Abundance of Plant-Associated Gammaproteobacteria Correlates with Immunostimulatory Activity of Angelica sinensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of A. sinensis Extracts for the Cell-Based Assay
2.3. Cell Culture
2.4. Cell Treatment and RNA Purification
2.5. Reverse Transcription and Quantitative Polymerase Chain Reaction
2.6. DNA Extraction from A. sinensis for 16S rRNA Amplicon Sequencing
2.7. 16S rRNA Amplicon Sequencing and Data Analysis
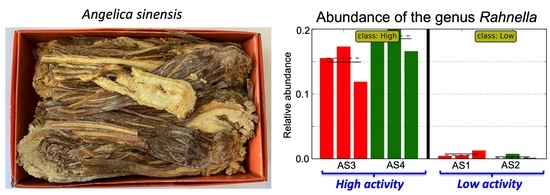
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Berg, G.; Koberl, M.; Rybakova, D.; Muller, H.; Grosch, R.; Smalla, K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [PubMed]
- Pugh, N.D.; Tamta, H.; Balachandran, P.; Wu, X.; Howell, J.; Dayan, F.E.; Pasco, D.S. The majority of in vitro macrophage activation exhibited by extracts of some immune enhancing botanicals is due to bacterial lipoproteins and lipopolysaccharides. Int. Immunopharmacol. 2008, 8, 1023–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamta, H.; Pugh, N.D.; Balachandran, P.; Moraes, R.; Sumiyanto, J.; Pasco, D.S. Variability in in vitro macrophage activation by commercially diverse bulk echinacea plant material is predominantly due to bacterial lipoproteins and lipopolysaccharides. J. Agric. Food Chem. 2008, 56, 10552–10556. [Google Scholar] [CrossRef]
- Pugh, N.D.; Jackson, C.R.; Pasco, D.S. Total bacterial load within Echinacea purpurea, determined using a new PCR-based quantification method, is correlated with LPS levels and in vitro macrophage activity. Planta Med. 2013, 79, 9–14. [Google Scholar] [CrossRef]
- Todd, D.A.; Gulledge, T.V.; Britton, E.R.; Oberhofer, M.; Leyte-Lugo, M.; Moody, A.N.; Shymanovich, T.; Grubbs, L.F.; Juzumaite, M.; Graf, T.N.; et al. Ethanolic Echinacea purpurea Extracts Contain a Mixture of Cytokine-Suppressive and Cytokine-Inducing Compounds, Including Some That Originate from Endophytic Bacteria. PLoS ONE 2015, 10, e0124276. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, D.; Kalpana, K.; Chrissian, C.; Sharma, A.; Takaoka, A.; Iacovidou, M.; Soll, C.E.; Aminova, O.; Heguy, A.; Cohen, L.; et al. Uncovering potential ‘herbal probiotics’ in Juzen-taiho-to through the study of associated bacterial populations. Bioorg. Med. Chem. Lett. 2015, 25, 466–469. [Google Scholar] [CrossRef]
- Huang, S.M.; Chien, L.Y.; Tai, C.J.; Chiou, J.F.; Chen, C.S. Effectiveness of 3-week intervention of Shi Quan Da Bu Tang for alleviating hematotoxicity among patients with breast carcinoma receiving chemotherapy. Integr. Cancer Ther. 2013, 12, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Omatsu, T.; Matsumoto, C.; Tsuchiya, N.; Yamamoto, M.; Naito, Y.; Yoshikawa, T. Protective effect of the Japanese traditional medicine juzentaihoto on myelosuppression induced by the anticancer drug TS-1 and identification of a potential biomarker of this effect. BMC Complement. Altern. Med. 2012, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Zee-Cheng, R.K. Shi-quan-da-bu-tang (ten significant tonic decoction), SQT. A potent Chinese biological response modifier in cancer immunotherapy, potentiation and detoxification of anticancer drugs. Methods Find. Exp. Clin. Pharmacol. 1992, 14, 725–736. [Google Scholar]
- Sho, Y.; Fujisaki, K.; Sakashita, H.; Yamaguchi, K.; Tahara, K.; Kubozono, O.; Ido, A.; Tsubouchi, H. Orally administered Kampo medicine, Juzen-taiho-to, ameliorates anemia during interferon plus ribavirin therapy in patients with chronic hepatitis C. J. Gastroenterol. 2004, 39, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Y.; Hoshida, S.; Furukawa, M.; Ito, M. Effects of Japanese herbal medicine, Juzen-taiho-to, in otitis-prone children—A preliminary study. Acta Otolaryngol. 2009, 129, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, H.; Mimura, T.; Honda, N. Orally administrated Juzen-taiho-to/TJ-48 ameliorates erythropoietin (rHuEPO)-resistant anemia in patients on hemodialysis. Hemodial. Int. 2008, 12 (Suppl. 2), S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hamalainen, A.M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 842–853. [Google Scholar] [CrossRef] [Green Version]
