Design, Syntheses, and Bioevaluations of Some Novel N2-Acryloylbenzohydrazides as Chemostimulants and Cytotoxic Agents
Abstract
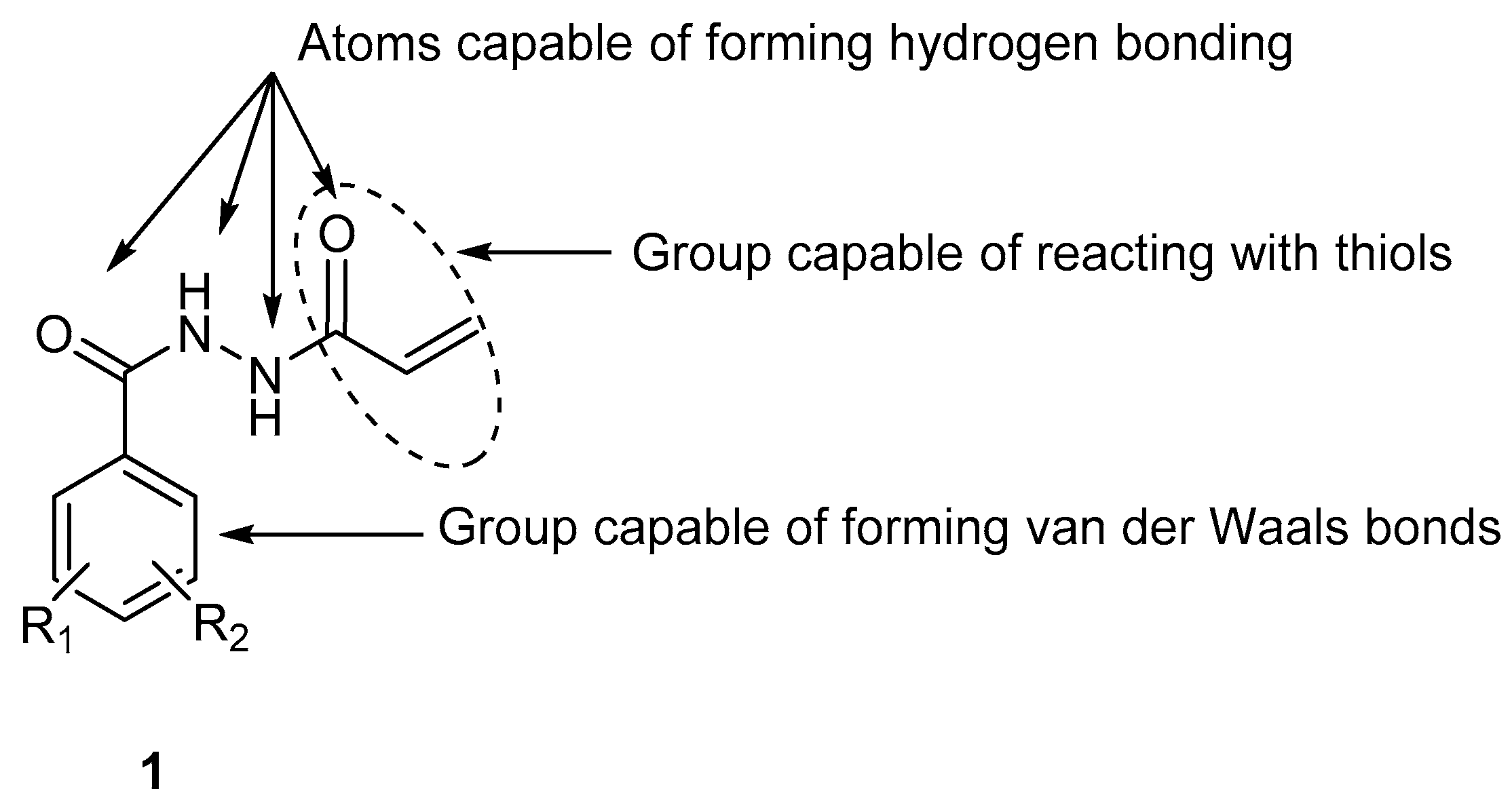
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Compounds
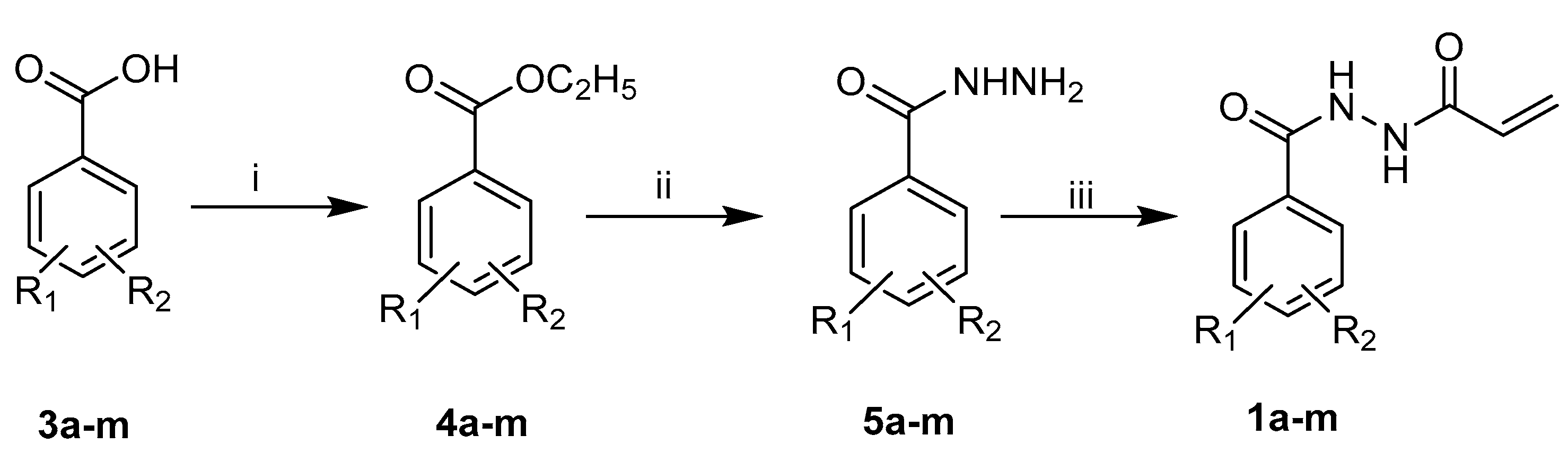
2.1.1. Synthesis of N1-aroyl-N2-acryloylhydrazides 1a–m
N2-Acryloylbenzohydrazide 1a
N2-Acryloyl-4-fluorobenzohydrazide 1b
N2-Acryloyl-4-bromobenzohydrazide 1c
N2-Acryloyl-4-chlorobenzohydrazide 1d
N2-Acryloyl-2-chlorobenzohydrazide 1e
N2-Acryloyl-3-chlorobenzohydrazide 1f
N2-Acryloyl-3,4-dichlorobenzohydrazide 1g
N2-Acryloyl-2,5-dichlorobenzohydrazide 1h
N2-Acryloyl-3-methylbenzohydrazide 1i
N2-Acryloyl-2-methylbenzohydrazide 1j
N2-Acryloyl-4-methylbenzohydrazide 1k
N2-Acryloyl-4-methoxylbenzohydrazide 1l
N2-Acryloyl-3,4-dimethoxylbenzohydrazide 1m
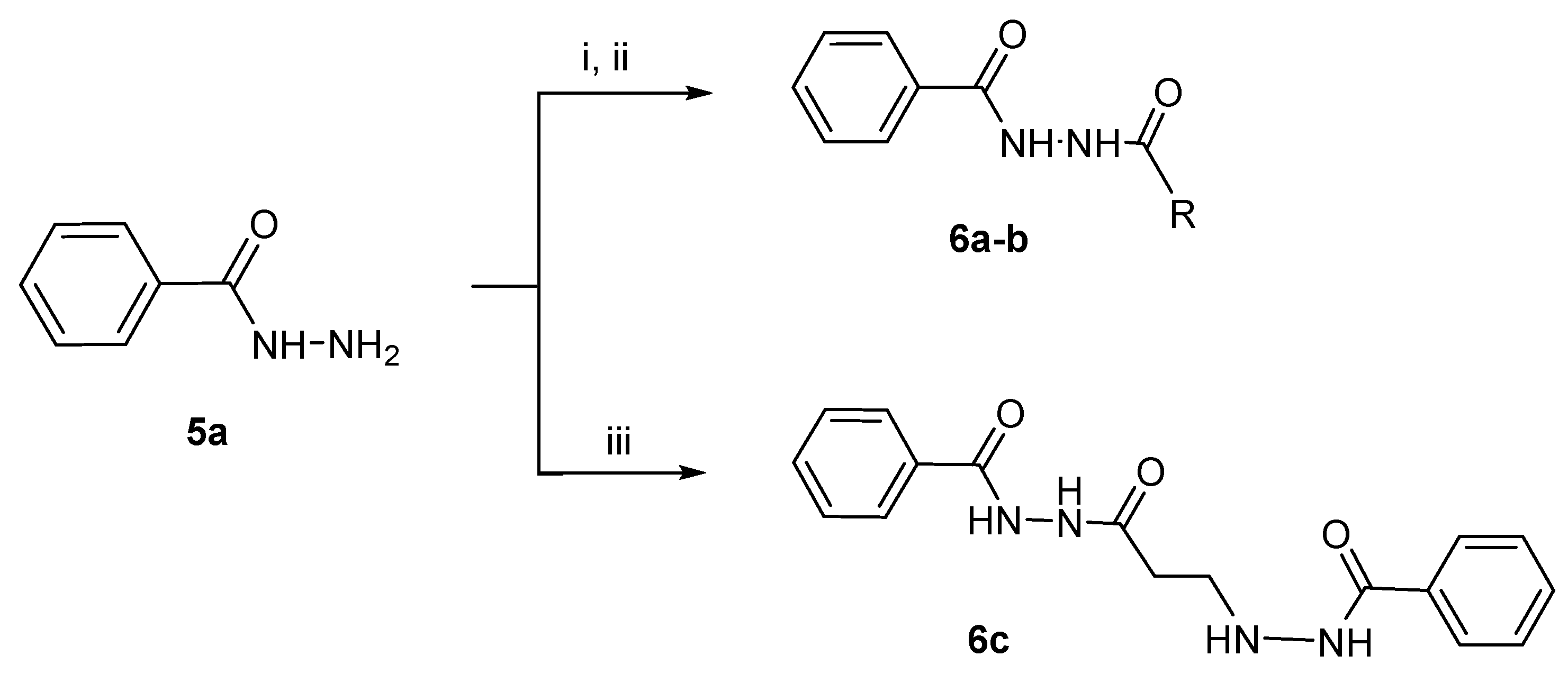
2.1.2. Synthesis of the N2-Acylbenzohydrazides 6a–c
N2-Propionylbenzohydrazide 6a
N2-Acetylbenzohydrazide 6b
N2-[3-(Benzoylaminoamino)-1-oxopropyl]-N1-benzoylhydrazine 6c
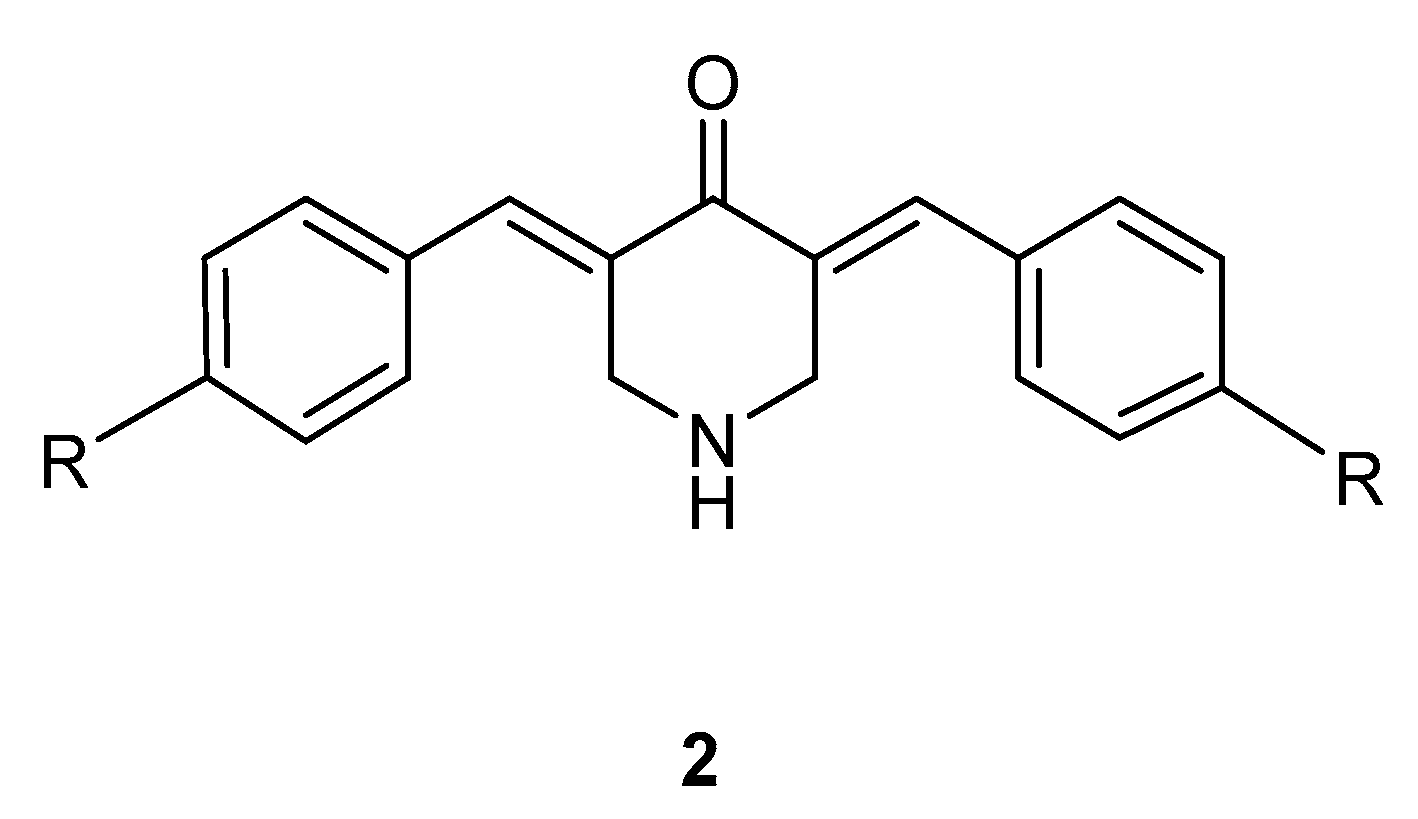
2.1.3. Synthesis of the 3,5-bis(benzylidene)-4-piperidones 2a–f
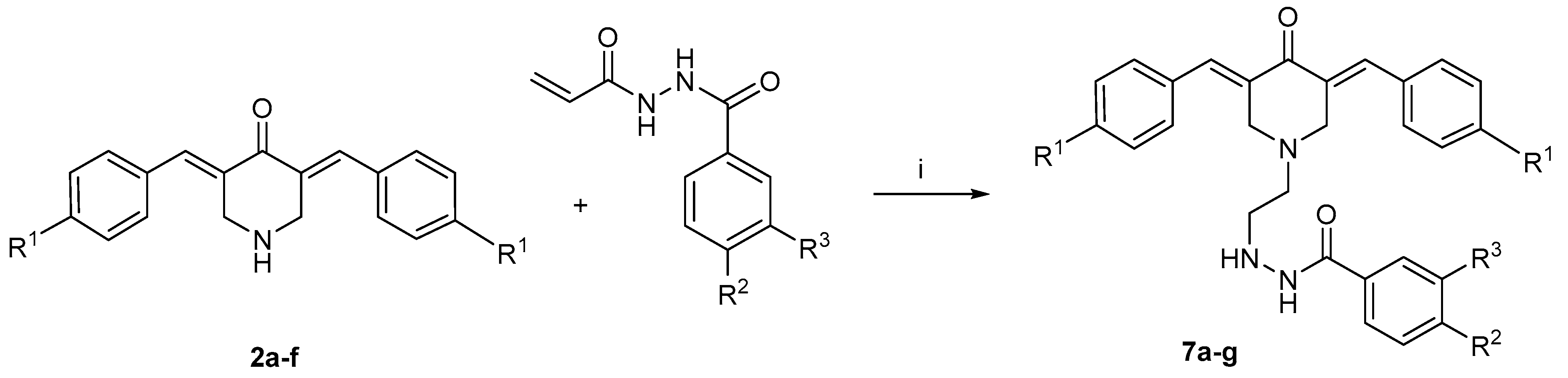
2.1.4. Synthesis of the N-alkyl-3,5-bis(benzylidene)-4-piperidones 7a–g
3,5-Bis(benzylidene)-1-[3-(phenylcarbonylaminoamino)-3-oxo-1-propyl]-4-piperidone 7a
3,5-Bis(benzylidene)-1-[3-(3,4-dimethoxylphenylcarbonylaminoamino)-3-oxo-1-propyl]-4-piperidone 7b
3,5-Bis(4-chlorobenzylidene)-1-[3-(3,4-dimethoxyphenylcarbonylaminoamino)-3-oxo-1-propyl]-4-piperidone 7c
3,5-Bis(4-fluorobenzylidene)-1-[3-(3,4-dimethoxyphenylcarbonylaminoamino)-3-oxo-1-propyl]-4-piperidone 7d
