Hospital-Acquired Serum Ionized Calcium Derangements and Their Associations with In-Hospital Mortality
Abstract
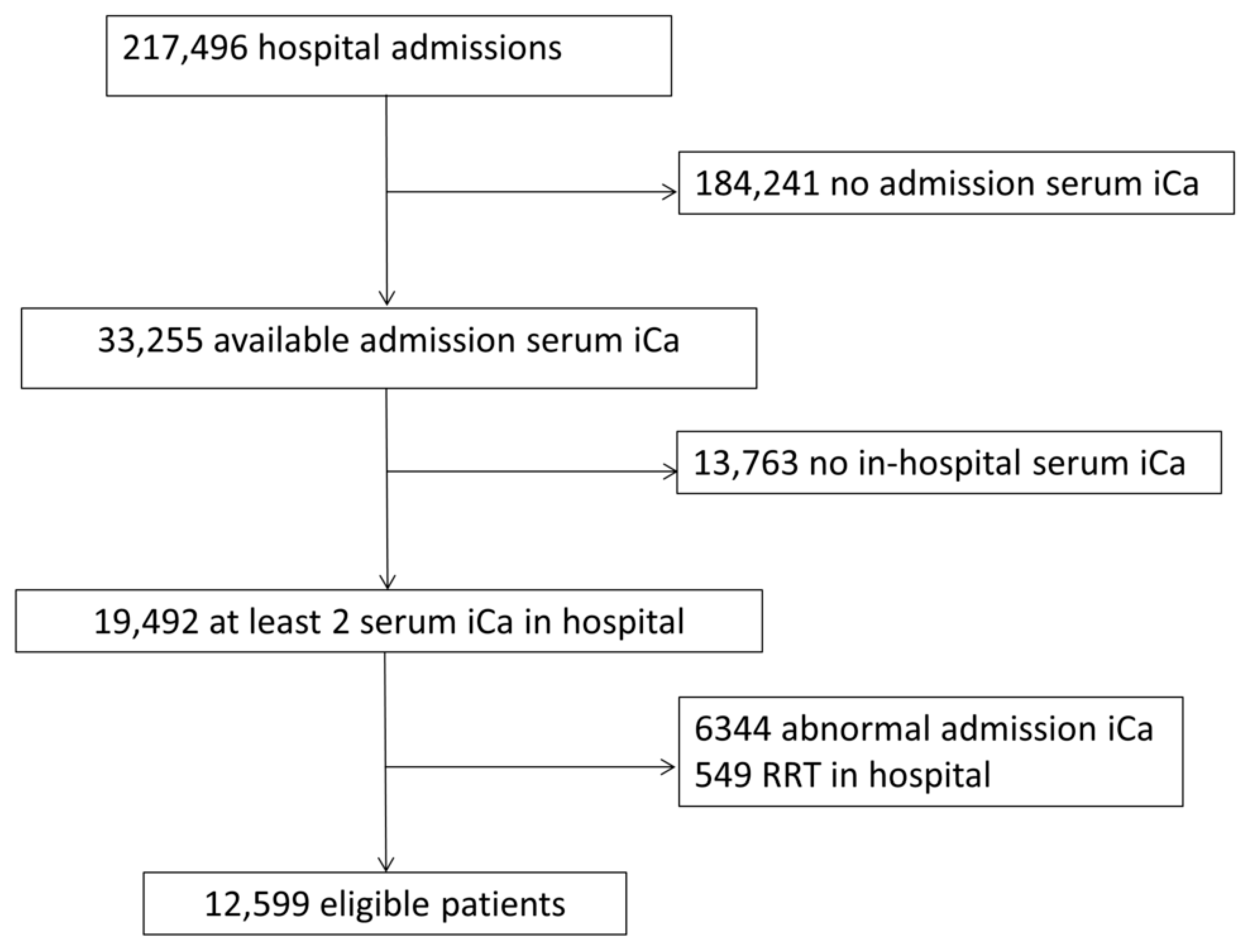
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definition of In-Hospital Hypocalcemia and Hypercalcemia
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Incidence of In-Hospital Hypocalcemia and Hypercalcemia
3.2. Association of In-Hospital Hypocalcemia and Hypercalcemia with Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5 (Suppl. 1), S23–S30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, T.K.; Carlson, R.W.; Geheb, M.A. Prevalence and clinical implications of hypocalcemia in acutely ill patients in a medical intensive care setting. Am. J. Med. 1988, 84, 209–214. [Google Scholar] [CrossRef]
- Akirov, A.; Gorshtein, A.; Shraga-Slutzky, I.; Shimon, I. Calcium levels on admission and before discharge are associated with mortality risk in hospitalized patients. Endocrine 2017, 57, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Chewcharat, A.; Mao, M.A.; Bathini, T.; Vallabhajosyula, S.; Thirunavukkarasu, S.; Kashani, K.B. Impact of admission serum ionized calcium levels on risk of acute kidney injury in hospitalized patients. Sci. Rep. 2020, 10, 12316. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W.; Chewcharat, A.; Mao, M.A.; Kashani, K.B. Serum ionised calcium and the risk of acute respiratory failure in hospitalised patients: A single-centre cohort study in the USA. BMJ Open 2020, 10, e034325. [Google Scholar] [CrossRef] [Green Version]
- Phillips, P.; Pain, R. Correcting the calcium. Br. Med. J. 1977, 1, 598. [Google Scholar] [CrossRef]
- Gauci, C.; Moranne, O.; Fouqueray, B.; de la Faille, R.; Maruani, G.; Haymann, J.P.; Jacquot, C.; Boffa, J.J.; Flamant, M.; Rossert, J.; et al. Pitfalls of measuring total blood calcium in patients with CKD. J. Am. Soc. Nephrol. 2008, 19, 1592–1598. [Google Scholar] [CrossRef] [Green Version]
- Comorbid conditions and correlations with mortality risk among 3399 incident hemodialysis patients. Am. J. Kidney Dis. 1992, 20, 32–38.
- Ladenson, J.H.; Lewis, J.W.; Boyd, J.C. Failure of total calcium corrected for protein, albumin, and pH to correctly assess free calcium status. J. Clin. Endocrinol. Metab. 1978, 46, 986–993. [Google Scholar] [CrossRef]
- Oberleithner, H.; Greger, R.; Lang, F. The effect of respiratory and metabolic acid-base changes on ionized calcium concentration: In vivo and in vitro experiments in man and rat. Eur. J. Clin. Investig. 1982, 12, 451–455. [Google Scholar] [CrossRef]
