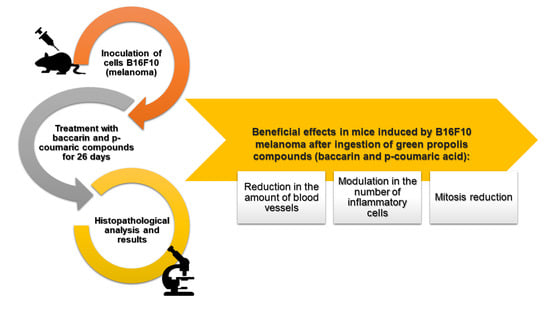
Green Propolis Compounds (Baccharin and p-Coumaric Acid) Show Beneficial Effects in Mice for Melanoma Induced by B16f10
Abstract:
1. Introduction
2. Material and Methods
2.1. Baccharin and p-Coumaric Acid Isolation from Green Propolis
2.2. Animal Care
2.3. Cell Culture
2.4. Experimental Design
- Group 1—Control: received Phosphate buffer saline (PBS) v.o;
- Group 2—PBS + baccharin: PBS injection + treatment with baccharin v.o.;
- Group 3—PBS + p-coumaric: PBS injection + treatment with p-coumaric acid v.o.;
- Group 4—Melanoma: Injection of B16F10 cells + PBS v.o.;
- Group 5—Melanoma + baccharin: Injection of B16F10 cells + treatment with baccharin v.o.
- Group 6–Melanoma + p-coumaric: Injection of B16F10 cells + treatment with p-coumaric acid v.o.
2.5. Inflammatory Cells Counting in Peripheral Blood
2.6. Analysis of Tumor Growth
2.7. Histological and Morphometric Evaluation
2.8. Statistical Analysis
3. Results
3.1. Tumor Growth and Monitoring of Mice
3.2. Effects of Baccharin and p-Coumaric Acid on Mitotic Cells
3.3. Effects of Baccharin and p-Coumaric Acid in Tumor Angiogenesis
3.4. Effects of Baccharin and p-Coumaric Acid in Inflammatory Tissue Cells
3.5. Effects of Baccharin and p-Coumaric Acid on Inflammatory Cells in Blood
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schadendorf, D.; van Akkooi, A.C.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bono, A.; Tomatis, S.; Bartoli, C.; Tragni, G.; Radaelli, G.; Maurichi, A.; Marchesini, R. The ABCD system of melanoma detection. Cancer 1999, 85, 72–77. [Google Scholar] [CrossRef]
- Edwards, B.K.; Howe, H.L.; Ries, L.A.; Thun, M.J.; Rosenberg, H.M.; Yancik, R.; Wingo, P.A.; Jemal, A.; Feigal, E.G. Annual Report to the Nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer 2002, 94, 2766–2792. [Google Scholar] [CrossRef]
- Iranzo, C.C.; Rubia-Ortí, J.E.; Castillo, S.S.; Firmino-Canhoto, J. Lesões cutâneas malignas e pré-malignas: Conhecimentos, hábitos e campanhas de prevenção solar. Acta Paul. Enferm. 2015, 28, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Whiteman, D.C.; Whiteman, C.A.; Green, A.C. Childhood sun exposure as a risk factor for melanoma: A systematic review of epidemiologic studies. Cancer Causes Control 2001, 12, 69–82. [Google Scholar] [CrossRef]
- Evans, M.S.; Madhunapantula, S.R.V.; Robertson, G.P.; Drabick, J.J. current and future trials of targeted therapies in cutaneous melanoma. Adv. Exp. Med. Biol. 2012, 779, 223–255. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins Patologia Baásica; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Chinembiri, T.; du Plessis, L.; Gerber, M.; Hamman, J.; du Plessis, J. review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Yuan, B.-C.; Ma, Y.-S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2016, 55, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Salatino, A.; Teixeira, É.W.; Negri, G.; Message, D. Origin and chemical variation of Brazilian propolis. Evid. Based Complementary Altern. Med. 2005, 2, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Filho, R.N.; Batista, F.S.; Ribeiro, D.R.; Melo, G.C.; Reis, F.P.; Melo, A.U.; Gomes, M.Z.; Cardoso, J.C.; Albuquerque, R.L., Jr. Chemopreventive effect of Brazilian green propolis on experimental dermal carcinogenesis in murine model. Int. J. Morphol. 2014, 32, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Alencar, S.M.; Aguiar, C.L.; Paredes-Guzmán, J.; Park, Y.K. Composição química de Baccharis dracunculifolia, fonte botânica das própolis dos estados de São Paulo e Minas Gerais. Ciência Rural 2005, 35, 909–915. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent progress in pharmacological research of propolis. Phytother. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Maruyama, H.; Matsumoto, K.; Ohguchi, K.; Nishizawa, K.; Sakamoto, T.; Araki, Y.; Mishima, S.; Nozawa, Y. Cell Growth inhibitory effect of cinnamic acid derivatives from propolis on human tumor cell lines. Biol. Pharm. Bull. 2003, 26, 1057–1059. [Google Scholar] [CrossRef] [Green Version]
