisms to Measure Environmental Impacts. Nat. Educ. Knowl. 2010, 3, 8. [Google Scholar]
- Parmar, T.K.; Rawtani, D.; Agrawal, Y.K. Bioindicators: The natural indicator of environmental pollution. Front. Life Sci. 2016, 9, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Gimbert, F.; Moyen, C.; Chanez, E.; Petitjean, Q.; Al-Ashoor, A.; Aleya, L.; Verneaux, V. Les larves de Chironomidae dans les approches écotoxicologiques d’évaluation de la qualité des milieux aquatiques. In Eaux Industrielles Contaminées: Réglementation, Paramètres Chimiques et Biologiques & Procédés D’épuration Innovants; Morin-Crini, N., Crini, G., Eds.; Presses Universitaires de Franche-Comté: Besançon, France, 2017; pp. 207–240. [Google Scholar]
- Ferrari, B.J.; Faburé, J. Field assessment of reproduction-related traits of chironomids using a newly developed emergence platform (E-Board). Ecotoxicol. Environ. Saf. 2017, 137, 186–193. [Google Scholar] [CrossRef]
- Gimbert, F.; Petitjean, Q.; Al-Ashoor, A.; Cretenet, C.; Aleya, L. Encaged Chironomus riparius larvae in assessment of trace metal bioavailability and transfer in a landfill leachate collection pond. Environ. Sci. Pollut. Res. 2017, 25, 11303–11312. [Google Scholar] [CrossRef]
- Gimbert, F.; Geffard, A.; Guédron, S.; Dominik, J.; Ferrari, B.J. Mercury tissue residue approach in Chironomus riparius: Involvement of toxicokinetics and comparison of subcellular fractionation methods. Aquat. Toxicol. 2016, 171, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peijnenburg, W.; Posthuma, L.; Eijsackers, H.; Allen, H. A Conceptual Framework for Implementation of Bioavailability of Metals for Environmental Management Purposes. Ecotoxicol. Environ. Saf. 1997, 37, 163–172. [Google Scholar] [CrossRef]
- Paller, M.H.; Knox, A.S. Bioavailability of Metals in Contaminated Sediments. In E3S Web of Conferences; EDP Sciences: Aiken, SC, USA, 2013; p. 02001. [Google Scholar]
- Contini, D. Notice Explicative, Carte Géol. France (1/50 000), Feuille Lure (443); BRGM: Orléans, France, 2000; p. 68. [Google Scholar]
- Mathieu, G. Analyse stratigraphique de la série carbonifère dans le bassin houiller de Ronchamp. Comptes Rendus Acad. Sci. 1947, 225, 1016–1018. [Google Scholar]
- ISO 14869-1. Soil Quality—Dissolution for the Determination of Total Element Content—Part 1: Dissolution with Hydrofluoric and Perchloric Acids; International Organization for Standardization: Geneva, Switzerland, 2001. [Google Scholar]
- OECD. Test No. 218: Sediment-Water Chironomid Toxicity Using Spiked Sediment. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2004; pp. 1–21. [Google Scholar]
- Péry, A.R.; Mons, R.; Flammarion, P.; Lagadic, L.; Garric, J. A modelling approach to link food availability, growth emergence, and reproduction for the midge chironomos riparius. Environ. Toxicol. Chem. 2002, 21, 2507. [Google Scholar] [CrossRef]
- Péry, A.R.; Geffard, A.; Conrad, A.; Mons, R.; Garric, J. Assessing the risk of metal mixtures in contaminated sediments on Chironomus riparius based on cytosolic accumulation. Ecotoxicol. Environ. Saf. 2008, 71, 869–873. [Google Scholar] [CrossRef]
- Rainbow, P.S.; Luoma, S.N.; Wang, W.-X. Trophically available metal—A variable feast. Environ. Pollut. 2011, 159, 2347–2349. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 31 May 2021).
- James, A.; Bonnomet, V.; Morin, A.; Fribourg-Blanc, B. Implementation of Requirements on Priority Substances within the Context of the Water Framework Directive. Prioritiza-tion Process: Monitoring-Based Ranking. 2009. Available online: https://www.scienceopen.com/document?vid=69ddb09c-08bd-4829-b377-2fa57ce22c96 (accessed on 31 May 2021).
