Pharmacological Modulation of Behaviour, Serotonin and Dopamine Levels in Daphnia magna Exposed to the Monoamine Oxidase Inhibitor Deprenyl
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
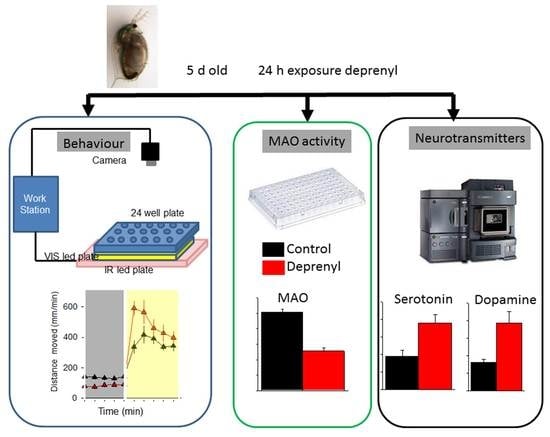
2.2. Experimental Procedures
2.3. Behavioural Analysis
2.4. Daphnia Monoamine Oxidase (MAO) Activity
2.5. Extraction and Analysis of Neurotransmitters
2.6. Statistical Analysis
3. Results
3.1. Behaviour
3.2. Biochemical and Neurotransmitter Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burks, R.L.; Jeppesen, E.; Lodge, D.M. Littoral zone structures as Daphnia refugia against fish predators. Limnol. Oceanogr. 2001, 46, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Bedrossiantz, J.; Martínez-Jerónimo, F.; Bellot, M.; Raldua, D.; Gómez-Canela, C.; Barata, C. A high-throughput assay for screening environmental pollutants and drugs impairing predator avoidance in Daphnia magna. Sci. Total Environ. 2020, 740. [Google Scholar] [CrossRef]
- Faria, M.; Prats, E.; Novoa-Luna, K.A.; Bedrossiantz, J.; Gómez-Canela, C.; Gómez-Oliván, L.M.; Raldúa, D. Development of a vibrational startle response assay for screening environmental pollutants and drugs impairing predator avoidance. Sci. Total Environ. 2019, 650, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Busch, W.; Schmidt, S.; Kühne, R.; Schulze, T.; Krauss, M.; Altenburger, R. Micropollutants in European rivers: A mode of action survey to support the development of effect-based tools for water monitoring. Environ. Toxicol. Chem. 2016, 35, 1887–1899. [Google Scholar] [CrossRef] [Green Version]
- Fong, P.P.; Ford, A.T. The biological effects of antidepressants on the molluscs and crustaceans: A review. Aquat. Toxicol. 2014, 151, 4–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, B.W.; Foran, C.M.; Richards, S.M.; Weston, J.; Turner, P.K.; Stanley, J.K.; Solomon, K.R.; Slattery, M.; La Point, T.W. Aquatic ecotoxicology of fluoxetine. Toxicol. Lett. 2003, 142, 169–183. [Google Scholar] [CrossRef]
- Orsini, L.; Gilbert, D.; Podicheti, R.; Jansen, M.; Brown, J.B.; Solari, O.S.; Spanier, K.I.; Colbourne, J.K.; Rush, D.; Decaestecker, E. Daphnia magna transcriptome by RNA-Seq across 12 environmental stressors. Sci. Data 2016, 3, 1–16. [Google Scholar] [CrossRef]
- Gunnarsson, L.; Jauhiainen, A.; Kristiansson, E.; Nerman, O.; Larsson, D.G.J. Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ. Sci. Technol. 2008, 42, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Rivetti, C.; Campos, B.; Piña, B.; Raldúa, D.; Kato, Y.; Watanabe, H.; Barata, C. Tryptophan hydroxylase (TRH) loss of function mutations induce growth and behavioral defects in Daphnia magna. Sci. Rep. 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, B.; Rivetti, C.; Kress, T.; Barata, C.; Dircksen, H. Depressing Antidepressant: Fluoxetine Affects Serotonin Neurons Causing Adverse Reproductive Responses in Daphnia magna. Environ. Sci. Technol. 2016, 50. [Google Scholar] [CrossRef]