- Berg, G.; Erlacher, A.; Grube, M. The edible plant microbiome: Importance and health issues. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer: Cham, Switzerland, 2015; pp. 419–426. [Google Scholar]
- Komaniecka, I.; Zdzisinska, B.; Kandefer-Szerszen, M.; Choma, A. Low endotoxic activity of lipopolysaccharides isolated from Bradyrhizobium, Mesorhizobium, and Azospirillum strains. Microbiol. Immunol. 2010, 54, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Haron, M.H.; Tyler, H.L.; Pugh, N.D.; Moraes, R.M.; Maddox, V.L.; Jackson, C.R.; Pasco, D.S. Activities and Prevalence of Proteobacteria Members Colonizing Echinacea purpurea Fully Account for Macrophage Activation Exhibited by Extracts of This Botanical. Planta Med. 2016, 82, 1258–1265. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Hong, S.; Kim, Y.T.; Ryu, S.; Kim, H.B.; Lee, J.H. Metagenomic Approach to Identifying Foodborne Pathogens on Chinese Cabbage. J. Microbiol. Biotechnol. 2018, 28, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [Green Version]
- Navas-Molina, J.A.; Peralta-Sanchez, J.M.; Gonzalez, A.; McMurdie, P.J.; Vazquez-Baeza, Y.; Xu, Z.; Ursell, L.K.; Lauber, C.; Zhou, H.; Song, S.J.; et al. Advancing our understanding of the human microbiome using QIIME. Methods Enzymol. 2013, 531, 371–444. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Gruning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [Green Version]
- Takaoka, A.; Iacovidou, M.; Hasson, T.H.; Montenegro, D.; Li, X.; Tsuji, M.; Kawamura, A. Biomarker-guided screening of Juzen-taiho-to, an oriental herbal formulation for immunostimulation. Planta Med. 2014, 80, 283–289. [Google Scholar] [CrossRef]
- Hanshew, A.S.; Mason, C.J.; Raffa, K.F.; Currie, C.R. Minimization of chloroplast contamination in 16S rRNA gene pyrosequencing of insect herbivore bacterial communities. J. Microbiol. Methods 2013, 95, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LEfSe on the Huttenhower Galaxy Server. Available online: http://huttenhower.sph.harvard.edu/galaxy/ (accessed on 22 May 2019).
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, N.; Guo, X.; Zhang, Y.; Ye, B. Comparative analysis of bacterial community structure in the rhizosphere of maize by high-throughput pyrosequencing. PLoS ONE 2017, 12, e0178425. [Google Scholar] [CrossRef] [PubMed]
- Ceuppens, S.; Delbeke, S.; De Coninck, D.; Boussemaere, J.; Boon, N.; Uyttendaele, M. Characterization of the Bacterial Community Naturally Present on Commercially Grown Basil Leaves: Evaluation of Sample Preparation Prior to Culture-Independent Techniques. Int. J. Environ. Res. Public Health 2015, 12, 10171–10197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, L.; Wu, X.; Li, H.; Liao, Q.; Zhang, X.; Sun, Z.; Li, W. Analysis of Microbial Diversity in Soil under Ginger Cultivation. Scientifica 2017, 2017, 8256865. [Google Scholar] [CrossRef] [PubMed]
- Erlacher, A.; Cardinale, M.; Grube, M.; Berg, G. Biotic stress shifted structure and abundance of Enterobacteriaceae in the lettuce microbiome. PLoS ONE 2015, 10, e0118068. [Google Scholar] [CrossRef]
- Dong, L.; Cheng, R.; Xiao, L.; Wei, F.; Wei, G.; Xu, J.; Wang, Y.; Guo, X.; Chen, Z.; Chen, S. Diversity and composition of bacterial endophytes among plant parts of Panax notoginseng. Chin. Med. 2018, 13, 41. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.P.; Gillespie, J.J.; Sobral, B.W.; Nordberg, E.K.; Snyder, E.E.; Shallom, J.M.; Dickerman, A.W. Phylogeny of gammaproteobacteria. J. Bacteriol. 2010, 192, 2305–2314. [Google Scholar] [CrossRef]
- Varbanets, L.D.; Zdorovenko, E.L.; Ostapchuk, A.N. Chemical characteristics and endotoxic activity of the lipopolysaccharide of Rahnella aquatilis 2–95. Microbiology 2008, 77, 298–304. [Google Scholar] [CrossRef]
