Negative Consequences on the Growth, Morphometry, and Community Structure of the Kelp Macrocystis pyrifera (Phaeophyceae, Ochrophyta) by a Short Pollution Pulse of Heavy Metals and PAHs
Abstract
:1. Introduction
2. Materials and Methods
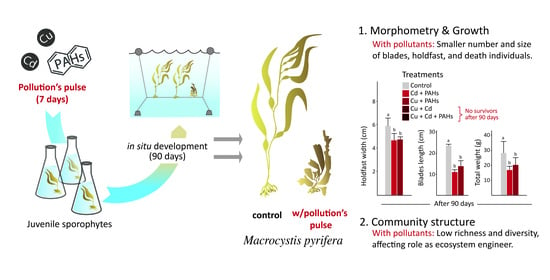
2.1. Exposure of M. pyrifera to Treatment
2.2. Growth and Morphometry
2.3. Community Structure
2.4. Statistical Analyses
3. Results
3.1. Growth and Morphometry of M. pyrifera
3.2. Community Structure Associated with M. pyrifera
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnston, E.L.; Mayer-Pinto, M.; Crowe, T.P. Chemical contaminant effects on marine ecosystem functioning. J. Appl. Ecol. 2015, 52, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Oyarzo-Miranda, C.; Latorre, N.; Meynard, A.; Rivas, J.; Bulboa, C.; Contreras-Porcia, L. Coastal pollution from the industrial park Quintero Bay of central Chile: Effects on abundance, morphology, and development of the kelp Lessonia spicata (Phaeophyceae). PLoS ONE 2020, 15, e0240581. [Google Scholar] [CrossRef]
- Zapata, M.; Lang, M.; Riso, R.; Moraga, D.; Riquelme, C. Trace metal and biomarker levels in tissues of Argopecten purpuratus in the north of Chile, and the potential use of this species as a bioindicator of metallic stress. Aquat. Living Resour. 2012, 25, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Valdés, J.; Guiñez, M.; Castillo, A.; Vega, S.E. Cu, Pb, and Zn content in sediments and benthic organisms from San Jorge Bay (northern Chile): Accumulation and biotransference in subtidal coastal systems. Cienc. Mar. 2014, 40, 45–58. [Google Scholar] [CrossRef] [Green Version]
- Parra, S.; Bravo, M.A.; Quiroz, W.; Querol, X.; Paipa, C. Distribution and pollution assessment of trace elements in marine sediments in the Quintero Bay (Chile). Mar. Pollut. Bull. 2015, 99, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Latorre-Padilla, N.; Meynard, A.; Oyarzun, F.X.; Contreras-Porcia, L. Ingestion of contaminated kelps by the herbivore Tetrapygus niger: Negative effects on food intake, growth, fertility, and early development. Mar. Pollut. Bull. 2021, 167, 112365. [Google Scholar] [CrossRef]
- Medina, M.; Andrade, S.; Faugeron, S.; Lagos, N.; Mella, D.; Correa, J.A. Biodiversity of rocky intertidal benthic communities associated with copper mine tailing discharges in northern Chile. Mar. Pollut. Bull. 2005, 50, 396–409. [Google Scholar] [CrossRef]
- Ansari, T.M.; Marr, I.L.; Tariq, N. Heavy metals in marine pollution perspective-a mini review. J. Appl. Sci. 2004, 4, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ma, Z.; Van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef]
- Recabarren-Villalón, T.; Orazi, M.M.; Ronda, A.C.; Marcovecchio, J.E.; Arias, A.H. Hidrocarburos Aromáticos Policíclicos (HAPs) en ambientes marinos: Una revisión de América. JAINA Cost. Mar. Cam. Clim. 2019, 1, 19–40. [Google Scholar]
- Meador, J.P.; Casillas, E.; Sloan, C.A.; Varanasi, U. Comparative bioaccumulation of polycyclic aromatic hydrocarbons from sediment by two infaunal invertebrates. Mar. Ecol. Prog. Ser. 1955, 123, 107–124. [Google Scholar] [CrossRef] [Green Version]
- Sinaei, M.; Mashinchian, A. Polycyclic aromatic hydrocarbons in the coastal sea water, the surface sediment and Mudskipper Boleophthalmus dussumieri from coastal areas of the Persian Gulf: Source investigation, composition pattern and spatial distribution. J. Environ. Health Sci. Eng. 2014, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Golovanova, I.L. Effects of heavy metals on the physiological and biochemical status of fishes and aquatic invertebrates. Inland Water Biol. 2008, 1, 93–101. [Google Scholar] [CrossRef]