3,5-Bis(4-nitrobenzylidene)-1-[3-(3,4-dimethoxyphenylcarbonylaminoamino)-3-oxo-1-propyl]-4-piperidone 7e
3,5-Bis(4-methoxybenzylidene)-1-[3-(3,4-dimethoxyphenylcarbonylaminoamino)-3-oxo-1-propyl]-4-piperidone 7f
3,5-Bis(4-methylbenzylidene)-1-[3-(3,4-dimethoxyphenylcarbonylaminoamino)-3-oxo-1-propyl]-4-piperidone 7g
2.2. Bioevaluations
2.2.1. Cytotoxicity Assays
2.2.2. Evaluation of 1l on the Mitochondrial Membrane Potential of Ramos Cells
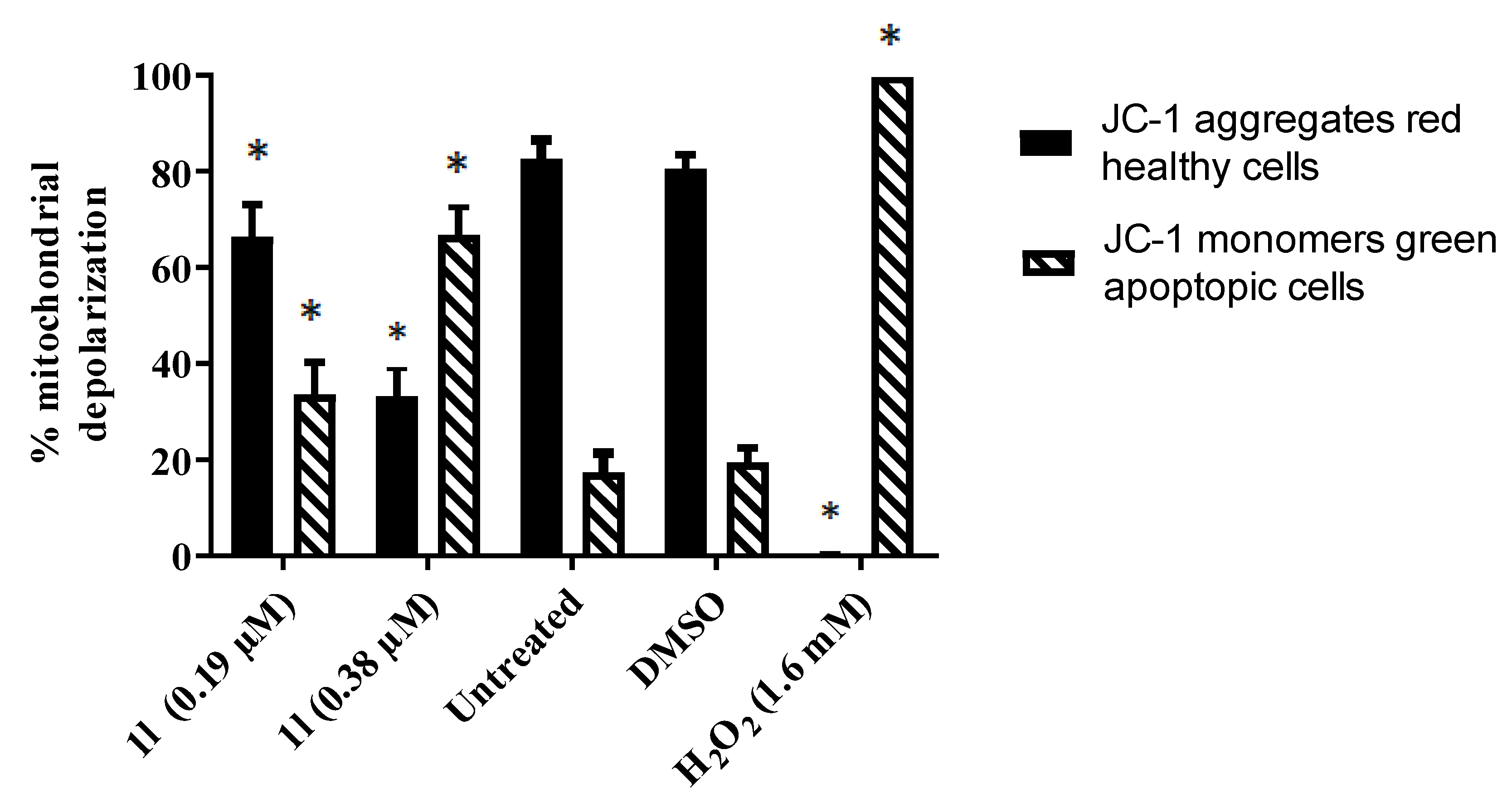
2.2.3. Effect of Compounds on the Mitochondrial Membrane Potential of HCT 116 cells
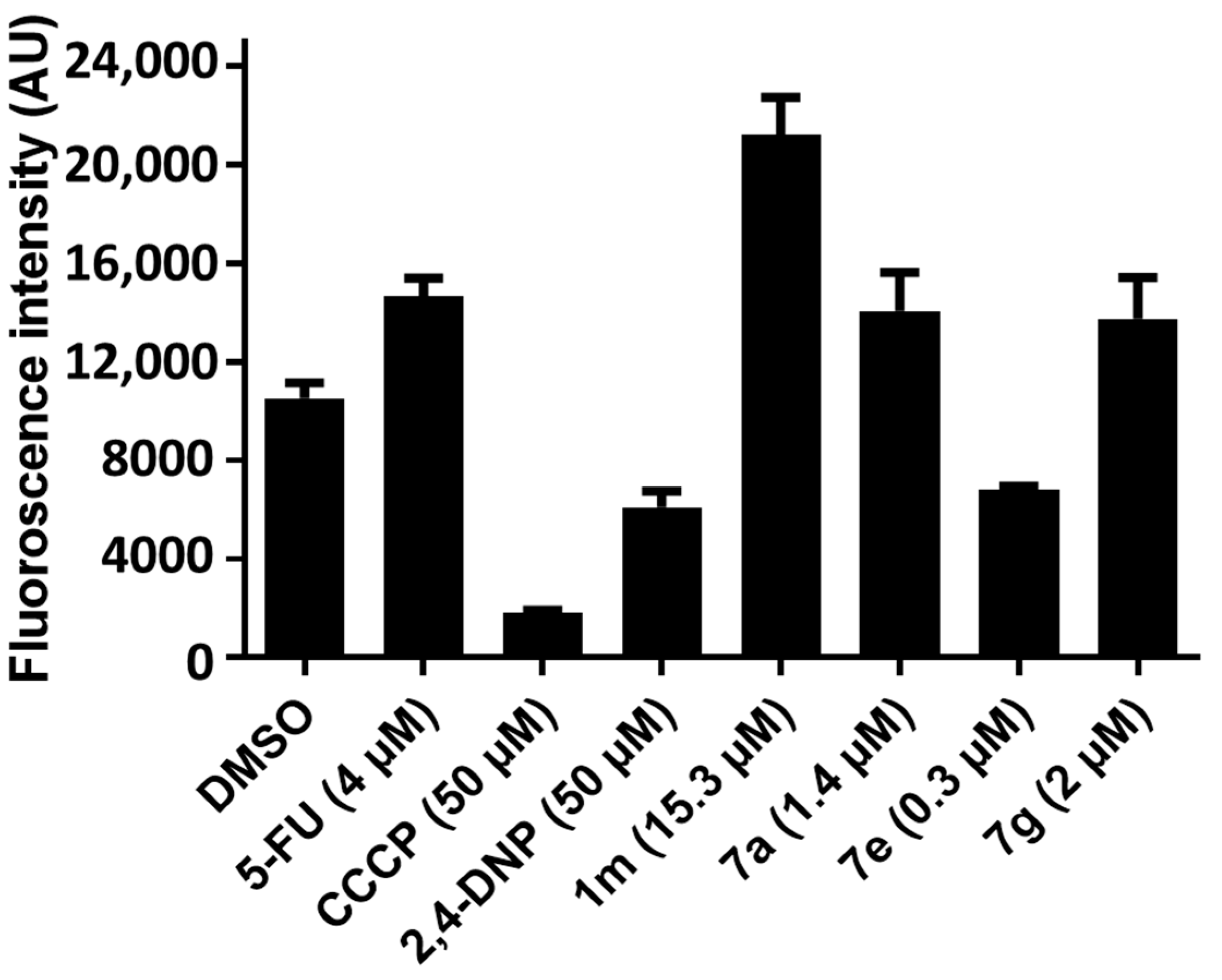
2.2.4. Evaluation of the ROS levels in HCT 116 Cells
2.3. Quantitative Structure-Activity Relationships
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, J.S.; Reddy, B.V.S.; Baishya, G. Green protocol for conjugate addition of thiols to α,β-unsaturated ketones using a [Bmim]PF6/H2O system. J. Org. Chem. 2003, 68, 2098–7100. [Google Scholar] [CrossRef]
- Hossain, M.; Das, U.; Umemura, N.; Sakagami, H.; Balzarini, J.; De Clercq, E.; Kawase, M.; Dimmock, J.R. Tumor-specific cytotoxicity and structure-activity relationships of novel 1-[3-(2-methoxyethylthio)propionyl]-3,5-bis(benzylidene)-4-piperidones. Bioorg. Med. Chem. 2016, 24, 2206–2214. [Google Scholar] [CrossRef]
- Robles-Escajeda, E.; Das, U.; Ortega, N.M.; Parra, K.; Francia, G.; Dimmock, J.R.; Varela-Ramirez, A.; Aguilera, R.J. A novel curcumin-like dienone induces apoptosis in triple-negative breast cancer cells. Cell. Oncol. 2016, 39, 265–277. [Google Scholar] [CrossRef]