- De Deckere, E.; De Cooman, W.; Leloup, V.; Meire, P.; Schmitt, C.; Von Der Ohe, P.C. Development of sediment quality guidelines for freshwater ecosystems. J. Soils Sediments 2011, 11, 504–517. [Google Scholar] [CrossRef]
- Clark, E.V.; Daniels, W.L.; Zipper, C.E.; Eriksson, K. Mineralogical influences on water quality from weathering of surface coal mine spoils. Appl. Geochem. 2018, 91, 97–106. [Google Scholar] [CrossRef]
- Mehta, N.; Kocar, B.D. Geochemical conditions conducive for retention of trace elements and radionuclides during shale–fluid interactions. Environ. Sci. Process. Impacts 2019, 21, 1764–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, M.; Moncur, M.; Bain, J.G.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Geochemical and mineralogical aspects of sulfide mine tailings. Appl. Geochem. 2015, 57, 157–177. [Google Scholar] [CrossRef]
- Blowes, D.; Ptacek, C.; Jambor, J.; Weisener, C.; Paktunc, D.; Gould, W.; Johnson, D. The Geochemistry of Acid Mine Drainage. In Treatise on Geochemistry; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 131–190. [Google Scholar]
- Kappler, A.; Straub, K.L. Geomicrobiological Cycling of Iron. Mol. Geomicrobiol. 2005, 59, 85–108. [Google Scholar] [CrossRef]
- Carlson, L.; Bigham, J.M.; Schwertmann, U.; Kyek, A.; Wagner, F. Scavenging of As from Acid Mine Drainage by Schwertmannite and Ferrihydrite: A Comparison with Synthetic Analogues. Environ. Sci. Technol. 2002, 36, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Máša, B.; Pulišová, P.; Bezdicka, P.; Michalková, E.; Subrt, J. Ochre precipitates and acid mine drainage in a mine environment. Ceram. Silik. 2012, 56, 9–14. [Google Scholar]
- Hammarstrom, J.; Seal, R.; Meier, A.; Kornfeld, J. Secondary sulfate minerals associated with acid drainage in the eastern US: Recycling of metals and acidity in surficial environments. Chem. Geol. 2005, 215, 407–431. [Google Scholar] [CrossRef] [Green Version]
- García-Lorenzo, M.L.; Marimón, J.; Navarro-Hervás, M.C.; Perezsirvent, C.; Martínez-Sánchez, M.J.; Molina-Ruiz, J. Impact of acid mine drainages on surficial waters of an abandoned mining site. Environ. Sci. Pollut. Res. 2016, 23, 6014–6023. [Google Scholar] [CrossRef]
- BRGM. Identification des zones à risque de fond géochimique élevé en éléments traces dans les cours d’eau et les eaux souterraines du bassin Rhône-Méditerranée et Corse. 2005. 85–88. Available online: http://infoterre.brgm.fr/rapports/RP-54031-FR.pdf (accessed on 8 August 2018).