- Contreras, L.; Mella, D.; Moenne, A.; Correa, J.A. Differential responses to copper-induced oxidative stress in the marine macroalgae Lessonia nigrescens and Scytosiphon lomentaria (Phaeophyceae). Aquat. Toxicol. 2009, 94, 94–102. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, B. Toxic effects of fluoranthene and copper on marine diatom Phaeodactylum tricornutum. J. Environ. Sci. 2008, 20, 1363–1372. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Chen, X.; Lv, B.; Wang, X.; Wang, D. Phenanthrene and pyrene disturbed the growth of Microcystis aeruginosa as co-cultured with Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. Int. 2020, 27, 45957–45964. [Google Scholar] [CrossRef]
- Espinoza-González, C.; Meynard, A.; Núñez, A.; Castañeda, F.; Oyarzo-Miranda, O.; Rivas, J.; Contreras-Porcia, L. Assessment of the independent and combined effects of Copper and Polycyclic Aromatic Hydrocarbons on gametophytes and sporophytes development of the kelp Lessonia spicata (Phaeophyceae, Ochrophyta). J. Appl. Phycol. 2021, in press. [Google Scholar] [CrossRef]
- Meynard, A.; Espinoza-González, C.; Núñez, A.; Castañeda, F.; Contreras-Porcia, L. Synergistic, antagonistic, and additive effects of heavy metals (copper and cadmium) and polycyclic aromatic hydrocarbons (PAHs) under binary and tertiary combinations in key habitat-forming kelp species of Chile. Environ. Sci. Pollut. Res. 2021, 28, 18300–18307. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qu, M.; Ding, J.; Zhang, Y.; Wang, Y.; Di, Y. BaP-metals co-exposure induced tissue-specific antioxidant defense in marine mussels Mytilus coruscus. Chemosphere 2018, 205, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C.; Altieri, A.H.; Silliman, B.R.; Bertness, M.D. Interactions among foundation species and their consequences for community organization, biodiversity, and conservation. BioScience 2011, 61, 782–789. [Google Scholar] [CrossRef] [Green Version]
- Borst, A.C.; Verberk, W.C.; Angelini, C.; Schotanus, J.; Wolters, J.W.; Christianen, M.J.; van der Zee, E.M.; van der Heide, T. Foundation species enhance food web complexity through non-trophic facilitation. PLoS ONE 2018, 13, e0199152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, J.A.; Burkholder, D.A.; Heithaus, M.R.; Fourqurean, J.W.; Fraser, M.W.; Statton, J.; Kendrick, G.A. Extreme temperatures, foundation species, and abrupt ecosystem change: An example from an iconic seagrass ecosystem. Glob. Chang. Biol. 2015, 21, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Romero, G.Q.; Gonçalves-Souza, T.; Vieira, C.; Koricheva, J. Ecosystem engineering effects on species diversity across ecosystems: A meta-analysis. Biol. Rev. 2014, 90, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, C.; Olivier, F.; Paterson, D.M.; Meziane, T.; Hubas, C. Organisms as cooperative ecosystem engineers in intertidal flats. J. Sea Res. 2014, 92, 92–101. [Google Scholar] [CrossRef]
- Miller, R.J.; Page, H.M.; Reed, D.C. Trophic versus structural effects of a marine foundation species, giant kelp (Macrocystis pyrifera). Oecologia 2015, 179, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Schiel, D.; Foster, M. The Biology and Ecology of Giant Kelp Forest, 2nd ed.; University of California Press: Berkeley, CA, USA, 2015. [Google Scholar]
- Ríos, C.; Arntz, W.E.; Gerdes, D.; Mutschke, E.; Montiel, A. Spatial and temporal variability of the benthic assemblages associated to the holdfasts of the kelp Macrocystis pyrifera in the Straits of Magellan, Chile. Polar Biol. 2007, 31, 89–100. [Google Scholar] [CrossRef]
- Miller, R.J.; Lafferty, K.D.; Lamy, T.; Kui, L.; Rassweiler, A.; Reed, D.C. Giant kelp, Macrocystis pyrifera, increases faunal diversity through physical engineering. Proc. R. Soc. B 2018, 285, 20172571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer-Pinto, M.; Ledet, J.; Crowe, T.P.; Johnston, E.L. Sublethal effects of contaminants on marine habitat-forming species: A review and meta-analysis. Biol. Rev. 2020, 95, 1554–1573. [Google Scholar] [CrossRef]
