Semi-Synthesis and Evaluation of Sargahydroquinoic Acid Derivatives as Potential Antimalarial Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental
2.2. Extraction and Isolation of Natural Products
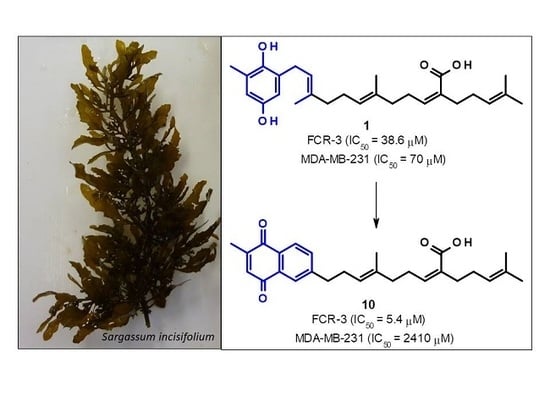
2.3. Sargaquinoic Acid (3) and Sarganaphthoquinoic Acid (10)
2.4. Sargaquinoic Acid Methyl ester (5)
2.5. Diacetyl Sargahydroquinoic Acid (2)
2.6. Sargaquinol (6) and Sargachromendiol (8)
2.7. Z-sargaquinal (4)
2.8. Antiplasmodial Assays
2.9. Cytotoxicity Assay
3. Results and Discussion
3.1. Isolation and Synthetic Modification of Sargahydroquinoic Acid Derivatives
3.2. Biological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okombo, J.; Chibale, K. Recent updates in the discovery and development of novel antimalarial drug candidates. Med. Chem. Commun. 2018, 9, 437–453. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Amato, R.; Pearson, R.D.; Almagro-Garcia, J.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Drury, E.; Stalker, J.; Miotto, O.; et al. Origins of the current outbreak of multidrug-resistant malaria in southeast Asia: A retrospective genetic study. Lancet Infect. Dis. 2018, 18, 337–345. [Google Scholar] [CrossRef]
- Fernández-Álvaro, E.; Hong, W.D.; Nixon, G.L.; O’Neill, P.M.; Calderón, F. Antimalarial chemotherapy: Natural product inspired development of preclinical and clinical candidates with diverse mechanisms of action. J. Med. Chem. 2016, 59, 5587–5603. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, A.F.; Bolton, J.J.; Lategan, C.A.; Smith, P.J.; Beukes, D.R. Fucoxanthin, tetraprenylated toluquinone and toluhydroquinone metabolites from Sargassum heterophyllum inhibit the in vitro growth of the malaria parasite Plasmodium falciparum. Z. Naturforsch. 2008, 63c, 848–852. [Google Scholar] [CrossRef]
- Segawa, M.; Shirahama, H. New plastoquinones from the brown alga Sargassum sagamianum var. yezoense. Chem. Lett. 1987, 1365–1366. [Google Scholar] [CrossRef]
- Kusumi, T.; Ishitsuka, M.; Kinoshita, T.; Kakisawa, H.; Shibata, Y. Structures of new plastoquinones from the brown alga Sargassum serratifolium. Chem. Lett. 1979, 8, 277–278. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, R.E.; Canfield, C.J.; Haynes, J.D.; Chulay, J.D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979, 16, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Perez-Castorena, A.; Arciniegas, A.; Apan, M.T.R.; Villasenor, J.L.; de Vivar, A. Evaluation of the anti-inflammatory and antioxidant activities of the plastoquinones derivatives isolated from Roldana barba-johanis. Planta Med. 2002, 68, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-H.; Su, J.-H.; Sung, P.-J.; Wang, G.-H.; Dai, H. Novel meroditerpenoid-related metabolites from the formosan soft coral Nephthea chabrolii. J. Nat. Prod. 2004, 67, 2048–2052. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.T.S.; Pullar, M.A.; Khalil, I.M.; Allouche, E.; Barker, D.; Copp, B.R. Bio-inspired dimerisation of prenylated quinones directed towards the synthesis of the meroterpenoid natural products, the scabellones. Tetrahedron Lett. 2015, 56, 1486–1488. [Google Scholar] [CrossRef]
- Alonso, M.A.; Lopez-Alvarado, P.; Avendano, C.; Menendez, J.C. Regioselective Diels-Alder reactions of 3-vinylindoles with quinones. Lett. Org. Chem. 2004, 1, 20–22. [Google Scholar] [CrossRef]
- de Koning, C.B.; Rousseau, A.L.; van Otterlo, W.A.L. Modern methods for the synthesis of substituted naphthalenes. Tetrahedron 2003, 59, 7–36. [Google Scholar] [CrossRef]
- Couladouros, E.A.; Plyta, Z.F.; Papageorgiou, V.P. A general procedure for the efficient synthesis of (alkylamino)naphthoquinones. J. Org. Chem. 1996, 61, 3031–3033. [Google Scholar] [CrossRef] [PubMed]
