Analgesic Efficacy of a Combination of Fentanyl and a Japanese Herbal Medicine “Yokukansan” in Rats with Acute Inflammatory Pain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Administration of Drugs
2.3. Assessment of Analgesia
2.3.1. Formalin Test
2.3.2. Immunofluorescent Staining
2.4. Cell Culture
2.5. Whole-Cell Patch-Clamp Recording
2.6. Statistical Analysis
3. Results
3.1. Formalin Test
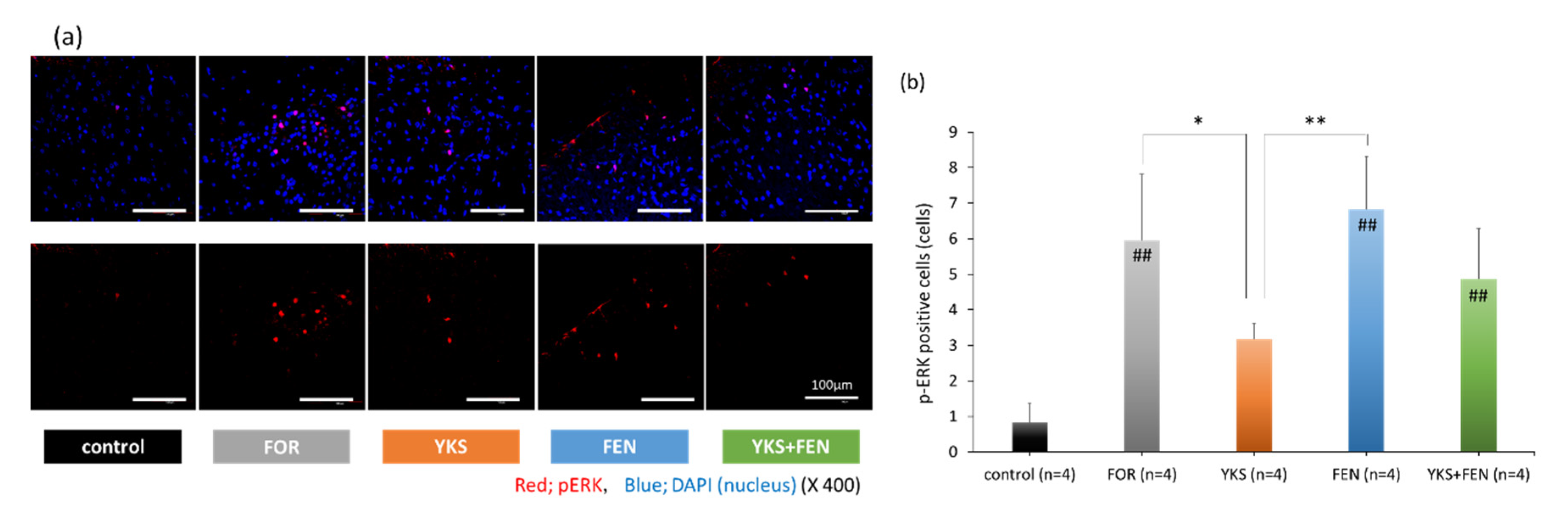
3.2. Immunofluorescent Staining of pERK(+) Cells
3.3. Whole-Cell Patch-Clamp Recording of TRPA1 Currents
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jowkar, S.; Khosravi, M.B.; Sahmeddini, M.A.; Eghbal, M.H.; Samadi, K. Preconditioning effect of remifentanil versus fentanyl in prevalence of early graft dysfunction in patients after liver transplant: A randomized clinical trial. Exp. Clin. Transplant. 2020, 18, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.; Martinez, V. Opioid-induced hyperalgesia in patients after surgery: A systematic review and a meta-analysis. Br. J. Anaesth. 2014, 112, 991–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, E.H.; Tran, D.H.; Lam, S.W.; Irwin, M.G. Remifentanil tolerance and hyperalgesia: Short-term gain, long-term pain? Anaesthesia 2016, 71, 1347–1362. [Google Scholar] [CrossRef] [PubMed]
- Chia, Y.Y.; Liu, K.; Wang, J.J.; Kuo, M.C.; Ho, S.T. Intraoperative high dose fentanyl induces postoperative fentanyl tolerance. Can. J. Anaesth. 1999, 46, 872–877. [Google Scholar] [CrossRef]
- Rupniewska-Ladyko, A.; Malec-Milewska, M.A. High dose of fentanyl may accelerate the onset of acute postoperative pain. Anesthesiol. Pain Med. 2019, 9, e94498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, H.K.; Ma, Y.; Xie, H. TRPV1 and spinal astrocyte activation contribute to remifentanil-induced hyperalgesia in rats. Neuroreport 2019, 30, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhang, L.; Zhao, H.; Song, F.; Chen, G.; Zhu, H. The role of p38MAPK activation in spinal dorsal horn in remifentanil-induced postoperative hyperalgesia in rats. Neurol. Res. 2016, 38, 929–936. [Google Scholar] [CrossRef]
- Lv, C.C.; Xia, M.L.; Shu, S.J.; Chen, F.; Jiang, L.S. Attenuation of remifentanil-induced hyperalgesia by betulinic acid associates with inhibiting oxidative stress and inflammation in spinal dorsal horn. Pharmacology 2018, 102, 300–306. [Google Scholar] [CrossRef]
- Li, T.; Wang, H.; Wang, J.; Chen, Y.; Yang, C.; Zhao, M.; Wang, G.; Yang, Z. Annexin 1 inhibits remifentanil-induced hyperalgesia and NMDA receptor phosphorylation via regulating spinal CXCL12/CXCR4 in rats. Neurosci. Res. 2019, 144, 48–55. [Google Scholar] [CrossRef]
- Lu, A.; Lei, H.; Li, L.; Lai, L.; Liang, W.; Xu, S. Role of mitochondrial Ca2+ uniporter in remifentanil-induced postoperative allodynia. Eur. J. Neurosci. 2018, 47, 305–313. [Google Scholar] [CrossRef]