- Gad, A.; El-Dissouky, A.; Mansour, E.; El-Maghraby, A. Thermal stability of poly acryloyl benzoic hydrazide and its complexes with some transition metals. Polym. Degrad. Stab. 2000, 68, 153–158. [Google Scholar] [CrossRef]
- Clemence, F.; Joliveau-Maushart, C.; Meier, J.; Cerede, J.; Delevallee, F.; Benzoni, J.; Deraedt, R. Synthesis and analgesic activity in the 1,2,4-triazole series. Eur. J. Med. Chem. 1985, 20, 257–266. [Google Scholar]
- Dimmock, J.R.; Padmanilayam, M.P.; Puthucode, R.N.; Nazarali, A.J.; Motaganahalli, N.L.; Zello, G.A.; Quail, J.W.; Oloo, E.O.; Kraatz, H.B.; Prisciak, J.S.; et al. A Conformational and structure-activity relationship study of cytotoxic 3,5-bis(arylidene)-4-piperidones and related N-acryloyl analogues. J. Med. Chem. 2001, 44, 586–593. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protocols. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Santiago-Vázquez, Y.; Das, U.; Varela-Ramirez, A.; Baca, S.T.; Ayala-Marin, Y.; Lema, C.; Das, S.; Baryyan, A.; Dimmock, J.R.; Aguilera, R.J. Tumor-selective cytotoxicity of a novel pentadiene analogue on human leukemia/lymphoma cells. Clin. Cancer Drugs. 2016, 3, 138–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, L.; Calderon, R.I.; Varela-Ramirez, A.; Zhang, H.-Y.; Quan, Y.; Das, U.; Dimmock, J.R.; Skouta, R.; Aguilera, R.J. Induction of apoptosis via proteasome inhibition in leukemia/lymphoma cells by two potent piperidones. Cell. Oncol. 2018, 41, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Christensen, M.E.; Waterhouse, N.J. Measuring mitochondrial transmembrane potential by TMRE staining. Cold Spring Harb. Protoc. 2016. