Compounds from Terminalia mantaly L. (Combretaceae) Stem Bark Exhibit Potent Inhibition against Some Pathogenic Yeasts and Enzymes of Metabolic Significance
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Microbial Isolates
2.4. Plant Extraction and Screening of Anti-Yeast Activity
2.5. Isolation of Compounds and Screening for Activity
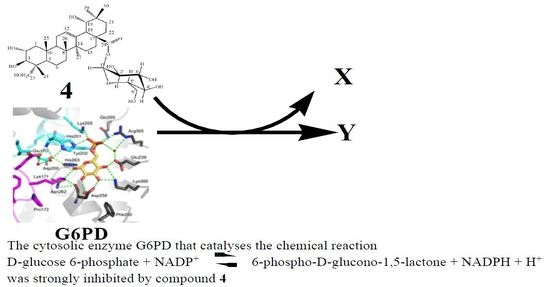
2.6. Purification of Glucose 6-Phosphate Dehydrogenase and Activity Determination
2.7. Purification of Carbonic Anhydrase Isoenzymes by Affinity Chromatography and Activity Determination
2.8. Purification of Glutathione S-Transferase Enzyme and Activity Determination
2.9. In Vitro Inhibition and Kinetic Studies
3. Results and Discussion
4. NMR Spectral Data of the Tested Compounds
5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arif, T.; Mandal, T.K.; Dabur, R. Natural products: Anti-fungal agents derived from Plants. In Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry; Pandalai, S.G., Ed.; Research Signpost: Trivandrum, India, 2011; pp. 283–311. [Google Scholar]
- Nelesh, G. HIV-associated opportunistic fungal infections: A guide to using the clinical microbiology laboratory. South. Arf. J. HIV Med. 2007, 1, 18–23. [Google Scholar]
- Álvaro-Meca, A.; Jensen, J.; Micheloud, D.; Asunción, D.; Gurbindo, D.; Resino, S. Rate of candidiasis among HIV infected children in Spain in the era of highly active antiretroviral therapy (1997–2008). BMC Inf. Dis. 2013, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Brissaud, O.; Guichoux, J.; Harambat, J.; Tandonnet, O.; Zaoutis, T. Invasive fungal disease in PICU: Epidemiology and risk factors. Ann. Intensiv. Care 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Ngouana, K.T.; Krasteva, D.; Drakulovski, P.; Toghueo, K.R.; Kouanfack, C.; Ambe, A.; Reynes, J.; Delaporte, E.; Boyom, F.F.; Mallié, M.; et al. Investigation of minor species Candida africana, Candida stellatoidea, and Candida dubliniensis in the Candida albicans complex among Yaoundé (Cameroon) HIV-infected patients. Mycoses 2015, 58, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ngouana, K.T.; Dongtsa, J.; Kouanfack, C.; Tonfack, C.; Foména, S.; Krasteva, D.; Drakulovski, P.; Aghokeng, A.; Mallié, M.; Delaporte, E.; et al. Cryptoccocal meningitis in Yaoundé (Cameroon) HIV infected patients: Diagnosis, frequency and susceptibility of Cryptococcus neoformans isolates to fluconazole. J. Mycol. Med. 2015, 25, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, K. Evaluation of the Antifungal Activity of Extracts of Bark of Commercial Species, Category P1 the Forest of Mopri, Tiassalé (Southern Ivory Coast). Master’s Thesis, University of Cocody-Abidjan, Abidjan, Côte D’Ivoire, 2006; pp. 23–25. [Google Scholar]
- Ngouana, T.K.; Mbouna, C.D.J.; Kuipou, R.M.T.; Tchuenmogne, M.A.T.; Zeuko’o, E.M.; Ngouana, V.; Mallié, M.; Bertout, S.; Boyom, F.F. Potent and Synergistic Extract Combinations from Terminalia Catappa, Terminalia Mantaly and Monodora tenuifolia against Pathogenic Yeasts. Medicines 2015, 2, 220–235. [Google Scholar] [CrossRef]
- Cock, I. The medicinal properties and phytochemistry of plants of the genus Terminalia (Combretaceae). Inflammopharmacology 2015, 23, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Valsaraj, R.; Pushpangadan, P.; Smitt, U.W.; Adsersen, A.; Christensen, S.B.; Sittie, A.; Nyman, U.; Nielsen, C.; Olsen, C.E. New anti-HIV-1, antimalarial and antifungal compounds from Terminalia bellerica. J. Nat. Prod. 1997, 60, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Srivastava, S.D.; Chouksey, B.K. New constituents of Terminalia alata. Fitoterapia 1999, 70, 390–394. [Google Scholar] [CrossRef]
