Clinical Outcome of Patients Submitted to Liver Resection in the Context of Metastatic Breast Cancer: A Study of a Tertiary Hospital Center
Abstract
:1. Introduction
2. Materials and Methods
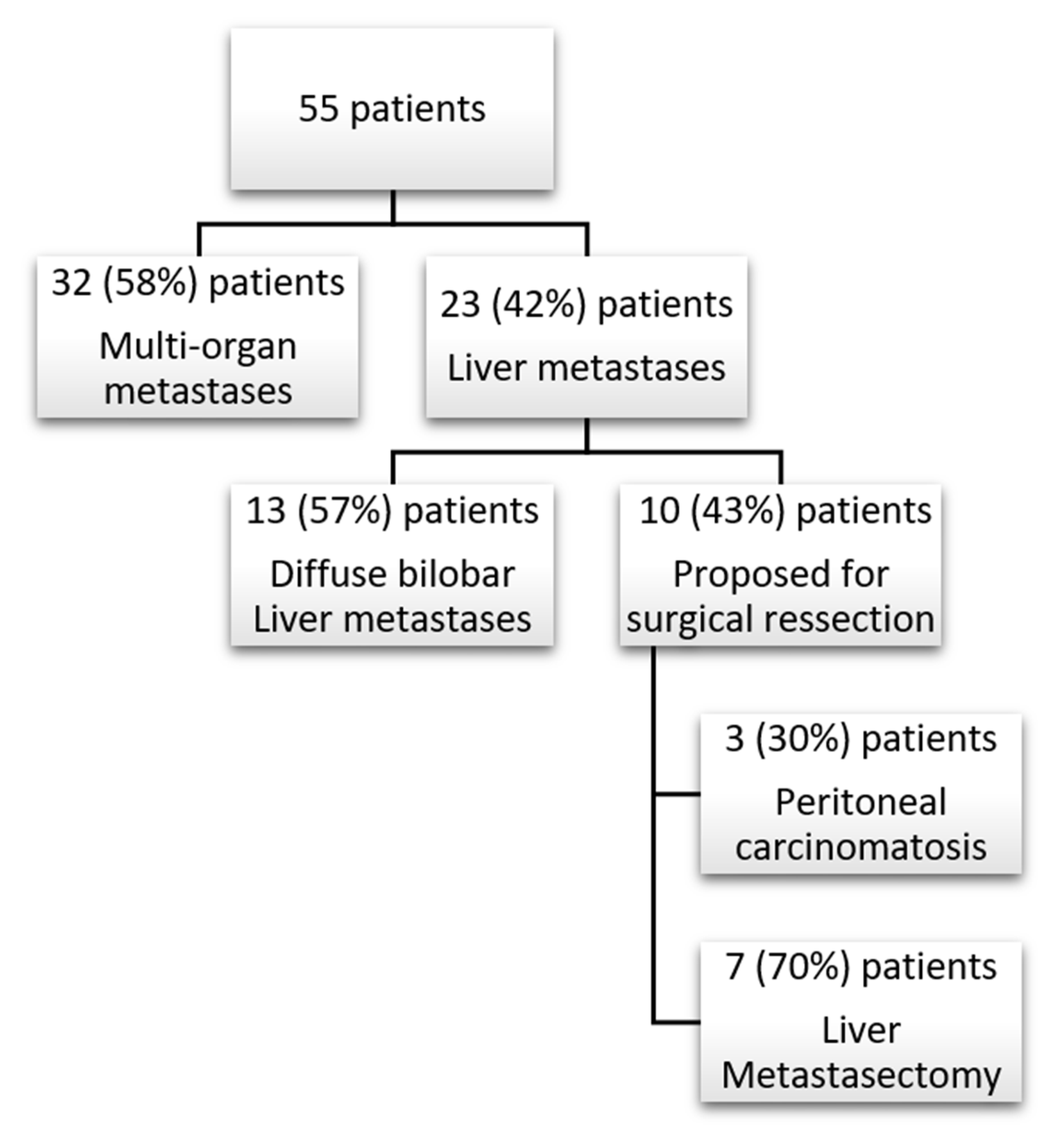
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- GLOBOCAN 2020. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2020. World Health Organization. International Agency for Research on Cancer. Available online: https://gco.iarc.fr (accessed on 2 September 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bacalbasa, N.; Dima, S.O.; Purtan-Purnichescu, R.; Herlea, V.; Popescu, I. Role of surgical treatment in breast cancer liver metastases: A single center experience. Anticancer Res. 2014, 34, 5563–5568. [Google Scholar] [PubMed]
- Elias, D.; Maisonnette, F.; Druet-Cabanac, M.; Ouellet, J.-F.; Guinebretiere, J.-M.; Spielmann, M.; Delaloge, S. An attempt to clarify indications for hepatectomy for liver metastases from breast cancer. Am. J. Surg. 2003, 185, 158–164. [Google Scholar] [CrossRef]
- Howlader, M.; Heaton, N.; Rela, M. Resection of liver metastases from breast cancer: Towards a management guideline. Int. J. Surg. 2011, 9, 285–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, D.; Lasser, P.; May-Levin, F.; el Malt, O.; Thomas, H.; Mouriesse, H. Surgical and chemotherapeutic treatment of hepatic metastases from carcinoma of the breast. Surg. Gynecol. Obstet. 1991, 172, 461–464. [Google Scholar] [PubMed]
- Elias, D.; Di Pietroantonio, D. Surgery for liver metastases from breast cancer. HPB 2006, 8, 97–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlastos, G.; Smith, D.L.; Singletary, S.E.; Mirza, N.Q.; Tuttle, T.M.; Popat, R.J.; Curley, S.A.; Ellis, L.M.; Roh, M.S.; Vauthey, J.-N. Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann. Surg. Oncol. 2004, 11, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Golse, N.; Adam, R. Liver Metastases From Breast Cancer: What Role for Surgery? Indications and Results. Clin. Breast Cancer 2017, 17, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Tasleem, S.; Bolger, J.C.; Kelly, M.E.; Boland, M.R.; Bowden, D.; Sweeney, K.J.; Malone, C. The