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.; Das, U.; Bandy, B.; Islam, A.; Nazarali, A.J.; Dimmock, J.R. Mitochondrial dysfunction contributes to the cytotoxicity of some 3,5-bis(benzylidene)-4-piperidone derivatives in colon HCT-116 cells. Bioorg. Med. Chem. Lett. 2013, 23, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.J. Subsituent Constants for Correlation Analysis in Chemistry and Biology; John Wiley and Sons: New York, NY, USA, 1979; p. 49. [Google Scholar]
- IBM SPSS Statistics for Windows; Released 2019, Version 26.0; IBM Corp.: Armonk, NY, USA, 2019.
- Bagkos, G.; Koufopoulos, K.; Piperi, C. Synthesis revisited: New avenues for the management of mitochondrial conditions. Curr. Pharm. Des. 2014, 20, 4570–4579. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R.; Lee, C.T.; Lopaschuk, G.D.; Puttagunta, L.; Bonnet, S.; et al. A mitochondrial-K+ channel axis is suppressed in cancer and its normalisation promotes apoptosis and inhibits cancer growth. Cancer Cell 2007, 11, 37–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorova, L.D.; Popkov, V.A.; Plothikov, E.Y.; Silachev, D.W. Mitochonrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]

| Compound | HCT 116 | MCF-7 | MDA-MB-231 | |||
|---|---|---|---|---|---|---|
| 10 µM | 100 µM | 10 µM | 100 µM | 10 µM | 100 µM | |
| 1a | 10.6 ± 1.70 | 94.1 ± 1.44 | 5.47 ± 3.52 | 51.1 ± 2.87 | −12.2 ± 1.50 | 52.1 ± 2.04 |
| 1b | −2.35 ± 2.24 | 60.7 ± 3.22 | −40.1 ± 3.34 | 33.2 ± 3.13 | −7.37 ± 1.52 | 39.9 ± 2.51 |
| 1c | −3.28 ± 1.50 | 87.9 ± 0.91 | −4.68 ± 3.17 | 46.6 ± 3.35 | −16.5 ± 1.45 | 88.7 ± 1.26 |
| 1d | −5.05 ± 2.36 | 82.3 ± 0.80 | 3.59 ± 2.08 | 55.9 ± 2.82 | −12.3 ± 4.05 | 49.1 ± 2.04 |