- Conrad, J.; Vogler, B.; Klaiber, I.; Roos, G.; Walter, U.; Kraus, W. Two triterpene esters from Terminalia macroptera bark. Phytochemistry 1998, 48, 647–650. [Google Scholar] [CrossRef]
- Conrad, J.; Vogler, B.; Reeb, S.; Klaiber, I.; Papajewski, S.; Roos, G.; Vasquez, E.; Setzer, M.C.; Kraus, W. Isoterchebulin and 4,6-O-isoterchebuloyl-d-glucose, Novel Hydrolyzable Tannins from Terminalia macroptera. J. Nat. Prod. 2001, 64, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Kandil, F.E.; Nassar, M.I. A tannin anti-cancer promotor from Terminalia arjuna. Phytochemistry 1998, 47, 1567–1568. [Google Scholar] [CrossRef]
- Mahato, S.B.; Nandy, A.K.; Kundu, A.H. Pentacyclic triterpenoids sapogenols and their glycosides from Terminalia bellerica. Tetrahedron 1992, 48, 2483–2484. [Google Scholar] [CrossRef]
- Singh, D.V.; Verma, R.K.; Singh, C.S.; Gupta, M.M. RP-LC determination of oleane derivatives in Terminalia arjuna. J. Pharm. Biomed. Anal. 2002, 28, 447–452. [Google Scholar] [CrossRef]
- Garcez, R.F.; Garcez, S.W.; Santana, A.L.B.D.; Alves, M.M.; Matos, M.F.C.C.; Scaliante, A.M. Bioactive flavonoids and triterpenes from Terminalia fagifolia (Combretaceae). J. Braz. Chem. Soc. 2006, 17, 1223–1228. [Google Scholar] [CrossRef]
- Yeh, G.C.; Daschner, P.J.; Lopaczynska, J.; MacDonald, C.J.; Ciolino, H.P. Modulation of glucose-6-phosphate dehydrogenase activity and expression is associated with aryl hydrocarbon resistance in vitro. J. Biol. Chem. 2001, 276, 34708–34713. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Kato, T.; Oshikawa, K.; Yamada, T.; Takayama, T.; Koike, T.; Sato, I. Glucose-6-phosphate dehydrogenase in rat lung alveolar epithelial cells. An ultrastructural enzyme-cytochemical study. Eur. J. Histochem. 2002, 46, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Preuss, J.; Richardson, A.D.; Pinkerton, A.; Hedrick, M.; Sergienko, E.; Rahlfs, S.; Bode, L. Identification and characterization of novel human glucose-6-phosphate dehydrogenase inhibitors. J. Biomol. Screen. 2013, 18, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Tripp, B.C.; Smith, K.; Ferry, J.G. Mini review: Carbonic anhydrase: New insights for an ancient enzyme. J. Biol. Chem. 2001, 276, 48615–48618. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Comakli, V.; Ciftci, M.; Kufrevioglu, O.I. Effects of some metal ions on rainbow trout erythrocytes glutathione S-transferase enzyme: An in vitro study. J. Enzyme Inhib. Med. Chem. 2013, 28, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B.; Danielson, U.H. Glutathione transferases—Structure and catalitic activity. CRC Crit. Rev. Biochem. 1988, 23, 283–337. [Google Scholar] [CrossRef] [PubMed]
- Matshushita, N.; Aritake, K.; Takada, A.; Hizue, M.; Hayashi, K.; Mitsui, K. Pharmacological studies on the novel antiallergenic drug HQL-79: 2. Elucidation of mechanisms for antiallergic and antiasthmatic effects. Jpn. J. Pharmacol. 1998, 78, 11–22. [Google Scholar] [CrossRef]