- Li, S.; Zeng, J.; Wan, X.; Yao, Y.; Zhao, N.; Yu, Y.; Yu, C.; Xia, Z. Enhancement of spinal dorsal horn neuron NMDA receptor phosphorylation as the mechanism of remifentanil induced hyperalgesia: Roles of PKC and CaMKII. Mol. Pain 2017, 13, 1–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Shi, L.; Zhang, J.; Kong, M.; Liu, Y.; Zhou, Y.; Xu, L.; He, J.; Ma, Z.; Gu, X. Neuron-restrictive silencer factor in periaqueductal gray contributes to remifentanil-induced postoperative hyperalgesia via repression of the mu-opioid receptor. J. Neurol. Sci. 2015, 352, 48–52. [Google Scholar] [CrossRef]
- Ye, L.; Xiao, L.; Yang, S.Y.; Duan, J.J.; Chen, Y.; Cui, Y.; Chen, Y. Cathepsin S in the spinal microglia contributes to remifentanil-induced hyperalgesia in rats. Neuroscience 2017, 344, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Xuerong, Y.; Yuguang, H.; Xia, J.; Hailan, W. Ketamine and lornoxicam for preventing a fentanyl-induced increase in postoperative morphine requirement. Anesth. Analg. 2008, 107, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.W.; Lindsay, S.L.; Ryall, D.M.; Kokri, M.S.; Eldabe, S.S.; Lear, G.A. Does intrathecal fentanyl produce acute cross-tolerance to i.v. morphine? Br. J. Anaesth. 1997, 78, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.; Drover, D.R.; Ginosar, Y.; Cohen, S.E.; Riley, E.T. Intrathecal fentanyl added to bupivacaine and morphine for cesarean delivery may induce a subtle acute opioid tolerance. Int. J. Obstet. Anesth. 2012, 21, 29–34. [Google Scholar] [CrossRef]
- Yildirim, V.; Doganci, S.; Cinar, S.; Eskin, M.B.; Ozkan, G.; Eksert, S.; Ince, M.E.; Dogrul, A. Acute high dose-fentanyl exposure produces hyperalgesia and tactile allodynia after coronary artery bypass surgery. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3425–3434. [Google Scholar]
- Richebé, P.; Rivat, C.; Laulin, J.P.; Maurette, P.; Simonnet, G. Ketamine improves the management of exaggerated postoperative pain observed in perioperative fentanyl-treated rats. Anesthesiology 2005, 102, 421–428. [Google Scholar] [CrossRef]
- Li, Q.B.; Chang, L.; Ye, F.; Luo, Q.H.; Tao, Y.X.; Shu, H.H. Role of spinal cyclooxygenase-2 and prostaglandin E2 in fentanyl-induced hyperalgesia in rats. Br. J. Anaesth. 2018, 120, 827–835. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Ye, F.; Luo, Q.; Tao, Y.; Shu, H. Increased hyperalgesia and proinflammatory cytokines in the spinal cord and dorsal root ganglion after surgery and/or fentanyl administration in rats. Anesth. Analg. 2018, 126, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yin, P.; Chen, J.; Jin, S.; Liu, J.; Luo, F. CaMKIIα may modulate fentanyl-induced hyperalgesia via a CeLC-PAG-RVM-spinal cord descending facilitative pain pathway in rats. PLoS ONE 2017, 12, e0177412. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wei, W. Role of gabapentin in preventing fentanyl- and morphine-withdrawal-induced hyperalgesia in rats. J. Anesth. 2012, 26, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Caires, S.; Steenkamp, V. Use of Yokukansan (TJ-54) in the treatment of neurological disorders: A review. Phytother. Res. 2010, 24, 1265–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikarashi, Y.; Mizoguchi, K. Neuropharmacological efficacy of the traditional Japanese Kampo medicine yokukansan and its active ingredients. Pharmacol. Ther. 2016, 166, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Tajima, K.; Kawagoe, I.; Kanai, M.; Mitsuhata, H. Efficacy of traditional herbal medicine, Yokukansan on patients with neuropathic pain. Masui. Jpn. J. Anesthesiol. 2009, 58, 1248–1255, (In Japanese, English Abstract). [Google Scholar]