role of liver resection in patients with metastatic breast cancer: A systematic review examining the survival impact. Ir. J. Med. Sci. 2018, 187, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Sadot, E.; Lee, S.Y.; Sofocleous, C.T.; Solomon, S.B.; Gönen, M.; Kingham, T.P.; Allen, P.J.; DeMatteo, R.P.; Jarnagin, W.R.; Hudis, C.A.; et al. Hepatic Resection or Ablation for Isolated Breast Cancer Liver Metastasis: A Case-control Study With Comparison to Medically Treated Patients. Ann. Surg. 2016, 264, 147–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schatka, I.; Tschernig, M.; Rogasch, J.M.M.; Bluemel, S.; Graef, J.; Furth, C.; Sehouli, J.; Blohmer, J.-U.; Gebauer, B.; Fehrenbach, U.; et al. Selective Internal Radiation Therapy in Breast Cancer Liver Metastases: Outcome Assessment Applying a Prognostic Score. Cancers 2021, 13, 3777. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Saxena, A.; Liauw, W.; Chu, F.; Morris, D.L. Hepatic resection for metastatic breast cancer: A systematic review. Eur. J. Cancer 2011, 47, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Aloia, T.; Krissat, J.; Bralet, M.-P.; Paule, B.; Giacchetti, S.; Delvart, V.; Azoulay, D.; Bismuth, H.; Castaing, D. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann. Surg. 2006, 244, 897–908. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, Q.; Feng, Y.; Li, Z.; Pan, Q.; Zhao, Y.; Zhu, W.; Zhang, N.; Zhou, J.; Wang, L.; et al. Resection of liver metastases from breast cancer: A multicentre analysis. Clin. Transl. Oncol. 2020, 22, 512–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Resected LM | Non-Resected LM | Multi-Organ Metastases | |
|---|---|---|---|
| Age (med, IQR) | 48 (39–58) | 42.5 (25–55) | 49.5 (43.25–54.00) |
| Primary tumor size (med, IQR) | 29 (19–30) | 29.5 (25–31.25) | 26 (14.5–40) |
| Primary tumor histology | |||
| DCIS | 0 | 0 | 1 |
| IDC | 4 | 12 | 22 |
| ILC | 1 | 1 | 4 |
| Other | 2 | 1 | 3 |
| Pathological T stage (pT/ypT) | |||
| Tis | 0 | 1 | 2 |
| 1 | 3 | 3 | 8 |
| 2 | 4 | 9 | 15 |
| 3 | 0 | 2 | 2 |
| 4 | 0 | 0 | 1 |
| Pathological N stage (pN/ypN) | |||
| 0 | 2 | 7 | 13 |
| 1 | 3 | 2 | 4 |
| 2 | 2 | 5 | 8 |
| 3 | 0 | 1 | 2 |
