The Etiology and Pathophysiology Genesis of Benign Prostatic Hyperplasia and Prostate Cancer: A New Perspective
Abstract
:1. Introduction
2. Testosterone-Vascular-Inflamm-Ageing Triad
3. Amyloidosis
4. Autophagy
5. Evolutionary Tumorigenesis Microenvironment
- (i)
- From about 40 years and onwards is the early period asymptomatic phase at the start of testosterone, vascular and inflamm-ageing, and their effects are mitigated by the prostate being largely functional. However, it is the beginning of nitric oxide down-regulating, oxidative stress, ischaemia hypoxia, chronic inflammation, amyloidosis corpora amylacea, autophagy induction, and remodeling degeneration.
- (ii)
- From about 50 years and onwards is the mid-period mild symptoms phase, which includes the development of lower urinary tract symptoms and benign prostatic hyperplasia [6]. This is due to the incremental prostate ageing degeneration effects of nitric oxide down-regulating, oxidative stress, ischaemia hypoxia, chronic inflammation, amyloidosis corpora amylacea, autophagy induction, and remodeling degeneration
- (iii)
- From about 60 years and onwards is the late period acute symptoms phase, which includes the co-morbidities of benign prostatic hyperplasia, erectile dysfunction, bladder outlet obstruction and adenocarcinoma growth. This is the threshold point at the start of “prostate reprogramming” and the “loss” of cell function, homeostasis and regulation pathways [192,193,194,195,196,197]. It marks the beginning of a prostate stagnation tumorigenesis inflammatory microenvironment with heterogeneous events [17] including inflammation [57,140,141,142,198], genetic aberrations [199,200,201,202,203,204,205], epigenetic dysregulation [206,207,208,209,210], autophagy dysregulation [86,87,89,90,211,212,213,214,215,216] and lysosomal dysfunction [217,218,219,220].
6. Prevention
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devlin, C.M.; Simms, M.S.; Maitland, N.J. Benign Prostatic Hyperplasia-what do we know? BJU Int. 2020, 127, 389–399. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Spring, D.J.; DePinho, R.A. Genetics and biology of prostate cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, P.P.; Banerjee, S.; Brown, T.R.; Zirkin, B.R. Androgen action in prostate function and disease. Am. J. Clin. Exp. Urol. 2018, 6, 62–77. [Google Scholar]
- Kucera, R.; Pecen, L.; Topolcan, O.; Dahal, A.R.; Costigliola, V.; Giordano, F.A.; Golubnitschaja, O. Prostate cancer management: Long-term beliefs, epidemic developments in the early twenty-first century and 3PM dimensional solutions. EPMA J. 2020, 11, 399–418. [Google Scholar] [CrossRef] [PubMed]
- Olmedo-Requena, R.; Lozano-Lorca, M.; Salcedo-Bellido, I.; Jiménez-Pacheco, A.; Vázquez-Alonso, F.; García-Caballos, M.; Sánchez, M.-J.; Jiménez-Moleón, J.-J. Compliance with the 2018 World Cancer Research Fund/American Institute for Cancer Research Cancer Prevention Recommendations and Prostate Cancer. Nutrients 2020, 12, 768. [Google Scholar] [CrossRef] [Green Version]