- Hayes, J.D.; McLellan, L.I.; Stockman, P.K.; Chalmers, J.; Beckett, G.J. Glutathione S-transferases in man: The relationship between rat and human enzymes. Biochem. Soc. Trans. 1987, 15, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Mga’ning, B.M.; Lenta, B.N.; Noungoue, D.T.; Antheaume, C.; Fongang, Y.F.; Ngouela, S.A.; Boyom, F.F.; Rosenthal, P.J.; Tsamo, E.; Sewald, N.; et al. Antiplasmodial sesquiterpenes from the seeds of Salacia longipes var. camerunensis. Phytochemistry 2013, 96, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, M.; Aslan, A.; Ahmed, I.; Comakli, V.; Demirdag, R.; Uzun, N. Purification of glucose-6-phosphate dehydrogenase and glutathione reductase enzymes from the gill tissue of Lake Van fish and analyzing the effects of some chalcone derivatives on enzyme activities. Fish Physiol. Biochem. 2016, 42, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E. Red Cell Metabolism: A Manual of Biochemical Methods, 3rd ed.; Grune and Stratton Inc.: Orlando, FL, USA, 1984; pp. 68–70. [Google Scholar]
- Ekinci, D.; Cavdar, H.; Talaz, O.; Sentürk, M.; Supuran, C.T. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms I and II. Bioorg. Med. Chem. 2010, 18, 3559–3563. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, J.A.; Mehta, S.; Edsall, J.T. Esterase activities of human carbonic anhydrases B and C. J. Biol. Chem. 1976, 242, 4221–4229. [Google Scholar]
- Hunaiti, A.A.; Soud, M. Effect of lead concentration on the level of glutathione, glutathione S-transferase, reductase and peroxidase in human blood. Sci. Total Environ. 2000, 248, 45–50. [Google Scholar] [CrossRef]
- Güvercin, S.; Erat, M.; Sakiroglu, H. Determination of some kinetic and characteristic properties of glutathione S-transferase from bovine erythrocytes. Protein Pept. Lett. 2008, 15, 6–12. [Google Scholar] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [PubMed]
- Liu, M.; Katerere, R.D.; Gray, I.A.; Seidel, V. Phytochemistry and antifungal studies on Terminalia mollis and Terminalia brachystemina. Fitoterapia 2009, 80, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Nandy, A.K.; Podder, G.; Sahu, N.P.; Mahato, S.B. Triterpenoids and their glycosides from Terminalia bellerica. Phytochemistry 1989, 28, 2769–2772. [Google Scholar] [CrossRef]
- Jossang, A.; Seuleiman, M.; Maidou, E.; Bodo, B. Pentacyclic triterpenes from Combretum nigricans. Phytochemistry 1996, 41, 591–594. [Google Scholar] [CrossRef]
- Kojima, H.; Tominaga, H.; Sato, S.; Ogura, H. Pentacyclic triterpenoids from Prunella vulgaris. Phytochemistry 1987, 26, 1107–1111. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Dawuti, G.; Aibai, S. Antifungal activity of ellagic acid in vitro and in vivo. Phytother. Res. 2015, 29, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.Y.; Lin, J.Y.; Tang, K. Human glucose-6-phophate dehydrogenase (G6PD). Gene transforms NIH 3T3 cells and induces tumors in nude mice. Int. J. Cancer 2000, 85, 857–864. [Google Scholar] [CrossRef]
- Gupta, S.; Cordeiro, T.A.; Michels, P.A.M. Glucose-6-phosphate dehydrogenase is the target for the trypanocidal action of human steroids. Mol. Biochem. Parasitol. 2011, 176, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.N.; Dawson, M.; Fairweather, E.E.; Hamilton, S.N.; Hitchin, R.J.; James, I.D.; Jones, D.S.; Jordan, M.A.; Lyones, J.A.; Small, F.H. Novel steroid inhibitors of glucose 6-phosphate dehydrogenase. J. Med. Chem. 2012, 55, 4431–4445. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.S.; Park, J.; Shin, J.M.; Cho, D.; Cho, S.Y.; Shin, D.W.; Ham, M.; Kim, J.B.; Lee, T.R. Cathechin gallates are NADP+-competitive inhibitors of glucose-6-phosphate dehydrogenase and other enzymes that employ NADP+ as a coenzyme. Bioorg. Med. Chem. 2008, 16, 3580–3586. [Google Scholar] [CrossRef] [PubMed]