- Zdorovenko, E.L.; Varbanets, L.D.; Zatonsky, G.V.; Kachala, V.V.; Zdorovenko, G.M.; Shashkov, A.S.; Knirel, Y.A. Structure of the O-specific polysaccharide of the lipopolysaccharide of Rahnella aquatilis 95 U003. Carbohydr. Res. 2008, 343, 2494–2497. [Google Scholar] [CrossRef] [PubMed]
- Zdorovenko, E.L.; Varbanets, L.D.; Zatonsky, G.V.; Ostapchuk, A.N. Structure of the O-polysaccharide of the lipopolysaccharide of Rahnella aquatilis 1–95. Carbohydr. Res. 2004, 339, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Zdorovenko, E.L.; Varbanets, L.D.; Zatonsky, G.V.; Ostapchuk, A.N. Structures of two putative O-specific polysaccharides from the Rahnella aquatilis 3–95 lipopolysaccharide. Carbohydr. Res. 2006, 341, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Zdorovenko, E.L.; Varbanets, L.D.; Zatonsky, G.V.; Zdorovenko, G.M.; Shashkov, A.S.; Knirel, Y.A. Isolation and structure elucidation of two different polysaccharides from the lipopolysaccharide of Rahnella aquatilis 33071T. Carbohydr. Res. 2009, 344, 1259–1262. [Google Scholar] [CrossRef]
- Heinrich, M. Quality and safety of herbal medical products: Regulation and the need for quality assurance along the value chains. Br. J. Clin. Pharmacol. 2015, 80, 62–66. [Google Scholar] [CrossRef]
- Zhou, X.; Li, C.G.; Chang, D.; Bensoussan, A. Current Status and Major Challenges to the Safety and Efficacy Presented by Chinese Herbal Medicine. Medicines 2019, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Bensoussan, A.; Lee, S.; Murray, C.; Bourchier, S.; van der Kooy, F.; Pearson, J.L.; Liu, J.; Chang, D.; Khoo, C.S. Choosing chemical markers for quality assurance of complex herbal medicines: Development and application of the herb MaRS criteria. Clin. Pharmacol. Ther. 2015, 97, 628–640. [Google Scholar] [CrossRef]
- Song, X.Y.; Li, Y.D.; Shi, Y.P.; Jin, L.; Chen, J. Quality control of traditional Chinese medicines: A review. Chin. J. Nat. Med. 2013, 11, 596–607. [Google Scholar] [CrossRef]
- Giacomelli, N.; Yongping, Y.; Huber, F.K.; Ankli, A.; Weckerle, C.S. Angelica sinensis (Oliv.) Diels: Influence of Value Chain on Quality Criteria and Marker Compounds Ferulic Acid and Z-Ligustilide. Medicines 2017, 4, 14. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, J.A.; Qian, D.; Guo, J.; Song, B.; Yang, M. Assessment and comparison of immunoregulatory activity of four hydrosoluble fractions of Angelica sinensisin vitro on the peritoneal macrophages in ICR mice. Int. Immunopharmacol. 2010, 10, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Tabira, T. Juzen-Taiho-to, an herbal medicine, promotes the differentiation of transplanted bone marrow cells into microglia in the mouse brain injected with fibrillar amyloid beta. Tohoku J. Exp. Med. 2014, 233, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Kataoka, S.; Anan, M.; Ueda, A.; Mutoh, T.; Tabira, T. The therapeutic effects of the herbal medicine, Juzen-taiho-to, on amyloid-beta burden in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2010, 20, 427–439. [Google Scholar] [CrossRef]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunol. 2007, 8, 1. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalpana, K.; Montenegro, D.; Romero, G.; Peralta, X.; Akgol Oksuz, B.; Heguy, A.; Tsuji, M.; Kawamura, A. Abundance of Plant-Associated Gammaproteobacteria Correlates with Immunostimulatory Activity of Angelica sinensis. Medicines 2019, 6, 62. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines6020062
Kalpana K, Montenegro D, Romero G, Peralta X, Akgol Oksuz B, Heguy A, Tsuji M, Kawamura A. Abundance of Plant-Associated Gammaproteobacteria Correlates with Immunostimulatory Activity of Angelica sinensis. Medicines. 2019; 6(2):62. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines6020062
Chicago/Turabian StyleKalpana, Kriti, Diego Montenegro, Giovanna Romero, Ximena Peralta, Betul Akgol Oksuz, Adriana Heguy, Moriya Tsuji, and Akira Kawamura. 2019. "Abundance of Plant-Associated Gammaproteobacteria Correlates with Immunostimulatory Activity of Angelica sinensis" Medicines 6, no. 2: 62. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines6020062