- Sauter, T.C.; Lindner, G.; Ahmad, S.S.; Leichtle, A.B.; Fiedler, G.M.; Exadaktylos, A.K.; Haider, D.G. Calcium Disorders in the Emergency Department: Independent Risk Factors for Mortality. PLoS ONE 2015, 10, e0132788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thongprayoon, C.; Cheungpasitporn, W.; Hansrivijit, P.; Medaura, J.; Chewcharat, A.; Mao, M.A.; Bathini, T.; Vallabhajosyula, S.; Thirunavukkarasu, S.; Erickson, S.B. Impact of Changes in Serum Calcium Levels on In-Hospital Mortality. Medicina 2020, 56, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheungpasitporn, W.; Thongprayoon, C.; Mao, M.A.; Kittanamongkolchai, W.; Sakhuja, A.; Erickson, S.B. Impact of admission serum calcium levels on mortality in hospitalized patients. Endocr. Res. 2018, 43, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Hamano, T.; Doi, Y.; Oka, T.; Kajimoto, S.; Kubota, K.; Yasuda, S.; Shimada, K.; Matsumoto, A.; Hashimoto, N.; et al. Hidden Hypocalcemia as a Risk Factor for Cardiovascular Events and All-Cause Mortality among Patients Undergoing Incident Hemodialysis. Sci. Rep. 2020, 10, 4418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obi, Y.; Park, C.; Soohoo, M.; Sumida, K.; Hamano, T.; Rhee, C.M.; Kovesdy, C.P.; Kalantar-Zadeh, K.; Streja, E. Association of Pre-ESRD Serum Calcium With Post-ESRD Mortality Among Incident ESRD Patients: A Cohort Study. J. Bone Miner. Res. 2018, 33, 1027–1036. [Google Scholar] [CrossRef]
- Inaguma, D.; Koide, S.; Takahashi, K.; Hayashi, H.; Hasegawa, M.; Yuzawa, Y.; Tanaka, A.; Murata, M.; Shinjo, H.; Otsuka, Y.; et al. Relationship between serum calcium level at dialysis initiation and subsequent prognosis. Renal Replace. Ther. 2017, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Miura, S.; Yoshihisa, A.; Takiguchi, M.; Shimizu, T.; Nakamura, Y.; Yamauchi, H.; Iwaya, S.; Owada, T.; Miyata, M.; Abe, S.; et al. Association of Hypocalcemia With Mortality in Hospitalized Patients With Heart Failure and Chronic Kidney Disease. J. Card Fail. 2015, 21, 621–627. [Google Scholar] [CrossRef]
- Lu, J.L.; Molnar, M.Z.; Ma, J.Z.; George, L.K.; Sumida, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Racial Differences in Association of Serum Calcium with Mortality and Incident Cardio- and Cerebrovascular Events. J. Clin. Endocrinol. Metab. 2016, 101, 4851–4859. [Google Scholar] [CrossRef] [Green Version]
- Thongprayoon, C.; Cheungpasitporn, W.; Chewcharat, A.; Mao, M.A.; Thirunavukkarasu, S.; Kashani, K.B. Hospital mortality and long-term mortality among hospitalized patients with various admission serum ionized calcium levels. Postgrad. Med. 2020, 132, 385–390. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Mao, M.A.; Kittanamongkolchai, W.; Sakhuja, A.; Erickson, S.B. Admission serum phosphate levels predict hospital mortality. Hosp. Pract. 2018, 46, 121–127. [Google Scholar] [CrossRef]