- Nixon, G.L.; Moss, D.M.; Shone, A.E.; Lalloo, D.G.; Fisher, N.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Antimalarial pharmacology and therapeutics of atovaquone. J. Antimicrob. Chemother. 2013, 68, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P. Mode of action and mechanisms of resistance for antimalarial drugs. Pharmacol. Ther. 2001, 89, 207–219. [Google Scholar] [CrossRef]
- Vennerstrom, J.L.; Eaton, J.W. Oxidants, oxidant drugs, and malaria. J. Med. Chem. 1988, 31, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Brandão, G.C.; Rocha Missias, F.C.; Arantes, L.M.; Soares, L.F.; Roy, K.K.; Doerksen, R.J.; Braga de Oliveira, A.; Pereira, G.R. Antimalarial naphthoquinones. Synthesis via click chemistry, in vitro activity, docking to PfDHOD and SAR of lapachol-based compounds. Eur. J. Med. Chem. 2018, 145, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, C.; Persico, M.; Senese, M.; Aiello, A.; Casertano, M.; Luciano, P.; Basilico, N.; Parapini, S.; Paladino, A.; Fattorusso, C.; et al. Exploring the antimalarial potential of the methoxy-thiazinoquinone scaffold: Identification of a new lead candidate. Bioorg. Chem. 2019, 85, 240–252. [Google Scholar] [CrossRef] [PubMed]



| Carbon Number | δC | Type | δH, mult, J (Hz) | COSY | HMBC |
|---|---|---|---|---|---|
| 1 | 185.4 | C | - | ||
| 2 | 130.2 | C | - | ||
| 3 | 132.2 | C | - | ||
| 4 | 185.4 | C | - | ||
| 5 | 136.0 | CH | 6.81, s | H-7 | |
| 6 | 149.0 | C | - | ||
| 7 | 16.4 | CH3 | 2.18, s, | H-5 | C-6, C-5 |
| 1’ | 126.7 | CH | 8.00, d, 7.9 | H-2’ | C-2, C-1 |
| 2’ | 133.8 | CH | 7.51, d, 7.9 | H-4’, H-20’ | C-1, C-20’ |
| 3’ | 148.2 | C | - | ||
| 4’ | 36.2 | CH2 | 2.77, t, 7.6 | H-5’ | C-5’, C-3’ |
| 5’ | 29.1 | CH2 | 2.36, m | H-4’, H-6’ | C-4’, C-6’, C-7’ |
| 6’ | 123.2 | CH | 5.17, m | ||
| 7’ | 136.0 | C | - | ||
| 8’ | 39.0 | CH2 | 2.08, m | H-9’ | C-6’ |
| 9’ | 28.2 | CH2 | 2.57, m | H-10’ | C-8’, C-10’ |
| 10’ | 145.0 | CH | 5.96, t, 7.3 | H-9’ | C-8’ |
| 11’ | 130.6 | C | - | ||
| 12’ | 27.8 | CH2 | 2.26, m | H-13’, H-14’ (lr) | C-13’, C-14’ |
| 13’ | 28.2 | CH2 | 2.11, m | H-14’ | C-15’, C-11’ |
| 14’ | 123.4 | CH | 5.17, t, 7.0 | H-13’, H-14’ (lr) | |
| 15’ | 132.3 | C | - | ||
| 16’ | 25.6 | CH3 | 1.68, s | H-14’ (lr) | C-15’, C-14’ |
| 17’ | 17.7 | CH3 | 1.59, s | H-14’ (lr) | C-16’ |
| 18’ | 171.9 | C | - | ||
| 19’ | 15.9 | CH3 | 1.58, s | H-6’ | C-6’ |
| 20’ | 125.9 | CH | 7.86, s | H-4’ | C-4’, C-4 |
| Compound | IC50 (μM) | Selectivity Index | ||
|---|---|---|---|---|
| D10 1 | FCR-3 | MDA-MB-231 | ||
| Sargahydroquinoic acid (1) | 15.2 | 38.6 | 70 | 1.8 |
| Sargahydroquinoic acid di-acetate (2) | - | 84.3 | 286 | 3.4 |
| Sargaquinoic acid (3) | 12.0 | 10.8 | 658 | 60.9 |
| 10Z-sargaquinal (4) | - | 72.6 | 211 | 2.9 |
| Sargaquinoic acid methyl ester (5) | - | 8.2 | 70 | 8.6 |
| Sargaquinol (6) | - | 93.1 | 99 | 1.1 |
| Sargachromenol (7) | - | 114.8 | 56 | 0.5 |
| Sargachromendiol (8) | - | 34.2 | 187 | 5.5 |
| 10E-sargaquinal (9) | 2.0 | 104.4 | 69 | 0.7 |
| Sarganaphthoquinone (10) | - | 5.4 | 2410 | 443 |
| Quinine | 0.17 | - | - | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munedzimwe, T.C.; van Zyl, R.L.; Heslop, D.C.; Edkins, A.L.; Beukes, D.R. Semi-Synthesis and Evaluation of Sargahydroquinoic Acid Derivatives as Potential Antimalarial Agents. Medicines 2019, 6, 47. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines6020047
Munedzimwe TC, van Zyl RL, Heslop DC, Edkins AL, Beukes DR. Semi-Synthesis and Evaluation of Sargahydroquinoic Acid Derivatives as Potential Antimalarial Agents. Medicines. 2019; 6(2):47. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines6020047
Chicago/Turabian StyleMunedzimwe, Tatenda C., Robyn L. van Zyl, Donovan C. Heslop, Adrienne L. Edkins, and Denzil R. Beukes. 2019. "Semi-Synthesis and Evaluation of Sargahydroquinoic Acid Derivatives as Potential Antimalarial Agents" Medicines 6, no. 2: 47. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines6020047