- Palma, P.; Ledo, L.; Alvarenga, P. Ecotoxicological endpoints, are they useful tools to support ecological status assessment in strongly modified water bodies? Sci. Total. Environ. 2016, 541, 119–129. [Google Scholar] [CrossRef] [PubMed]
- De Bisthoven, L.J.; Gerhardt, A.; Soares, A.M.V.M. Effects of acid mine drainage on larval chironomus (diptera, chironomidae) measured with the multispecies freshwatter biomonitor®. Environ. Toxicol. Chem. 2004, 23, 1123–1128. [Google Scholar] [CrossRef]
- Péry, A.R.; Garric, J. Modelling Effects of Temperature and Feeding Level on the Life Cycle of the Midge Chironomus Riparius: An Energy-Based Modelling Approach. Hydrobiology 2006, 553, 59–66. [Google Scholar] [CrossRef]
- Haas, E.M.; Kraak, M.H.A. Analyzing the causes for the persistence of chironomids in floodplain lake sediments. Archiv für Hydrobiol. 2005, 162, 211–228. [Google Scholar] [CrossRef]
- Kooijman, S.A.L.M.; Baas, J.; Bontje, D.; Broerse, M.; van Gestel, C.A.M.; Jager, T. Ecotoxicological Applications of Dynamic Energy Budget Theory. Wildl. Ecotoxicol. 2009, 237–259. [Google Scholar] [CrossRef]
- Warren, L.A.; Haack, E.A. Biogeochemical controls on metal behaviour in freshwater environments. Earth Sci. Rev. 2001, 54, 261–320. [Google Scholar] [CrossRef]
- Dumas, J.; Hare, L. The Internal Distribution of Nickel and Thallium in Two Freshwater Invertebrates and its Relevance to Trophic Transfer. Environ. Sci. Technol. 2008, 42, 5144–5149. [Google Scholar] [CrossRef]
- Lu, Y.; E Allen, H. Partitioning of copper onto suspended particulate matter in river waters. Sci. Total. Environ. 2001, 277, 119–132. [Google Scholar] [CrossRef]
- Baudin, J.; Nucho, R. 60Co accumulation from sediment and planktonic algae by midge larvae (Chironomus luridus). Environ. Pollut. 1992, 76, 133–140. [Google Scholar] [CrossRef]
- Timmermans, K.R.; Peeters, W.; Tonkes, M. Cadmium, zinc, lead and copper inChironomus riparius (Meigen) larvae (Diptera, Chironomidae): Uptake and effects. Hydrobiology 1992, 241, 119–134. [Google Scholar] [CrossRef]
- Burgos, M.; Rainbow, P. Uptake, Accumulation and Excretion byCorophium volutator(Crustacea: Amphipoda) of Zinc, Cadmium and Cobalt Added to Sewage Sludge. Estuarine Coast. Shelf Sci. 1998, 47, 603–620. [Google Scholar] [CrossRef]
- Amiard, J.-C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef] [PubMed]
- Toušová, Z.; Kuta, J.; Hynek, D.; Adam, V.; Kizek, R.; Bláha, L.; Hilscherová, K. Metallothionein modulation in relation to cadmium bioaccumulation and age-dependent sensitivity of Chironomus riparius larvae. Environ. Sci. Pollut. Res. 2016, 23, 10504–10513. [Google Scholar] [CrossRef]
- House, J.E. Chemistry of Coordination Compounds; Inorganic Chemistry Academic Press: Burlington, MA, USA, 2008; pp. 582–583. [Google Scholar]
- Belowitz, R.; Leonard, E.M.; O’Donnell, M.J. Effects of exposure to high concentrations of waterborne Tl on K and Tl concentrations in Chironomus riparius larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 166, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Belowitz, R.; O’Donnell, M.J. Ion-selective microelectrode measurements of Tl+ and K+ transport by the gut and associated epithelia in Chironomus riparius. Aquat. Toxicol. 2013, 138–139, 70–80. [Google Scholar] [CrossRef]