| 1e | 4.30 ± 1.38 | 75.7 ± 1.65 | 8.66 ± 1.81 | 75.8 ± 2.76 | −7.15 ± 2.29 | 91.9 ± 4.53 |
| 1f | 13.5 ± 1.79 | 95.7 ± 1.71 | −15.5 ± 3.38 | 85.9 ± 2.70 | 15.6 ± 3.14 | 98.7 ± 0.92 |
| 1g | 6.72 ± 1.97 | >100 | −50.5 ± 3.27 | 97.4 ± 2.60 | −19.4 ± 3.01 | 139.8 ± 1.03 |
| 1h | 7.12 ± 1.51 | 87.7 ± 1.62 | 14.7 ± 3.68 | 54.2 ± 3.42 | −5.95 ± 4.21 | 122.3 ± 2.90 |
| 1i | 15.43 ± 2.13 | 93.9 ± 3.35 | −82.8 ± 3.48 | 44.2 ± 2.96 | −5.91 ± 3.86 | 53.3 ± 2.89 |
| 1j | 10.4 ± 1.61 | 62.1 ± 2.48 | 53.3 ± 3.07 | >100 | −2.64 ± 2.37 | 46.8 ± 3.63 |
| 1k | 11.5 ± 2.99 | >100 | 55.3 ± 3.05 | >100 | −8.78 ± 1.52 | 74.7 ± 2.96 |
| 1l | 8.62 ± 2.79 | 85.1 ± 3.28 | 60.6 ± 3.29 | 99.4 ± 2.34 | −4.95 ± 3.12 | 47.7 ± 3.19 |
| 1m | 46.9 ± 2.55 | 97.9 ± 1.92 | −16.1 ± 3.51 | >100 | −15.89 ± 1.96 | 50.5 ± 1.42 |
| 6a | −10.5 ± 2.93 | 9.35 ± 2.53 | −19.6 ± 3.66 | −5.69 ± 2.54 | −25.3 ± 1.82 | −11.6 ± 2.52 |
| 6b | −4.89 ± 2.52 | 13.6 ± 3.03 | −93.8 ± 4.15 | −58.7 ± 3.24 | −22.6 ± 2.65 | −15.3 ± 3.46 |
| 6c | 18.6 ± 3.98 | 96.9 ± 1.86 | 16.1 ± 3.55 | 97.9 ± 2.68 | −16.0 ± 2.91 | 95.6 ± 4.22 |
| 5-FU | 84.7 ± 2.53 | 94.1 ± 0.96 | 91.2 ± 3.03 | 99.3 ± 2.87 | 17.4 ± 2.97 | >100 |
| Melphalan | 33.9 ± 1.81 | 98.7 ± 1.12 | >100 | >100 | 28.6 ± 2.48 | >100 |
| Compound | Neoplastic Cells | Non-Malignant CRL 1790 Cells | SI a | |
|---|---|---|---|---|
| Cell Line | IC50 (µM) | IC50 (µM) | ||
| 1a | HCT 116 | 36.9 ± 2.84 | >100 | >2.70 |
| 1m | HCT 116 | 15.3 ± 0.42 | >100 | >6.54 |
| 6a | HCT 116 | >100 | >100 | ~1.00 |
| 5-FU | HCT 116 | 3.77 ± 0.48 | 10.9 ± 1.43 | 2.92 |
| Melphalan | HCT 116 | 30.7 ± 2.73 | >100 | >3.25 |