- Wysolmerski, J.J.; Broadus, A.E. Hypercalcemia of malignancy: The central role of parathyroid hormone-related protein. Annu. Rev. Med. 1994, 45, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Mao, M.A.; Harrison, A.M.; Erickson, S.B. Elevated admission serum calcium phosphate product as an independent risk factor for acute kidney injury in hospitalized patients. Hosp. Pract. 2019, 47, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Mao, M.A.; Erickson, S.B. Calcium-phosphate product and its impact on mortality in hospitalized patients. Nephrology 2020, 25, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Mao, M.A.; Sakhuja, A.; Erickson, S.B. Admission calcium levels and risk of acute kidney injury in hospitalised patients. Int. J. Clin. Pract. 2018, 72, e13057. [Google Scholar] [CrossRef] [PubMed]
- Baylis, P.H.; Milles, J.J.; Wilkinson, R.; Heath, D.A. Vasopressin function in hypercalcaemia. Clin. Endocrinol. 1981, 15, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Ryu, W.S.; Kim, B.J.; Yoon, B.W. Elevated calcium after acute ischemic stroke: Association with a poor short-term outcome and long-term mortality. J. Stroke 2015, 17, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Alonso, A.; Michos, E.D.; Loehr, L.R.; Astor, B.C.; Coresh, J.; Folsom, A.R. Serum magnesium, phosphorus, and calcium are associated with risk of incident heart failure: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2014, 100, 756–764. [Google Scholar] [CrossRef]
- Nair, P.; Lee, P.; Reynolds, C.; Nguyen, N.D.; Myburgh, J.; Eisman, J.A.; Center, J.R. Significant perturbation of vitamin D-parathyroid-calcium axis and adverse clinical outcomes in critically ill patients. Intensive Care Med. 2013, 39, 267–274. [Google Scholar] [CrossRef]
- Lind, L.; Carlstedt, F.; Rastad, J.; Stiernström, H.; Stridsberg, M.; Ljunggren, O.; Wide, L.; Larsson, A.; Hellman, P.; Ljunghall, S. Hypocalcemia and parathyroid hormone secretion in critically ill patients. Crit. Care Med. 2000, 28, 93–99. [Google Scholar] [CrossRef]
- Zaloga, G.P.; Chernow, B. The multifactorial basis for hypocalcemia during sepsis. Studies of the parathyroid hormone-vitamin D axis. Ann. Intern. Med. 1987, 107, 36–41. [Google Scholar] [CrossRef]