- Krantzberg, G.; Stokes, P.M. Metal Regulation, Tolerance, and Body Burdens in the Larvae of the Genus Chironomus. Can. J. Fish. Aquat. Sci. 1989, 46, 389–398. [Google Scholar] [CrossRef]
- Mogren, C.L.; Von Kiparski, G.R.; Parker, D.R.; Trumble, J.T. Survival, reproduction, and arsenic body burdens in Chironomus riparius exposed to arsenate and phosphate. Sci. Total. Environ. 2012, 425, 60–65. [Google Scholar] [CrossRef]
- Arambourou, H.; Gismondi, E.; Branchu, P.; Beisel, J.-N. Biochemical and morphological responses inChironomus riparius (Diptera, Chironomidae) larvae exposed to lead-spiked sediment. Environ. Toxicol. Chem. 2013, 32, 2558–2564. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, X.; Wei, X. Mine drainage: Remediation technology and resource recovery. Water Environ. Res. 2020, 92, 1533–1540. [Google Scholar] [CrossRef]
- Name, T.; Sheridan, C. Remediation of acid mine drainage using metallurgical slags. Miner. Eng. 2014, 64, 15–22. [Google Scholar] [CrossRef]
- Bwapwa, J.; Jaiyeola, A.; Chetty, R. Bioremediation of acid mine drainage using algae strains: A review. South Afr. J. Chem. Eng. 2017, 24, 62–70. [Google Scholar] [CrossRef]
- Mang, K.C.; Ntushelo, K. Phytoextraction and Phytostabilisation Approaches of heavy metal remediation in acid mine drainage with case studies: A Review. Appl. Ecol. Environ. Res. 2019, 17, 6129–6149. [Google Scholar] [CrossRef]







| Station | Al | As | Cd | Co | Cu | Ni | Pb | Tl | Zn | pH | Conducti-vity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Far upstream | 51.8 ± 21.4 | 5.52 ± 0.10 | 0.08 ± 0.02 | 0.23 ± 0.00 | <DL | 0.90 ± 0.06 | 0.28 ± 0.04 | 0.01 ± 0.00 | 41.0 ± 10.7 | 7.28 ± 0.03 | 116 ± 13 |
| Near usptream | 90.7 ± 24.8 | 9.19 ± 5.40 | 0.51 ± 0.61 | 0.33 | 14.6 | 3.28 ± 3.16 | 0.77 ± 0.64 | 0.04 ± 0.04 | 205 ± 217 | 7.19 ± 0.11 | 122 ± 18 |
| AMD Tributary | 15,585 ± 6611 | 1.31 ± 0.81 | 226 ± 185 | 158 ± 25 | 116 ± 48 | 270 ± 119 | 109 ± 82 | 0.81 ± 0.70 | 27,825 ± 12,551 | 4.20 ± 0.06 | 655 ± 12 |
| Near downstream | 107 ± 50 | 4.71 ± 0.30 | 0.96 ± 0.32 | 1.68 ± 0.63 | 4.62 | 3.11 ± 0.79 | 0.60 ± 0.20 | 0.02 ± 0.00 | 344 ± 110 | 6.76 ± 0.01 | 157 ± 47 |
| Far downstream | 211 ± 20 | 12.4 ± 4.3 | 4.79 ± 2.21 | 6.49 ± 2.93 | 12.7 ± 12.9 | 11.9 ± 4.3 | 2.46 ± 0.36 | 0.08 ± 0.01 | 1349 ± 555 | 7.29 ± 0.00 | 126 ± 19 |
| PNEC | 4.2 | 0.08 | 1.4 | 3.8 | 2.3 | 10.8 |
| Station | Al | As | Cd | Co | Cu | Ni | Pb | Tl | Zn |
|---|---|---|---|---|---|---|---|---|---|
| Far upstream | 13,906 | 50.7 | 3.31 | 9.34 | 36.6 | 17.9 | 55.8 | 0.51 | 747 |
| Near upstream | 17,610 | 84.8 | 5.30 | 14.6 | 38.5 | 22.0 | 63.4 | 0.56 | 1110 |
| Near downstream | 17,665 | 84.5 | 8.92 | 17.9 | 40.5 | 23.9 | 53.7 | 0.55 | 1784 |
| Far downstream | 21,044 | 129.6 | 28.4 | 42.4 | 67.0 | 35.9 | 71.9 | 0.62 | 3457 |
| Ratio far downstream/far upstream | 1.5 | 2.6 | 8.3 | 4.5 | 1.8 | 2.0 | 1.3 | 1.2 | 4.5 |