- Vickman, R.E.; Franco, O.E.; Moline, D.C.; Vander Griend, D.J.; Thumbikat, P.; Hayward, S.W. The role of the androgen receptor in prostate development and benign prostatic hyperplasia:A review. Asian J. Urol. 2020, 7, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-J.; Sung, F.-C.; Hsieh, P.-F.; Chang, H.-P.; Wu, K.-L.; Wu, H.-C. Metformin reduces prostate cancer risk among men with benign prostatic hyperplasia: A nationwide population-based cohort study. Cancer Med. 2019, 8, 2514–2523. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, M.H.A.; De Souza, D.B. Current evidence for the involvement of sex steroid receptors and sex hormones in benign prostatic hyperplasia. Res. Rep. Urol. 2019, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kensler, K.H.; Rebbeck, T.R. Cancer Progress and Priorities: Prostate Cancer. Am. Assoc. Cancer Res. 2020, 29, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Sinha, A.; Huang, V.; Livingstone, J.; Wang, J.; Fox, N.S.; Kurganovs, N.; Ignatchenko, V.; Fritsch, K.; Donmez, N.; Heisler, L.E.; et al. The Proteogenomic Landscape of Curable Prostate Cancer. Cancer Cell 2019, 35, 414–427. [Google Scholar] [CrossRef] [Green Version]
- Charmpi, K.; Guo, T.; Zhong, Q.; Wagner, U.; Sun, R.; Toussaint, N.C.; Fritz, C.E.; Yuan, C.; Chen, H.; Rupp, N.J.; et al. Convergent network effects along the axis of gene expression during prostate cancer progression. Genome Biol. 2020, 21, 302. [Google Scholar] [CrossRef] [PubMed]
- Liss, M.A.; Leach, R.J.; Sanda, M.G.; Semmes, O.J. Prostate Cancer Biomarker Development: National Cancer Institute’s Early Detection Research Network Prostate Cancer Collaborative Group Review. Am. Assoc. Cancer Res. 2020, 29, 2454–2462. [Google Scholar] [CrossRef]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; De Marzo, A.M.; Nelson, P.S.; Yegnasubramanian, S. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 2020. [Google Scholar] [CrossRef]
- Siddappa, M.; Wani, S.A.; Long, M.D.; Leach, D.A.; Mathé, E.A.; Bevan, C.L.; Campbell, M.J. Identification of transcription factor co-regulators that drive prostate cancer progression. Sci. Rep. 2020, 10, 20332. [Google Scholar] [CrossRef]
- Tonry, C.; Finn, S.; Armstrong, J.; Pennington, S.R. Clinical proteomics for prostate cancer: Understanding prostate cancer pathology and protein biomarkers for improved disease management. Clin. Proteom. 2020, 17, 41. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maitland, N.J.; Frame, F.M.; Rane, J.K.; Erb, H.H.; Packer, J.R.; Archer, L.K.; Pellacani, D. Resolution of Cellular Heterogeneity in Human Prostate Cancers: Implications for Diagnosis and Treatment. Adv. Exp. Med. Biol. 2019, 1164, 207–224. [Google Scholar] [CrossRef]
- Morton, A.; Williams, M.; Perera, M.; Teloken, P.E.; Donato, P.; Ranasinghe, S.; Chung, E.; Bolton, D.; Yaxley, J.; Roberts, M.J. Management of benign prostatic hyperplasia in the 21st century: Temporal trends in Australian population-based data. BJU Int. 2020, 126, 18–26. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare Cancer Data in Australia. Available online: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/cancer-rankings-data-visualisation (accessed on 8 June 2021).