- Yamaguchi, K. Traditional Japanese herbal medicines for treatment of odontopathy. Front. Pharmacol. 2015, 6, 176. [Google Scholar] [CrossRef] [Green Version]
- Sugasawa, Y. Effect of Yokukansan, Japanese Herbal Medicine, on Phantom-limb pain. Middle East J. Anaesthesiol. 2016, 23, 499–500. [Google Scholar]
- Akiyama, H.; Hasegawa, Y. Effectiveness of the traditional Japanese Kampo medicine Yokukansan for chronic migraine: A case report. Medicine (Baltimore) 2019, 98, e17000. [Google Scholar] [CrossRef]
- Suzuki, Y.; Mitsuhata, H.; Yuzurihara, M.; Kase, Y. Antiallodynic effect of herbal medicine yokukansan on peripheral neuropathy in rats with chronic constriction injury. Evid. Based Complement. Altern. Med. 2012, 2012, 953459. [Google Scholar] [CrossRef] [Green Version]
- Ebisawa, S.; Andoh, T.; Shimada, Y.; Kuraishi, Y. Yokukansan improves mechanical allodynia through the regulation of interleukin-6 expression in the spinal cord in mice with neuropathic pain. Evid. Based Complement. Altern. Med. 2015, 2015, 870687. [Google Scholar] [CrossRef] [Green Version]
- Suga, H.; Sunagawa, M.; Ikemoto, H.; Nakanishi, T.; Fujiwara, A.; Okada, M. The analgesic and anti-stress effects of a Kampo medicine (Yokukansan) in rats with chronic constriction injury—A comparative study with kamishoyosan. J. Integr. Med. Ther. 2015, 2, 5. [Google Scholar] [CrossRef]
- Honda, Y.; Sunagawa, M.; Yoneyama, S.; Ikemoto, H.; Nakanishi, T.; Iwanami, H.; Hisamitsu, T. Analgesic and anti-stress effects of Yokukansan in rats with adjuvant arthritis. Kampo Med. 2013, 64, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, M.; Sunagawa, M.; Okada, M.; Ikemoto, H.; Suga, H.; Katayama, A.; Otake, H.; Hisamitsu, T. Yokukansan, a Kampo medicine, prevents the development of morphine tolerance through the inhibition of spinal glial cell activation in rats. Integr. Med. Res. 2016, 5, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Katayama, A.; Kanada, Y.; Tsukada, M.; Akanuma, Y.; Takemura, H.; Ono, T.; Suga, H.; Mera, H.; Hisamitsu, T.; Sunagawa, M. Yokukansan (Kampo medicinal formula) prevents the development of morphine tolerance by inhibiting the secretion of orexin A. Integr. Med. Res. 2018, 7, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Z.; Ikarashi, Y.; Kase, Y. Isoliquiritigenin is a novel NMDA receptor antagonist in kampo medicine yokukansan. Cell Mol. Neurobiol. 2011, 31, 1203–1212. [Google Scholar] [CrossRef]
- Kawakami, Z.; Omiya, Y.; Mizoguchi, K. Comparison of the effects of Yokukansan and Yokukansankachimpihange on glutamate uptake by cultured astrocytes and glutamate-induced excitotoxicity in cultured PC12 cells. Evid. Based Complement. Altern. Med. 2019, 2019, 9139536. [Google Scholar] [CrossRef] [Green Version]