- Fuertes, I.; Barata, C. Characterization of neurotransmitters and related metabolites in Daphnia magna juveniles deficient in serotonin and exposed to neuroactive chemicals that affect its behavior: A targeted LC-MS/MS method. Chemosphere 2021, 263, 127814. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Prats, E.; Bellot, M.; Gomez-Canela, C.; Raldúa, D. Pharmacological modulation of serotonin levels in zebrafish larvae: Lessons for identifying environmental neurotoxicants targeting the serotonergic system. Toxics 2021, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.J.; Cummings, J.L. Parkinson’s Disease: Neurobehavioral Aspects; Oxford University Press: Oxford, UK, 1992; ISBN 0195069692. [Google Scholar]
- Riederer, P.; Youdim, M.B.H.; Rausch, W.D.; Birkmayer, W.; Jellinger, K.; Seemann, D. On the mode of action of L-deprenyl in the human central nervous system. J. Neural Transm. 1978, 43, 217–226. [Google Scholar] [CrossRef]
- Sloley, B.D. Metabolism of Monoamines in Invertebrates: The Relative Importance of Monoamine Oxidase in Different Phyla. Neurotoxicology 2004, 25, 175–183. [Google Scholar] [CrossRef]
- Kadir, H.A.; Knowles, C.O. Oxidative deamination and N-acetylation of biogenic amines by homogenates of bulb mites (Rhizoglyphus echinopus). Comp. Biochem. Physiol. Part C Comp. 1989, 94, 465–468. [Google Scholar] [CrossRef]
- Kaufman, R.; Sloley, D. Catabolism of dopamine and 5-hydroxytryptamine by monoamine oxidase in the ixodid tick, Amblyomma hebraeum. Insect Biochem. Mol. Biol. 1996, 26, 101–109. [Google Scholar] [CrossRef]
- Basova, N.E.; Basova, I.N.; Yagodina, O. V Monoamine Oxidase Activity in the Hepatopancreas of the Kamchatka Crab Paralithodes camtschaticus: A Substrate–Inhibitor Specificity. J. Evol. Biochem. Physiol. 2018, 54, 345–352. [Google Scholar] [CrossRef]
- Setini, A.; Pierucci, F.; Senatori, O.; Nicotra, A. Molecular characterization of monoamine oxidase in zebrafish (Danio rerio). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 140, 153–161. [Google Scholar] [CrossRef]
- Sallinen, V.; Sundvik, M.; Reenilä, I.; Peitsaro, N.; Khrustalyov, D.; Anichtchik, O.; Toleikyte, G.; Kaslin, J.; Panula, P. Hyperserotonergic phenotype after monoamine oxidase inhibition in larval zebrafish. J. Neurochem. 2009, 109, 403–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finberg, J.P.M.; Rabey, J.M. Inhibitors of MAO-A and MAO-B in psychiatry and neurology. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Dong, B.; Ishii, K.; Kinemuchi, H. Brain Dialysis: In Vivo Metabolism of Dopamine and Serotonin by Monoamine Oxidase A but Not B in the Striatum of Unrestrained Rats. J. Neurochem. 1986, 46, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R. Cellular mechanisms of learning and the biological basis of individuality. Princ. Neural Sci. 1991, 3, 1009–1031. [Google Scholar]
- Bortolato, M.; Godar, S.C.; Davarian, S.; Chen, K.; Shih, J.C. Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase b-deficient mice. Neuropsychopharmacology 2009, 34, 2746–2757. [Google Scholar] [CrossRef] [Green Version]