| 1j | MCF-7 | 9.14 ± 1.31 | >100 | >10.9 |
| 1k | MCF-7 | 5.54 ± 0.51 | >100 | >18.1 |
| 1l | MCF-7 | 3.18 ± 0.53 | >100 | >31.5 |
| 5-FU | MCF-7 | 2.00 ± 0.25 | 10.8 ± 1.64 | 5.42 |
| Melphalan | MCF-7 | 12.0 ± 1.43 | >100 | >8.32 |
| 5-FU | MDA-MB-231 | 15.9 ± 1.65 | 10.8 ± 1.64 | 0.68 |
| Melphalan | MDA-MB-231 | 52.9 ± 2.32 | >100 | >1.90 |
| Entry | Compounds | Live Cells % | Reduction in Live Cells % | Cal a | Found b | p Value | ||
|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 1m (48 h) | 2a (24 h) | |||||
| 1 | 1m 5 µM + 2a 0.1 µM | 70 ± 2.13 | 63 ± 0.35 | 11 | 8 | 19 | 23 | >0.05 |
| 2 | 1m 10 µM + 2a 0.1 µM | 68 ± 1.82 | 59 ± 1.99 | 29 | 8 | 37 | 27 | >0.05 |
| 3 | 1m 15 µM + 2a 0.1 µM | 55 ± 0.71 | 45 ± 3.32 | 54 | 8 | 62 | 41 | <0.05 |
| 4 | 1m 5 µM + 2a 0.2 µM | 39 ± 1.19 | 12 ± 0.18 | 11 | 22 | 33 | 74 | <0.05 |
| 5 | 1m 10 µM + 2a 0.2 µM | 27 ± 0.21 | 10 ± 0.26 | 29 | 22 | 51 | 76 | <0.05 |
| 6 | 1m 15 µM + 2a 0.2 µM | 17 ± 1.48 | 0 | 54 | 22 | 76 | 100 | <0.05 |
| 7 | 1m 5 µM + 2a 0.4 µM | 19 ± 1.25 | 0 | 11 | 43 | 54 | 100 | <0.05 |
| 8 | 1m 10 µM + 2a 0.4 µM | 11 ± 1.40 | 0 | 29 | 43 | 72 | 100 | <0.05 |
| 9 | 1m 15 µM + 2a 0.4 µM | 5 ± 0.29 | 0 | 54 | 43 | 97 | 100 | >0.05 |
| 10 | 1m 5 µM + 5-FU 3 µM | 53 ± 2.39 | 37 ± 1.73 | 11 | 13 | 24 | 49 | <0.05 |
| 11 | 1m 10 µM + 5-FU 3 µM | 50 ± 1.21 | 32 ± 0.43 | 29 | 13 | 42 | 54 | >0.05 |
| 12 | 1m 15 µM + 5-FU 3 µM | 32 ± 1.48 | 20 ± 0.55 | 54 | 13 | 67 | 66 | >0.05 |
| 13 | 1m 5 µM | 88 ± 2.82 | 75 ± 4.89 | -- | -- | -- | -- | -- |
| 14 | 1m 10 µM | 67 ± 1.67 | 57 ± 1.76 | -- | -- | -- | -- | -- |