- Takeda, A.; Itoh, H.; Tamano, H.; Yuzurihara, M.; Oku, N. Suppressive effect of Yokukansan on excessive release of glutamate and aspartate in the hippocampus of zinc-deficient rats. Nutr. Neurosci. 2008, 11, 41–46. [Google Scholar] [CrossRef]
- Vissers, K.C.; Geenen, F.; Biermans, R.; Meert, T.F. Pharmacological correlation between the formalin test and the neuropathic pain behavior in different species with chronic constriction injury. Pharmacol. Biochem. Behav. 2006, 84, 479–486. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [Green Version]
- Sałat, K.; Filipek, B. Antinociceptive activity of transient receptor potential channel TRPV1, TRPA1, and TRPM8 antagonists in neurogenic and neuropathic pain models in mice. J. Zhejiang Univ. Sci. B 2015, 16, 167–178. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.R.; Gereau, R.W., IV; Malcangio, M.; Strichartz, G.R. MAP kinase and pain. Brain Res. Rev. 2009, 60, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Nagayasu, K.; Nishitani, N.; Shirakawa, H.; Sekiguchi, K.; Ikarashi, Y.; Kase, Y.; Kaneko, S. Yokukansan inhibits morphine tolerance and physical dependence in mice: The role of α₂A-adrenoceptor. Neuroscience 2012, 227, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Derouiche, S.; Maruyama, K.; Tominaga, M. Emerging perspectives on pain management by modulation of TRP channels and ANO1. Int. J. Mol. Sci. 2019, 20, 3411. [Google Scholar] [CrossRef] [Green Version]
- Forster, A.B.; Reeh, P.W.; Messlinger, K.; Fischer, M.J. High concentrations of morphine sensitize and activate mouse dorsal root ganglia via TRPV1 and TRPA1 receptors. Mol. Pain 2009, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Kanada, Y.; Katayama, A.; Ikemoto, H.; Takahashi, K.; Tsukada, M.; Nakamura, A.; Ishino, S.; Hisamitsu, T.; Sunagawa, M. Inhibitory effect of the Kampo medicinal formula Yokukansan on acute stress-induced defecation in rats. Neuropsychiatr. Dis. Treat. 2018, 14, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Gamal-Eltrabily, M.; Espinosa de Los Monteros-Zúñiga, A.; Manzano-García, A.; Martínez-Lorenzana, G.; Condés-Lara, M.; González-Hernández, A. The Rostral Agranular Insular Cortex, a New Site of Oxytocin to Induce Antinociception. J. Neurosci. 2020, 40, 5669–5680. [Google Scholar] [CrossRef]
- Zhuang, Z.Y.; Gerner, P.; Woolf, C.J.; Ji, R.R. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005, 114, 149–159. [Google Scholar] [CrossRef]
- Karim, F.; Wang, C.C.; Gereau, R.W., IV. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J. Neurosci. 2001, 21, 3771–3779. [Google Scholar] [CrossRef]
- Tsuda, M.; Ishii, S.; Masuda, T.; Hasegawa, S.; Nakamura, K.; Nagata, K.; Yamashita, T.; Furue, H.; Tozaki-Saitoh, H.; Yoshimura, M.; et al. Reduced pain behaviors and extracellular signal-related protein kinase activation in primary sensory neurons by peripheral tissue injury in mice lacking platelet-activating factor receptor. J. Neurochem. 2007, 102, 1658–1668. [Google Scholar] [CrossRef]