- Chernow, B.; Bamberger, S.; Stoiko, M.; Vadnais, M.; Mills, S.; Hoellerich, V.; Warshaw, A.L. Hypomagnesemia in patients in postoperative intensive care. Chest 1989, 95, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hébert, P.; Mehta, N.; Wang, J.; Hindmarsh, T.; Jones, G.; Cardinal, P. Functional magnesium deficiency in critically ill patients identified using a magnesium-loading test. Crit. Care Med. 1997, 25, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.; Chan, Y.W.; Keung, W.; Yan, B.P. Electrophysiological mechanisms of long and short QT syndromes. Int. J. Cardiol. Heart Vasc. 2017, 14, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurley, K.; Baggs, D. Hypocalcemic cardiac failure in the emergency department. J. Emerg. Med. 2005, 28, 155–159. [Google Scholar] [CrossRef] [PubMed]
| Variables | All | Serum Ionized Calcium during Hospitalization | ||||
|---|---|---|---|---|---|---|
| Normal | Hypocalcemia Only | Hypercalcemia Only | Both Hypo- and Hypercalcemia | p-Value | ||
| N | 12,599 | 5739 | 5316 | 458 | 1086 | |
| Age (year) | 63 ± 17 | 63 ± 17 | 62 ± 16 | 66 ± 16 | 62 ± 16 | <0.001 |
| Male sex | 7250 (58) | 3181 (55) | 3265 (61) | 232 (51) | 572 (53) | <0.001 |
| Caucasian | 11,584 (92) | 5294 (92) | 4902 (92) | 425 (93) | 963 (89) | 0.001 |
| Principal diagnosis | <0.001 | |||||
| - Cardiovascular | 4281 (34) | 1138 (20) | 2321 (44) | 141 (31) | 681 (63) | |
| - Hematology/oncology | 2570 (20) | 1363 (24) | 1023 (19) | 79 (17) | 105 (10) | |
| - Infectious disease | 365 (3) | 176 (3) | 137 (3) | 27 (6) | 25 (2) | |
| - Endocrine/metabolic | 252 (2) | 149 (3) | 80 (2) | 16 (3) | 7 (1) | |
| - Respiratory | 548 (4) | 346 (6) | 151 (3) | 28 (6) | 23 (2) | |
| - Gastrointestinal | 1041 (8) | 611 (11) | 338 (6) | 46 (10) | 46 (4) | |
| - Genitourinary | 271 (2) | 156 (3) | 81 (2) | 22 (5) | 12 (1) | |
| - Injury and poisoning | 1733 (14) | 961 (17) | 637 (12) | 55 (12) | 80 (7) | |
| - Other | 1538 (12) | 839 (15) | 548 (10) | 44 (10) | 107 (10) | |
| Comorbidity | ||||||
| - Coronary artery disease | 2940 (23) | 1307 (23) | 1241 (23) | 127 (28) | 265 (24) | 0.08 |
| - Congestive heart failure | 1057 (8) | 443 (8) | 444 (8) | 46 (10) | 124 (11) | <0.001 |
| - Peripheral artery disease | 555 (4) | 265 (5) | 211 (4) | 28 (6) | 51 (5) | 0.09 |
| - Stroke | 1032 (8) | 498 (9) | 376 (7) | 46 (10) | 112 (10) | <0.001 |
| - Diabetes mellitus | 2755 (22) | 1333 (23) | 1094 (21) | 114 (25) | 214 (20) | 0.001 |
| - COPD | 1383 (11) | 672 (12) | 525 (10) | 65 (14) | 121 (11) | 0.002 |
| - Cirrhosis | 287 (2) | 127 (2) | 113 (2) | 12 (3) | 35 (3) | 0.15 |
| Charlson Comorbidity Score | 2.0 ± 2.4 | 2.2 ± 2.5 | 1.8 ± 2.3 | 2.6 ± 2.7 | 1.6 ± 2.0 | <0.001 |
| eGFR (ml/min/1.73 m2) | 77 ± 28 | 77 ± 29 | 77 ± 26 | 65 ± 32 | 76 ± 25 | <0.001 |
| Acute kidney injury | 2611 (21) | 869 (15) | 1215 (23) | 140 (31) | 387 (36) | <0.001 |
| ICU admission | 8691 (69) | 2947 (51) | 4369 (82) | 314 (69) | 1061 (98) | <0.001 |