| 15 | 1m 15 µM | 61 ± 3.49 | 32 ± 0.94 | -- | -- | -- | -- | -- |
| 16 | 2a 0.1 µM | 83 ± 2.28 | 78 ± 2.12 | -- | -- | -- | -- | -- |
| 17 | 2a 0.2 µM | 69 ± 1.38 | 52 ± 1.15 | -- | -- | -- | -- | -- |
| 18 | 2a 0.4 µM | 48 ± 1.20 | 32 ± 1.50 | -- | -- | -- | -- | -- |
| 19 | 5-FU 3 µM | 78 ± 2.44 | 55 ± 1.37 | -- | -- | -- | -- | -- |
| 20 | DMSO | 91 ± 1.30 | 86 ± 0.65 | -- | -- | -- | -- | -- |
| Compound | Aryl Substituent | IC50 (µM) | SI a | |||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | HCT 116 | CRL 1790 | ||
| 7a | H | H | H | 1.38 ± 0.25 | 12.3 ± 1.09 | 3.87 |
| 7b | H | 3-OCH3 | 4-OCH3 | 3.15 ± 0.33 | 9.63 ± 1.62 | 3.06 |
| 7c | 4-Cl | 3-OCH3 | 4-OCH3 | 3.40 ± 0.01 | 12.2 ± 1.22 | 3.60 |
| 7d | 4-F | 3-OCH3 | 4-OCH3 | 3.82 ± 0.11 | 11.4 ± 2.22 | 2.99 |
| 7e | 4-NO2 | 3-OCH3 | 4-OCH3 | 0.28 ± 0.01 | 4.57 ± 0.53 | 16.3 |
| 7f | 4-OCH3 | 3-OCH3 | 4-OCH3 | 2.87 ± 0.23 | 11.1 ± 0.52 | 3.86 |
| 7g | 4-CH3 | 3-OCH3 | 4-OCH3 | 1.94 ± 0.18 | 9.24 ± 0.52 | 4.76 |
| 2a | -- | -- | -- | 0.37 ± 0.06 | 13.5 ± 1.99 | 36.4 |
| 5-FU | -- | -- | -- | 4.11 ± 0.22 | 10.8 ± 1.64 | 2.64 |
| Melphalan | -- | -- | -- | 33.2 ± 1.70 | >100 | >3.02 |
| Compound | Neoplastic Cells, CC50 (µM) and SI a | Non-Malignant Cells, CC50 (µM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ramos | NALM-60 | CEM | HL-60 | JURKAT | RAJI | Avg | Hs27 | MCF10A | Avg | |
| 1a | 0.19 ± 0.01 (67.9) | 2.74 ± 0.59 (4.71) | 0.91 ± 0.48 (14.2) | 3.32 ± 0.26 (3.89) | 3.52 ± 0.36 (3.65) | 5.18 ± 0.35 (2.49) | 2.64 (16.1) | 12.1 ± 0.91 | 13.7 ± 1.10 | 12.9 |