- Kianmehr, Z.; Khorsandi, K.; Mohammadi, M.; Hosseinzadeh, R. Low-level laser irradiation potentiates anticancer activity of p-coumaric acid against human malignant melanoma cells. Melanoma Res. 2020, 30, 136–146. [Google Scholar] [CrossRef]
- Rodrigues, D.M.; De Souza, M.C.; Arruda, C.; Pereira, R.A.; Bastos, J.K. The role of Baccharis dracunculifolia and its chemical profile on green propolis production by Apis mellifera. J. Chem. Ecol. 2019, 46, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef]
- Mattia, G.; Puglisi, R.; Ascione, B.; Malorni, W.; Carè, A.; Matarrese, P. Cell death-based treatments of melanoma:conventional treatments and new therapeutic strategies. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, T.; Sun, G.; Sun, X.; Zhao, L.; Zhong, R.; Peng, Y. tumor energy metabolism and potential of 3-bromopyruvate as an inhibitor of aerobic glycolysis: Implications in tumor treatment. Cancers 2019, 11, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molassiotis, A.; Fernadez-Ortega, P.; Pud, D.; Ozden, G.; Scott, J.A.; Panteli, V.; Margulies, A.; Browall, M.; Magri, M.; Selvekerova, S.; et al. Use of complementary and alternative medicine in cancer patients: A European survey. Ann. Oncol. 2005, 16, 655–663. [Google Scholar] [CrossRef]
- Hodi, F.S.; Mihm, M.C.; Soiffer, R.J.; Haluska, F.G.; Butler, M.; Seiden, M.V.; Davis, T.; Henry-Spires, R.; MacRae, S.; Willman, A.; et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl. Acad. Sci. USA 2003, 100, 4712–4717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Zhou, J.; Dong, Z.; Tandon, S.; Kuk, D.; Panageas, K.S.; Wong, P.; Wu, X.; Naidoo, J.; Page, D.B.; et al. Pretreatment serum VEGF is associated with clinical response and overall survival in advanced melanoma patients treated with ipilimumab. Cancer Immunol. Res. 2014, 2, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Kandalaft, L.E.; Motz, G.T.; Busch, J.; Coukos, G. Angiogenesis and the tumor vasculature as antitumor immune modulators: The role of vascular endothelial growth factor and endothelin. Curr. Top. Microbiol. Immunol. 2010, 344, 129–148. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Nascimento, B.A.; Gardinassi, L.G.; Silveira, I.M.; Gallucci, M.G.; Tomé, M.A.; Oliveira, J.F.; Moreira, M.R.; Meirelles, A.F.; Faccioli, L.H.; Tefé-Silva, C.; et al. Arctium lappa extract suppresses inflammation and inhibits melanoma progression. Medicines 2019, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Garcia, N.P.; Ireno, L.C.; De Castro, M.P.; Reis, M.D.S.; Gardinassi, L.G.; Faccioli, L.H.; Tefé-Silva, C.; Zoccal, K.F. Antitumoral effect of lobelia inflata in an experimental mouse model of melanoma. Biomed. J. Sci. Tech. Res. 2020, 25, 18856–18864. [Google Scholar] [CrossRef]
- Ireno, L.C.; Garcia, N.P.; Moreira, M.R.A.; Castro, M.P.; Zoccal, K.F.; Tefé-Silva, C. Evaluation of the Lobelia inflata extract in the histopathological profile of melanoma in experimental model. Biomed. J. Sci. Tech. Res. 2020, 26, 19927–19934. [Google Scholar] [CrossRef]
- Watanabe, M.A.; Amarante, M.K.; Conti, B.J.; Sforcin, J.M. Cytotoxic constituents of propolis inducing anticancer effects: A review. J. Pharm. Pharmacol. 2011, 63, 1378–1386. [Google Scholar] [CrossRef]
- Watabe, M.; Hishikawa, K.; Takayanagi, A.; Shimizu, N.; Nakaki, T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFκB and activation of fas in human breast cancer MCF-7 cells. J. Biol. Chem. 2004, 279, 6017–6026. [Google Scholar] [CrossRef] [Green Version]
- Kouidhi, B.; Zmantar, T.; Bakhrouf, A. Anti-cariogenic and anti-biofilms activity of Tunisian propolis extract and its potential protective effect against cancer cells proliferation. Anaerobe 2010, 16, 566–571. [Google Scholar] [CrossRef]