- Van Swinderen, B.; Andretic, R. Dopamine in Drosophila: Setting arousal thresholds in a miniature brain. Proc. R. Soc. B Biol. Sci. 2011, 278, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.G.; Ullsperger, M. An update on the role of serotonin and its interplay with dopamine for reward. Front. Hum. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Kshama, D.; Hrishikeshavan, H.J.; Shanbhogue, R.; Munonyedi, U.S. Modulation of baseline behavior in rats by putative serotonergic agents in three ethoexperimental paradigms. Behav. Neural Biol. 1990, 54, 234–253. [Google Scholar] [CrossRef]
- Izquierdo, A.; Carlos, K.; Ostrander, S.; Rodriguez, D.; McCall-Craddolph, A.; Yagnik, G.; Zhou, F. Impaired reward learning and intact motivation after serotonin depletion in rats. Behav. Brain Res. 2012, 233, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Waider, J.; Popp, S.; Lange, M.D.; Kern, R.; Kolter, J.F.; Kobler, J.; Donner, N.C.; Lowe, K.R.; Malzbender, J.H.; Brazell, C.J.; et al. Genetically driven brain serotonin deficiency facilitates panic-like escape behavior in mice. Transl. Psychiatry 2017, 7, e1246. [Google Scholar] [CrossRef] [Green Version]
- Mather, M.; Clewett, D.; Sakaki, M.; Harley, C.W. Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav. Brain Sci. 2016, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| df | t,F | P | ||
|---|---|---|---|---|
| Experiment 1 | AUCh | 31 | 2.1 | 0.047 |
| AUCEPR | 31 | 0.4 | 0.657 | |
| Experiment 2 | AUCh | 31 | 3.3 | 0.003 |
| AUCEPR | 31 | 0.7 | 0.493 | |
| Experiment 3 | AUCh | 2,45 | 10.2 | <0.001 |
| AUCEPR | 2,45 | 0.8 | 0.459 | |
| Experiment 4 | AUCh | 2,56 | 10.0 | <0.001 |
| AUCEPR | 2,56 | 3.9 | 0.026 |
| df | F | P | ||
|---|---|---|---|---|
| Experiment 1 | Time | 1,22 | 16.6 | 0.001 |
| Treatment | 1,22 | 4.4 | 0.048 | |
| Time * Treatment | 1,22 | 0.0 | 0.923 | |
| Experiment 2 | Time | 1,46 | 12.6 | 0.001 |
| Treatment | 1,46 | 0.2 | 0.688 | |
| Time * Treatment | 1,46 | 4.2 | 0.047 | |
| Experiment 3 | Time | 1,69 | 10.9 | 0.002 |
| Treatment | 2,69 | 8.7 | <0.001 | |
| Time * Treatment | 2,69 | 5.7 | 0.005 | |
| Experiment 4 | Time | 1,68 | 17.9 | <0.001 |
| Treatment | 2,68 | 3.9 | 0.026 | |
| Time * Treatment | 2,68 | 4.3 | 0.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellot, M.; Faria, M.; Gómez-Canela, C.; Raldúa, D.; Barata, C. Pharmacological Modulation of Behaviour, Serotonin and Dopamine Levels in Daphnia magna Exposed to the Monoamine Oxidase Inhibitor Deprenyl. Toxics 2021, 9, 187. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9080187
Bellot M, Faria M, Gómez-Canela C, Raldúa D, Barata C. Pharmacological Modulation of Behaviour, Serotonin and Dopamine Levels in Daphnia magna Exposed to the Monoamine Oxidase Inhibitor Deprenyl. Toxics. 2021; 9(8):187. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9080187
Chicago/Turabian StyleBellot, Marina, Melissa Faria, Cristian Gómez-Canela, Demetrio Raldúa, and Carlos Barata. 2021. "Pharmacological Modulation of Behaviour, Serotonin and Dopamine Levels in Daphnia magna Exposed to the Monoamine Oxidase Inhibitor Deprenyl" Toxics 9, no. 8: 187. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9080187