| 1h | 0.29 ± 0.05 (47.2) | 3.34 ± 0.43 (4.10) | 3.37 ± 0.18 (4.07) | 5.62 ± 0.29 (2.44) | 2.50 ± 0.24 (5.48) | 5.50 ± 1.10 (2.49) | 3.44 (11.0) | 14.4 ± 0.24 | 12.9 ± 0.95 | 13.7 |
| 1d | 0.15 ± 0.02 (57.9) | 2.86 ± 0.55 (3.04) | 0.86 ± 0.07 (10.1) | 2.90 ± 0.76 (3.00) | 1.07 ± 0.09 (8.12) | 2.71 ± 0.09 (3.24) | 1.76 (14.2) | 10.5 ± 0.05 | 6.87 ± 0.48 | 8.69 |
| 1l | 0.19 ± 0.01 (94.2) | 3.25 ± 0.99 (5.51) | 1.42 ± 0.25 (12.6) | 1.86 ± 0.66 (9.62) | 2.17 ± 0.45 (8.25) | 2.93 ± 0.43 (6.11) | 1.97 (22.7) | 17.7 ± 0.62 | 18.1 ± 0.84 | 17.9 |
| Avg CC50 (µM) | 0.21 | 3.05 | 1.64 | 3.43 | 2.32 | 4.08 | 2.45 | 13.7 | 12.9 | 13.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakhani, K.; Borrego, E.A.; Cano, K.G.; Dimmock, J.R.; Aguilera, R.J.; Das, S.; Roayapalley, P.K.; Sharma, R.K.; Das, U. Design, Syntheses, and Bioevaluations of Some Novel N2-Acryloylbenzohydrazides as Chemostimulants and Cytotoxic Agents. Medicines 2021, 8, 27. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8060027
Lakhani K, Borrego EA, Cano KG, Dimmock JR, Aguilera RJ, Das S, Roayapalley PK, Sharma RK, Das U. Design, Syntheses, and Bioevaluations of Some Novel N2-Acryloylbenzohydrazides as Chemostimulants and Cytotoxic Agents. Medicines. 2021; 8(6):27. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8060027
Chicago/Turabian StyleLakhani, Kinjal, Edgar A. Borrego, Karla G. Cano, Jonathan R. Dimmock, Renato J. Aguilera, Swagatika Das, Praveen K. Roayapalley, Rajendra K. Sharma, and Umashankar Das. 2021. "Design, Syntheses, and Bioevaluations of Some Novel N2-Acryloylbenzohydrazides as Chemostimulants and Cytotoxic Agents" Medicines 8, no. 6: 27. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8060027