| Margin Status (R) | |||
| 0 | 6 | 14 | 27 |
| 1 | 1 | 1 | 0 |
| Lymph vessel invasion | |||
| No | 4 | 5 | 11 |
| Yes | 3 | 7 | 14 |
| Blood vessel invasion | |||
| No | 5 | 10 | 22 |
| Yes | 2 | 2 | 3 |
| Perineural invasion | |||
| No | 7 | 9 | 20 |
| Yes | 0 | 3 | 0 |
| ER (≥1%) | |||
| No | 0 | 2 | 1 |
| Yes | 7 | 13 | 31 |
| PR (≥1%) | |||
| No | 2 | 6 | 5 |
| Yes | 5 | 8 | 26 |
| HER2 | |||
| Neg | 6 | 13 | 30 |
| Pos | 1 | 2 | 2 |
| Molecular subtype | |||
| Luminal | 6 | 11 | 29 |
| Luminal-HER2 | 1 | 2 | 2 |
| Triple negative | 0 | 2 | 1 |
| Primary treatment | |||
| Surgery | 6 | 8 | 21 |
| Chemotherapy | 1 | 8 | 11 |
| Type of resection | |||
| Tumorectomy | 4 | 9 | 11 |
| Mastectomy | 3 | 7 | 19 |
| Adjuvant Chemoterapy | |||
| No | 1 | 1 | 4 |
| Yes | 6 | 14 | 26 |
| Hormone therapy | |||
| No | 0 | 2 | 2 |
| Yes | 7 | 13 | 29 |
| Trastuzumab | |||
| No | 6 | 12 | 30 |
| Yes | 1 | 2 | 2 |
| Radiotherapy | |||
| No | 0 | 2 | 8 |
| Yes | 6 | 14 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueiro, J.; Devezas, V.; Sousa, F.; Fernandes, C.; Osório, F.; Costa, S.; Magalhães, A.; Mora, H.; Gonçalves, D.; Santos-Sousa, H.; et al. Clinical Outcome of Patients Submitted to Liver Resection in the Context of Metastatic Breast Cancer: A Study of a Tertiary Hospital Center. Medicines 2021, 8, 61. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8110061
Nogueiro J, Devezas V, Sousa F, Fernandes C, Osório F, Costa S, Magalhães A, Mora H, Gonçalves D, Santos-Sousa H, et al. Clinical Outcome of Patients Submitted to Liver Resection in the Context of Metastatic Breast Cancer: A Study of a Tertiary Hospital Center. Medicines. 2021; 8(11):61. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8110061
Chicago/Turabian StyleNogueiro, Jorge, Vitor Devezas, Fabiana Sousa, Cristina Fernandes, Fernando Osório, Susy Costa, André Magalhães, Henrique Mora, Diana Gonçalves, Hugo Santos-Sousa, and et al. 2021. "Clinical Outcome of Patients Submitted to Liver Resection in the Context of Metastatic Breast Cancer: A Study of a Tertiary Hospital Center" Medicines 8, no. 11: 61. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8110061