- Tsuchiya, I.; Hosoya, T.; Ushida, M.; Kunimasa, K.; Ohta, T.; Kumazawa, S. Nymphaeol-A isolated from okinawan propolis suppresses angiogenesis and induces caspase-dependent apoptosis via inactivation of survival signals. Evid. Based Complementary Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.-R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate t-cell function to promote tumoral immune escape. Cancer Res. 2011, 72, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Callahan, M.K.; Postow, M.A.; Wolchok, J.D. Immunomodulatory therapy for melanoma: Ipilimumab and beyond. Clin. Dermatol. 2013, 31, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieva Moreno, M.I.; Zampini, I.C.; Ordóñez, R.M.; Jaime, G.S.; Vattuone, M.A.; Isla, M.I. evaluation of the cytotoxicity, genotoxicity, mutagenicity, and antimutagenicity of propolis from Tucuman, Argentina. J. Agric. Food Chem. 2005, 53, 8957–8962. [Google Scholar] [CrossRef]
- Assumpção, J.H.; Takeda, A.A.; Sforcin, J.M.; Rainho, C.A. Effects of propolis and phenolic acids on triple-negative breast cancer cell lines: Potential involvement of epigenetic mechanisms. Molecules 2020, 25, 1289. [Google Scholar] [CrossRef] [Green Version]
- Sforcin, J.M. Propolis and the immune system: A review. J. Ethnopharmacol. 2007, 113, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, D.; Car, H.; Borawska, M.H.; Nikliński, J. The anticancer activity of propolis. Folia Histochem. Cytobiol. 2012, 50, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balch, C.M.; Buzaid, A.C.; Soong, S.-J.; Atkins, M.B.; Cascinelli, N.; Coit, D.G.; Fleming, I.D.; Gershenwald, J.E.; Houghton, A.; Kirkwood, J.M.; et al. Final version of the american joint committee on cancer staging system for cutaneous melanoma. J. Clin. Oncol. 2001, 19, 3635–3648. [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.-J.; Thompson, J.F.; Ding, S.; Byrd, D.R.; Cascinelli, N.; Cochran, A.J.; Coit, D.G.; Eggermont, A.M.; et al. Multivariate analysis of prognostic factors among 2,313 patients with stage iii melanoma: Comparison of nodal micrometastases versus macrometastases. J. Clin. Oncol. 2010, 28, 2452–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.F.; Soong, S.-J.; Balch, C.M.; Gershenwald, J.E.; Ding, S.; Coit, D.G.; Flaherty, K.T.; Gimotty, P.A.; Johnson, T.; Johnson, M.M.; et al. Prognostic Significance of Mitotic Rate in Localized Primary Cutaneous Melanoma: An Analysis of Patients in the Multi-Institutional American Joint Committee on Cancer Melanoma Staging Database. J. Clin. Oncol. 2011, 29, 2199–2205. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.-C.; Kuo, W.-H.; Lee, Y.-J.; Lin, W.-L.; Chou, F.-P.; Tseng, T.-H. Inhibitory effect of caffeic acid phenethyl ester on the growth of C6 glioma cells in vitro and in vivo. Cancer Lett. 2006, 234, 199–208. [Google Scholar] [CrossRef]
- Boo, Y.C. p-Coumaric acid as an active ingredient in cosmetics: A review focusing on its antimelanogenic effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Carmeliet, P.; Dor, Y.; Herbert, J.-M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998, 394, 485–490. [Google Scholar] [CrossRef]
- Mattei, S.; Colombo, M.P.; Melani, C.; Silvani, A.; Parmiani, G.; Herlyn, M. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int. J. Cancer 1994, 56, 853–857. [Google Scholar] [CrossRef]
- Esquivel-Velázquez, M.; Ostoa-Saloma, P.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Castro, J.I.; Morales-Montor, J. The role of cytokines in breast cancer development and progression. J. Interf. Cytokine Res. 2015, 35, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahabeleshwar, G.H.; Byzova, T.V. Angiogenesis in melanoma. Semin. Oncol. 2007, 34, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.D.C.; Andrade, S.P.; Campos, P.P.; Barcelos, L.S.; Soriani, F.M.; AL Moura, S.; Ferreira, M.A.N.D. Brazilian green propolis modulates inflammation, angiogenesis and fibrogenesis in intraperitoneal implant in mice. BMC Complementary Altern. Med. 2014, 14, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, R.; Alexander, P. Cooperation of immune lymphoid cells with macrophages in tumour immunity. Nature 1970, 228, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Bonnotte, B.; Larmonier, N.; Favre, N.; Fromentin, A.; Moutet, M.; Martin, M.; Gurbuxani, S.; Solary, E.; Chauffert, B.; Martin, F. Identification of tumor-infiltrating macrophages as the killers of tumor cells after immunization in a rat model system. J. Immunol. 2001, 167, 5077–5083. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.K.; Allavena, P.; Mantovani, A. Tumor-associated macrophages: Functional diversity, clinical significance, and open questions. Semin. Immunopathol. 2013, 35, 585–600. [Google Scholar] [CrossRef]