- Ma, Y.; Bao, Y.; Wang, S.; Li, T.; Chang, X.; Yang, G.; Meng, X. Anti-inflammation effects and potential mechanism of saikosaponins by regulating nicotinate and nicotinamide metabolism and arachidonic acid metabolism. Inflammation 2016, 39, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Luo, C.; Wang, P.; He, Q.; Zhou, J.; Peng, H. Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF-κB pathways in LPS-stimulated RAW 264.7 cells. Exp. Ther. Med. 2013, 5, 1345–1350. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.X.; Dai, Y.Y.; Pan, Y.F.; Wu, X.M.; Yang, Y.; Bian, K.; Zhang, D.D. Total flavonoids from radix glycyrrhiza exert anti-inflammatory and antitumorigenic effects by inactivating iNOS signaling pathways. Evid. Based Complement. Altern. Med. 2018, 2018, 6714282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, E.J.; Park, G.H.; Song, K.S. Neuroprotective effects of liquiritigenin isolated from licorice roots on glutamate-induced apoptosis in hippocampal neuronal cells. Neurotoxicology 2013, 39, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Roeckel, L.A.; Le Coz, G.M.; Gavériaux-Ruff, C.; Simonin, F. Opioid-induced hyperalgesia: Cellular and molecular mechanisms. Neuroscience 2016, 338, 160–182. [Google Scholar] [CrossRef] [PubMed]


| Uncariae cum Uncis ramulus | 3.0 g |
| Cnidii rhizoma | 3.0 g |
| Bupleuri radix | 2.0 g |
| Atratylodis Lanceae rhizoma | 4.0 g |
| Poria | 4.0 g |
| Angelicae radix | 3.0 g |
| Glycyrrhizae radix | 1.5 g |
| Groups | Days 1–7 | Day 8 | ||
|---|---|---|---|---|
| 10 min before Test | Formalin Test | |||
| control | Powdered chow | Saline (i.p.) | Saline (50 µL; s.c.) | Observation of pain-related behavior (60 min) |
| FOR | Powdered chow | Saline (i.p.) | Formalin (5%, 50 µL; s.c.) | |
| YKS | Powdered chow mixed with YKS (3%) | Saline (i.p.) | Formalin (5%, 50 µL; s.c.) | |
| FEN | Powdered chow | Fentanyl (0.08 µg/kg; i.p.) | Formalin (5%, 50 µL; s.c.) | |
| YKS+FEN | Powdered chow mixed with YKS (3%) | Fentanyl (0.08 µg/kg; i.p.) | Formalin (5%, 50 µL; s.c.) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akanuma, Y.; Kato, M.; Takayama, Y.; Ikemoto, H.; Adachi, N.; Ohashi, Y.; Yogi, W.; Okumo, T.; Tsukada, M.; Sunagawa, M. Analgesic Efficacy of a Combination of Fentanyl and a Japanese Herbal Medicine “Yokukansan” in Rats with Acute Inflammatory Pain. Medicines 2020, 7, 75. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines7120075
Akanuma Y, Kato M, Takayama Y, Ikemoto H, Adachi N, Ohashi Y, Yogi W, Okumo T, Tsukada M, Sunagawa M. Analgesic Efficacy of a Combination of Fentanyl and a Japanese Herbal Medicine “Yokukansan” in Rats with Acute Inflammatory Pain. Medicines. 2020; 7(12):75. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines7120075
Chicago/Turabian StyleAkanuma, Yuko, Mami Kato, Yasunori Takayama, Hideshi Ikemoto, Naoki Adachi, Yusuke Ohashi, Wakako Yogi, Takayuki Okumo, Mana Tsukada, and Masataka Sunagawa. 2020. "Analgesic Efficacy of a Combination of Fentanyl and a Japanese Herbal Medicine “Yokukansan” in Rats with Acute Inflammatory Pain" Medicines 7, no. 12: 75. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines7120075