| Number of serum ionized calcium measurement in hospital | 3 (2–5) | 2 (2–3) | 4 (3–6) | 4 (3–7) | 5 (4–9) | <0.001 |
| Length of hospital stay (day) | 6 (4–9) | 5 (3–8) | 6 (4–10) | 7 (5–13) | 7 (5–11) | <0.001 |
| Admission serum ionized calcium (mg/dL) | 4.80 (4.70–4.93) | 4.85 (4.73–5.00) | 4.76 (4.68–4.86) | 4.99 (4.81–5.14) | 4.80 (4.70–4.90) | <0.001 |
| Lowest serum ionized calcium (mg/dL) | 4.57 (4.23–4.75) | 4.75 (4.66–4.85) | 4.28 (4.04–4.45) | 4.85 (4.70–5.01) | 4.11 (3.94–4.30) | <0.001 |
| Highest serum ionized calcium (mg/dL) | 4.98 (4.82–5.20) | 4.97 (4.85–5.10) | 4.90 (4.77–5.09) | 5.60 (5.46–5.81) | 5.73 (5.54–6.20) | <0.001 |
| Serum Ionized Calcium during Hospitalization | N | In-Hospital Mortality | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | Adjusted OR (95% CI) | p | |||
| In-hospital hypocalcemia | ||||||
| No | 6197 | 147 (2.4) | 1 (ref) | - | 1 (ref) | - |
| Yes | 6402 | 198 (3.09) | 1.31 (1.06–1.63) | 0.01 | 1.29 (1.01–1.66) | 0.04 |
| In-hospital hypercalcemia | ||||||
| No | 11,055 | 268 (2.4) | 1 (ref) | - | 1 (ref) | - |
| Yes | 1544 | 77 (5.0) | 2.11 (1.63–2.74) | <0.001 | 1.44 (1.06–1.95) | 0.02 |
| Groups | ||||||
| Normal | 5739 | 123 (2.1) | 1 (ref) | - | 1 (ref) | - |
| Hypocalcemia only | 5316 | 145 (2.7) | 1.28 (1.01–1.63) | 0.04 | 1.28 (1.01–1.64) | 0.04 |
| Hypercalcemia only | 458 | 24 (5.2) | 2.52 (1.61–3.95) | <0.001 | 1.64 (1.02–2.68) | 0.03 |
| Both hypo- and hypercalcemia | 1086 | 53 (4.9) | 2.34 (1.69–3.25) | <0.001 | 1.73 (1.14–2.62) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongprayoon, C.; Hansrivijit, P.; Petnak, T.; Mao, M.A.; Bathini, T.; Vallabhajosyula, S.; Lertjitbanjong, P.; Qureshi, F.; Erickson, S.B.; Cheungpasitporn, W. Hospital-Acquired Serum Ionized Calcium Derangements and Their Associations with In-Hospital Mortality. Medicines 2020, 7, 70. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines7110070
Thongprayoon C, Hansrivijit P, Petnak T, Mao MA, Bathini T, Vallabhajosyula S, Lertjitbanjong P, Qureshi F, Erickson SB, Cheungpasitporn W. Hospital-Acquired Serum Ionized Calcium Derangements and Their Associations with In-Hospital Mortality. Medicines. 2020; 7(11):70. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines7110070
Chicago/Turabian StyleThongprayoon, Charat, Panupong Hansrivijit, Tananchai Petnak, Michael A. Mao, Tarun Bathini, Saraschandra Vallabhajosyula, Ploypin Lertjitbanjong, Fawad Qureshi, Stephen B. Erickson, and Wisit Cheungpasitporn. 2020. "Hospital-Acquired Serum Ionized Calcium Derangements and Their Associations with In-Hospital Mortality" Medicines 7, no. 11: 70. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines7110070