- Missima, F.; Filho, A.A.; Nunes, G.A.; Bueno, P.C.; De Sousa, J.P.; Bastos, J.K.; Sforcin, J.M. Effect of Baccharis dracunculifolia D.C (Asteraceae) extracts and its isolated compounds on macrophage activation. J. Pharm. Pharmacol. 2007, 59, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Knežević, A.H.; Šver, L.; Terzić, S.; Bašić, I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J. Ethnopharmacol. 2004, 94, 307–315. [Google Scholar] [CrossRef]
- Soumoy, L.; Kindt, N.; Ghanem, G.; Saussez, S.; Journe, F. Role of macrophage migration inhibitory factor (MIF) in melanoma. Cancers 2019, 11, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singel, K.L.; Segal, B.H. Neutrophils in the tumor microenvironment: Trying to heal the wound that cannot heal. Immunol. Rev. 2016, 273, 329–343. [Google Scholar] [CrossRef] [Green Version]
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils responsive to endogenous IFN-β regulate tumor angiogenesis and growth in a mouse tumor model. J. Clin. Investig. 2010, 120, 1151–1164. [Google Scholar] [CrossRef]
- Mishalian, I.; Bayuh, R.; Levy, L.; Zolotarov, L.; Michaeli, J.; Fridlender, Z.G. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol. Immunother. 2013, 62, 1745–1756. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsh, S.J.; Rizos, H.; Scolyer, R.A.; Long, G.V. Resistance to combination BRAF and MEK inhibition in metastatic melanoma: Where to next? Eur. J. Cancer 2016, 62, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.O.; Schmidt, H.; Møller, H.J.; Donskov, F.; Høyer, M.; Sjoegren, P.; Christensen, I.J.; Steiniche, T. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer 2011, 118, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, L.F.; Vodnar, D.C. Whole grains and phenolic acids: A review on bioactivity, functionality, health benefits and bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2019, 59, 1275–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M.K. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation 2012, 36, 169–176. [Google Scholar] [CrossRef]
- Tan, L.Y.; Martini, C.; Fridlender, Z.G.; Bonder, C.S.; Brown, M.P.; Ebert, L.M. Control of immune cell entry through the tumour vasculature: A missing link in optimising melanoma immunotherapy? Clin. Transl. Immunol. 2017, 6, e134. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastaldello, G.H.; Cazeloto, A.C.V.; Ferreira, J.C.; Rodrigues, D.M.; Bastos, J.K.; Campo, V.L.; Zoccal, K.F.; Tefé-Silva, C. Green Propolis Compounds (Baccharin and p-Coumaric Acid) Show Beneficial Effects in Mice for Melanoma Induced by B16f10. Medicines 2021, 8, 20. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8050020
Gastaldello GH, Cazeloto ACV, Ferreira JC, Rodrigues DM, Bastos JK, Campo VL, Zoccal KF, Tefé-Silva C. Green Propolis Compounds (Baccharin and p-Coumaric Acid) Show Beneficial Effects in Mice for Melanoma Induced by B16f10. Medicines. 2021; 8(5):20. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8050020
Chicago/Turabian StyleGastaldello, Gabriel H., Ana Caroline V. Cazeloto, Juliana C. Ferreira, Débora Munhoz Rodrigues, Jairo Kennup Bastos, Vanessa L. Campo, Karina F. Zoccal, and Cristiane Tefé-Silva. 2021. "Green Propolis Compounds (Baccharin and p-Coumaric Acid) Show Beneficial Effects in Mice for Melanoma Induced by B16f10" Medicines 8, no. 5: 20. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8050020