| Reference values * | 7.90 | 0.93 | 14.0 | 11.0 | 25.0 | 146 |
| Sampling Point | Al | As | Cd | Co | Cu | Fe | Ni | Pb | S | Sb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Former settling pond | 51,142 | 627.7 | 0.50 | 18.1 | 200.3 | 69,809 | 27.5 | 3223.7 | 9064 | 140.9 | 1267.0 |
| Proximity to AMD | 46,544 | 727.8 | 6.87 | 19.1 | 47.8 | 50,041 | 28.5 | 424.7 | 5377 | 40.00 | 990.1 |
| East border of the tailing | 48,032 | 661.8 | 3.48 | 13.4 | 44.5 | 37,524 | 17.1 | 424.9 | 4217 | 28.04 | 300.5 |
| Foot of the tailing | 25,400 | 11.6 | 3.35 | 7.2 | 34.5 | 12,660 | 20.8 | 269.4 | 3995 | 19.60 | 383.2 |
| West border of the tailing | 42,086 | 214.3 | 1.12 | 12.6 | 38.5 | 25,440 | 21.7 | 262.5 | 3085 | 18.86 | 258.7 |
| Station | A (%) | Kg (d−1) | I (d) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Estimated Value | Upper Limit | Lower Limit | Estimated Value | Upper Limit | LOWER Limit | Estimated Value | Upper Limit | |
| Far upstream | 64.1 | 71.4 ab | 78.6 | 0.352 | 0.728 ab | 1.104 | 15.7 | 17.0 ab | 18.4 |
| Near upstream | 51.6 | 57.9 a | 64.3 | 0.840 | 1.001 a | 1.162 | 15.6 | 16.2 a | 16.8 |
| AMD Tributary | - | - | - | - | - | - | - | - | - |
| Near downstream | 67.7 | 71.7 b | 75.6 | 0.374 | 0.539 b | 0.704 | 16.6 | 17.4 ab | 18.2 |
| Far downstream | 78.8 | 82.2 c | 85.6 | 0.502 | 0.637 b | 0.772 | 17.7 | 18.0 b | 18.2 |
| Station | Fraction | Al | As | Cd | Co | Cu | Ni | Pb | Tl | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| Near downstream | Soluble | 2.0 ± 0.3 | 16.3 ± 0.4 | 63.8 ± 0.3 | 28.7 ± 3.6 | 61.6 ± 1.8 | 25.3 ± 3.7 | 6.1 ± 1.0 | 20.0 ± 3.8 | 37.8 ± 1.7 |
| AMD Tributary | 2.3 ± 0.1 | 5.4 ± 0.9 | 59.6 ± 3.5 | 23.1 ± 14.0 | 45.3 ± 3.6 | 5.5 ± 0.6 | 3.6 ± 0.6 | 44.9 ± 6.1 | 19.6 ± 2.2 | |
| Near downstream | Insoluble | 98.0 ± 0.3 | 83.7 ± 0.4 | 36.2 ± 0.3 | 71.3 ± 3.6 | 38.4 ± 1.8 | 74.7 ± 3.7 | 93.9 ± 1.0 | 80.0 ± 3.8 | 62.2 ± 1.7 |
| AMD Tributary | 97.7 ± 0.1 | 94.6 ± 0.9 | 40.5 ± 3.5 | 76.9 ± 14.0 | 54.7 ± 3.6 | 94.6 ± 0.6 | 96.4 ± 0.5 | 55.1 ± 6.1 | 80.4 ± 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gauthier-Manuel, H.; Radola, D.; Choulet, F.; Buatier, M.; Vauthier, R.; Morvan, T.; Chavanne, W.; Gimbert, F. A Multidisciplinary Approach for the Assessment of Origin, Fate and Ecotoxicity of Metal(loid)s from Legacy Coal Mine Tailings. Toxics 2021, 9, 164. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9070164
Gauthier-Manuel H, Radola D, Choulet F, Buatier M, Vauthier R, Morvan T, Chavanne W, Gimbert F. A Multidisciplinary Approach for the Assessment of Origin, Fate and Ecotoxicity of Metal(loid)s from Legacy Coal Mine Tailings. Toxics. 2021; 9(7):164. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9070164
Chicago/Turabian StyleGauthier-Manuel, Honorine, Diane Radola, Flavien Choulet, Martine Buatier, Raphaël Vauthier, Tatiana Morvan, Walter Chavanne, and Frédéric Gimbert. 2021. "A Multidisciplinary Approach for the Assessment of Origin, Fate and Ecotoxicity of Metal(loid)s from Legacy Coal Mine Tailings" Toxics 9, no. 7: 164. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9070164