- Adem, S.; Comakli, V.; Kuzu, M.; Demirdag, R. Investigation of the effects of some phenolic compounds on the Activities of glucose-6-phosphate dehydrogenase and 6-Phosphogluconate dehydrogenase from human erythrocytes. J. Biochem. Mol. Toxicol. 2014, 28, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Beyza, Ö.S.; Gülçin, İ.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem. Biol. Drug Des. 2010, 75, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Hayeshi, R.; Mutingwende, I.; Mavengere, W.; Masiyanise, V.; Mukanganyama, S. The inhibition of human glutathione S-transferases activity by plant polyphenolic compounds ellagic acid and curcumin. Food Chem. Toxicol. 2007, 45, 286–295. [Google Scholar] [CrossRef] [PubMed]

| Extract/Fractions | C. parapsilosis | C. albicans | C. krusei | |
|---|---|---|---|---|
| MIC * (µg/Ml ± SD) | ||||
| MeOH Extract | 24.00 ± 0.21 | 39.00 ± 0.33 | 39.00 ± 0.30 | |
| Fraction T1 | 1250.00 ± 1.23 | 2500.00 ± 0.98 | 2500.00 ± 1.03 | |
| Fraction T2 | 39.00 ± 0.38 | >5000 | >5000 | |
| Fraction T3 | 0.16 ± 0.02 | 0.64 ± 0.12 | 0.02 ± 0.09 | |
| Fraction T4 | >5000 | >5000 | >5000 | |
| Fraction of origin | ||||
| 3,3′-di-O-methylellagic acid 4′-O-α-rhamnopyranoside | T3 | 39.00 ± 0.88 (80.4 µM) | 9.70 ± 0.72 (20 µM) | >5000 (10,300 µM) |
| 3-O-methyl ellagic acid | T3 | 78.00 ± 0.92 (247.6 µM) | 156.00 ± 1.00 (495 µM) | 19.50 ± 0.57 (61.9 µM) |
| Arjungenin | T1, T2, T3 | >5000 (9487 µM) | >5000 (9487 µM) | >5000 (9487 µM) |
| Arjunglucoside | T2, T3 | 39.00 ± 0.13 (56.60 µM) | 9.70 ± 0.36 (14.07 µM) | 312.00 ± 1.04 (452 µM) |
| 2α,3α,24-trihydroxyolean-11,13(18)-dien-28-oic acid | T1, T3 | >5000 (9823 µM) | >5000 (9823 µM) | >5000 (9823 µM) |
| Fluconazole ** | 2.00 ± 0.01 (6.53 µM) | 8.00 ± 0.25 (26.14 µM) | 32.00 ± 0.42 (10.45 µM) | |
| Activity Parameter | G6PD | CAI | CAII | GST | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| IC50 a (µM) | n.a | n.a | n.a | 1.84 ± 0.31 | 53.31 ± 1.09 | 86.64 ± 0.93 | 3.28 ± 0.13 | 69.31 ± 1.13 | n.a | n.a | 1.03 ± 0.01 | 63.01 ± 1.15 | n.a | 1.51 ± 0.78 | 1.84 ± 0.73 | |
| Ki b (µM) | n.a | n.a | n.a | 0.19 ± 0.03 | 44.11 ± 1.12 | 71.68 ± 0.96 | 2.72 ± 0.64 | 55.78 ± 0.97 | n.a | n.a | 1.84 ± 0.11 | 42.00 ± 1.39 | n.a | 1.00 ± 0.03 | 0.19 ± 0.77 | |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchuente Tchuenmogne, M.A.; Kammalac, T.N.; Gohlke, S.; Kouipou, R.M.T.; Aslan, A.; Kuzu, M.; Comakli, V.; Demirdag, R.; Ngouela, S.A.; Tsamo, E.; et al. Compounds from Terminalia mantaly L. (Combretaceae) Stem Bark Exhibit Potent Inhibition against Some Pathogenic Yeasts and Enzymes of Metabolic Significance. Medicines 2017, 4, 6. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines4010006
Tchuente Tchuenmogne MA, Kammalac TN, Gohlke S, Kouipou RMT, Aslan A, Kuzu M, Comakli V, Demirdag R, Ngouela SA, Tsamo E, et al. Compounds from Terminalia mantaly L. (Combretaceae) Stem Bark Exhibit Potent Inhibition against Some Pathogenic Yeasts and Enzymes of Metabolic Significance. Medicines. 2017; 4(1):6. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines4010006
Chicago/Turabian StyleTchuente Tchuenmogne, Marthe Aimée, Thierry Ngouana Kammalac, Sebastian Gohlke, Rufin Marie Toghueo Kouipou, Abdulselam Aslan, Muslum Kuzu, Veysel Comakli, Ramazan Demirdag, Silvère Augustin Ngouela, Etienne Tsamo, and et al. 2017. "Compounds from Terminalia mantaly L. (Combretaceae) Stem Bark Exhibit Potent Inhibition against Some Pathogenic Yeasts and Enzymes of Metabolic Significance" Medicines 4, no. 1: 6. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines4010006