- Evan, L.K.; Edwards, M.S. Bioaccumulation of copper and zinc by the giant kelp Macrocystis pyrifera. Algae 2011, 26, 265–275. [Google Scholar] [CrossRef]
- Fink, L.A.; Manley, S.L. The use of kelp sieve tube sap metal composition to characterize urban runoff in southern California coastal waters. Mar. Pollut. Bull. 2011, 62, 2619–2632. [Google Scholar] [CrossRef] [PubMed]
- Westermeier, R.; Murúa, P.; Patiño, D.J.; Muñoz, L.; Müller, D.G. Holdfast fragmentation of Macrocystis pyrifera (integrifolia morph) and Lessonia berteroana in Atacama (Chile): A novel approach for kelp bed restoration. J. Appl. Phycol. 2016, 28, 2969–2977. [Google Scholar] [CrossRef]
- Contreras, L.; Bulboa, C.; Galbán, C.; Remonsellez, J.; Mella, D. Cultivo del alga parda Macrocystis pyrifera en la zona de Quintero y Puchuncaví: Evaluación de la productividad y potencial uso para biorremediación de metales pesados y compuestos orgánicos. 2017; N° 30397482-0. [Google Scholar]
- Pielou, E.C. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef]
- Hubálek, Z. Measures of species diversity in ecology: An evaluation. Folia Zool. 2000, 49, 241–260. [Google Scholar]
- Contreras-Porcia, L.; Meynard, A.; Lopez-Cristoffanini, C.; Latorre, N.; Kumar, M. Marine Metal Pollution and Effects on Seaweed Species. Chapter 3. In Systems Biology of Marine Ecosystems, 1st ed.; Kumar, M., Ralph, P., Eds.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2017; pp. 35–48. [Google Scholar]
- Kumar, M.; Kumari, P.; Gupta, V.; Anisha, P.A.; Reddy, C.R.K.; Jha, B. Differential responses to cadmium induced oxidative stress in marine macroalga Ulva lactuca (Ulvales, Chlorophyta). Biometals 2010, 23, 315–325. [Google Scholar] [CrossRef]
- Malea, P.; Rijstenbil, J.W.; Haritonidis, S. Effects of cadmium, zinc and nitrogen status on non-protein thiols in the macroalgae Enteromorpha spp. from the Scheldt Estuary (SW Netherlands, Belgium) and Thermaikos Gulf (N Aegean Sea, Greece). Mar. Environ. Res. 2006, 62, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Stauber, J.L.; Lim, R.P.; Petocz, P. Toxicity of metal mixtures to a tropical freshwater alga (Chlorella sp.): The effect of interactions between copper, cadmium, and zinc on metal cell binding and uptake. Environ. Toxicol. Chem. 2002, 21, 2412–2422. [Google Scholar] [CrossRef]
- Andrade, S.; Medina, M.H.; Moffett, J.W.; Correa, J.A. Cadmium-copper antagonism in seaweeds inhabiting coastal areas affected by copper mine waste disposals. Environ. Sci. Technol. 2006, 40, 4382–4387. [Google Scholar] [CrossRef]
- Lewis, M.; Pryor, R. Toxicities of oils, dispersants and dispersed oils to algae and aquatic plants: Review and database value to resource sustainability. Environ. Pollut. 2013, 180, 345–367. [Google Scholar] [CrossRef]
- Aksmann, A.; Tukaj, Z. The effect of anthracene and phenanthrene on the growth, photosynthesis, and SOD activity of the green alga Scenedesmus armatus depends on the PAR irradiance and CO2 level. Arch. Environ. Contam. Toxicol. 2004, 47, 177–184. [Google Scholar] [CrossRef]
- Gauthier, P.; Norwood, P.; Prepas, E.; Pyle, G. Metal–PAH mixtures in the aquatic environment: A review of co-toxic mechanisms leading to more-than-additive outcomes. Aquat. Toxicol. 2014, 154, 253–269. [Google Scholar] [CrossRef]
- Castilla, J.C. Food Webs and Functional Aspects of the Kelp, Macrocystis pyrifera, Community in the Beagle Channel, Chile; Siegfried, W.R., Condy, P.R., Laws, R.M., Eds.; Antarctic nutrient cycles and food webs; Springer: Berlin, Germany, 1985; pp. 407–414. [Google Scholar]
- Adami, M.L.; Gordillo, S. Structure and dynamics of the biota associated with Macrocystis pyrifera (Phaeophyta) from the Beagle Channel, Tierra del Fuego. Sci. Mar. 1999, 63, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Graiff, A.; Pantoja, J.F.; Tala, F.; Thiel, M. Epibiont load causes sinking of viable kelp rafts: Seasonal variation in floating persistence of giant kelp Macrocystis pyrifera. Mar. Biol. 2016, 163, 1–14. [Google Scholar] [CrossRef]
- Saunders, M.; Metaxas, A. Temperature explains settlement patterns of the introduced bryozoan Membranipora membranacea in Nova Scotia, Canada. Mar. Ecol. Prog. Ser. 2007, 344, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Fauchald, K.; Jumars, P.A. The diet of worms: A study of polychaete feeding guilds. Oceanogr. Mar. Biol. Annu. Rev. 1979, 17, 193–284. [Google Scholar]
- Almanza, V.; Buschmann, A.H.; Hernández-González, M.C.; Henríquez, L.A. Can giant kelp (Macrocystis pyrifera) forests enhance invertebrate recruitment in southern Chile? Mar. Biol. Res. 2012, 8, 855–864. [Google Scholar] [CrossRef]
- Madariaga, D.J.; Ortiz, M.; Thiel, M. Demography and feeding behavior of the kelp crab Taliepus marginatus in subdital habitats dominated by the kelps Macrocystis pyrifera and Lessonia trabeculata. Invertebr. Biol. 2013, 132, 133–144. [Google Scholar] [CrossRef]
- Contreras, L.; Medina, M.H.; Andrade, S.; Oppliger, V.; Correa, J.A. Effects of copper on early developmental stages of Lessonia nigrescens Bory (Phaeophyceae). Environ. Pollut. 2007, 145, 75–83. [Google Scholar] [CrossRef]
- Roberts, D.A.; Poore, A.G.; Johnston, E.L. Ecological consequences of copper contamination in macroalgae: Effects on epifauna and associated herbivores. Environ. Toxicol. Chem. 2006, 25, 2470–2479. [Google Scholar] [CrossRef]





| Phylum/Class | Taxa | Treatments | |||||
|---|---|---|---|---|---|---|---|
| Control | Cd + PAHs | Cu +PAHs | |||||
| Holdfast | Blades | Holdfast | Blades | Holdfast | Blades | ||
| Arthropoda | |||||||
| Malacostraca | Aora typical * | 77 | 9 | 7 | 1 | 10 | 3 |
| Sunamphitoe femorata * | - | 4 | - | 1 | - | 1 | |
| Pilumnoides perlatus | - | 1 | - | 1 | - | - | |
| Taliepus dentatus * | 4 | 7 | 3 | 2 | 2 | 1 | |
| Erichthonius sp. | 2 | - | 1 | - | - | - | |
| Amphipoda sp.1 | - | - | - | 2 | - | 2 | |
| Amphipoda sp.2 | - | 3 | - | - | - | - | |
| Amphipoda sp.3 | - | 2 | - | - | - | - | |
| Annelida | |||||||
| Polychaeta | Platynereis australis | 6 | 3 | 6 | 1 | 5 | 2 |
| Pseudonereis sp. | 1 | - | - | - | - | - | |
| Phyllodocidae | - | - | - | 2 | - | - | |
| Terebellidae | 1 | - | 3 | - | 2 | - | |
| Mollusca | |||||||
| Gastropoda | Eatoniella sp.* | 1 | - | - | - | - | - |
| Bryozoa | |||||||
| Gymnolaemata | Membranipora membranacea | - | 42 | - | 12 | - | 22 |
| Foraminifera | Foraminifera | - | - | - | - | - | 1 |
| Total individuals | 92 | 71 | 20 | 22 | 19 | 32 | |
| Total identities | 7 | 8 | 5 | 8 | 4 | 7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jara-Yáñez, R.; Meynard, A.; Acosta, G.; Latorre-Padilla, N.; Oyarzo-Miranda, C.; Castañeda, F.; Piña, F.; Rivas, J.; Bulboa, C.; Contreras-Porcia, L. Negative Consequences on the Growth, Morphometry, and Community Structure of the Kelp Macrocystis pyrifera (Phaeophyceae, Ochrophyta) by a Short Pollution Pulse of Heavy Metals and PAHs. Toxics 2021, 9, 190. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9080190
Jara-Yáñez R, Meynard A, Acosta G, Latorre-Padilla N, Oyarzo-Miranda C, Castañeda F, Piña F, Rivas J, Bulboa C, Contreras-Porcia L. Negative Consequences on the Growth, Morphometry, and Community Structure of the Kelp Macrocystis pyrifera (Phaeophyceae, Ochrophyta) by a Short Pollution Pulse of Heavy Metals and PAHs. Toxics. 2021; 9(8):190. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9080190
Chicago/Turabian StyleJara-Yáñez, Roddy, Andrés Meynard, Gladys Acosta, Nicolás Latorre-Padilla, Carolina Oyarzo-Miranda, Francisco Castañeda, Florentina Piña, Jorge Rivas, Cristian Bulboa, and Loretto Contreras-Porcia. 2021. "Negative Consequences on the Growth, Morphometry, and Community Structure of the Kelp Macrocystis pyrifera (Phaeophyceae, Ochrophyta) by a Short Pollution Pulse of Heavy Metals and PAHs" Toxics 9, no. 8: 190. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics9080190