- Gray, A.; Feldman, H.A.; Mc Kinlay, J.B.; Longcope, C. Age, Disease, and Changing Sex Hormone Levels in Middle-Aged Men: Results of the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 1991, 73, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.B.; Wittert, G.A. Endocrinology of the aging male. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 303–319. [Google Scholar] [CrossRef] [Green Version]
- Hotta, Y.; Kataoka, T.; Kimura, K. Testosterone Deficiency and Endothelial Dysfunction: Nitric Oxide, Asymmetric Dimethylarginine, and Endothelial Progenitor Cells. Sex. Med. Rev. 2019, 7, 661–668. [Google Scholar] [CrossRef]
- Campelo, A.E.; Cutini, P.H.; Massheimer, V.L. Testosterone modulates platelet aggregation and endothelial cell growth through nitric oxide pathway. J. Endocrinol. 2012, 213, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, K.L. Modulatory influence of sex hormones on vascular aging. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H522–H526. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y.; Taylor, N.; Xu, X. Family history of prostate cancer and age-related trend of testosterone levels among US males: NHANES 2003–2004. Andrology 2019, 7, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, A.B.; Amigoni, N.; Tafuri, A.; Rizzetto, R.; Shakir, A.; Tiso, L.; Cerrato, C.; Lacola, V.; Antoniolli, S.Z.; Gozzo, A.; et al. Endogenous testosterone as a predictor of prostate growing disorders in the aging male. Int. Urol. Nephrol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Crecelius, A.R.; Kirby, B.S.; Voyles, W.F.; Dinenno, F.A. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am. J. Physiol. Hear. Circ. Physiol. 2010, 299, H1633–H1641. [Google Scholar] [CrossRef] [Green Version]
- Seals, D.R.; Alexander, L.M. Vascular aging. J. Appl. Physiol. 2018, 125, 1841–1842. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Jin Cho, W.; Pyo, J.S. Immunohistochemical analysis of the impact of ischemic change in benign prostatic hyperplasia. Pathol. Res. Pract. 2020, 216, 152694. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of vascular aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxid. Med. Cell. Longev. 2019, 2019, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Udensi, U.K.; Tchounwou, P.B. Oxidative stress in prostate hyperplasia and carcinogenesis. J. Exp. Clin. Cancer Res. 2016, 35, 139. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Li, X.; Wang, R.; Yu, J.; Ye, M.; Mao, L.; Zhang, S.; Zheng, S. Association between Oxidative DNA Damage and Risk of Colorectal Cancer: Sensitive Determination of Urinary 8-Hydroxy-2′-deoxyguanosine by UPLC-MS/MS Analysis. Sci. Rep. 2016, 6, 32581. [Google Scholar] [CrossRef] [PubMed]
- Vital, P.; Castro, P.; Ittmann, M. Oxidative stress promotes benign prostatic hyperplasia. Prostate 2016, 76, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Kaya, E.; Ozgok, Y.; Zor, M.; Eken, A.; Bedir, S.; Erdem, O.; Ebiloglu, T.; Ergin, G. Oxidative stress parameters in patients with prostate cancer, benign prostatic hyperplasia and asymptomatic inflammatory prostatitis: A prospective controlled study. Adv. Clin. Exp. Med. 2017, 26, 1095–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohtake, S.; Kawahara, T.; Ishiguro, Y.; Takeshima, T.; Kuroda, S.; Izumi, K.; Miyamoto, H.; Uemura, H. Oxidative stress marker 8-hydroxyguanosine is more highly expressed in prostate cancer than in benign prostatic hyperplasia. Mol. Clin. Oncol. 2018, 9, 302–304. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.; Srivastava, J.K.; Shankar, E.; Kanwal, R.; Nawab, A.; Sharma, H.; Bhaskaran, N.; Ponsky, L.E.; Fu, P.; MacLennan, G.T.; et al. Oxidative stress and antioxidant status in high-risk prostate cancer subjects. Diagnostics 2020, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinger, A.; Cho, W.C.; Ben-Yehuda, A. Cancer and aging - the inflammatory connection. Aging Dis. 2017, 8, 611–627. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, G.C.; Accardi, G.; Monastero, R.; Nicoletti, F.; Libra, M. Ageing: From inflammation to cancer. Immun. Ageing 2018, 15, 1. [Google Scholar] [CrossRef]
- Vital, P.; Castro, P.; Tsang, S.; Ittmann, M. The senescence-associated secretory phenotype promotes benign prostatic hyperplasia. Am. J. Pathol. 2014, 184, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Song, C.S.; Chatterjee, B. Stimulation of prostate cells by the senescence phenotype of epithelial and stromal cells: Implication for benign prostate hyperplasia. FASEB BioAdv. 2019, 1, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Shendrik, I.; Peacocke, M.; Peehl, D.; Buttyan, R.; Ikeguchi, E.F.; Katz, A.E.; Benson, M.C. Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology 2000, 56, 160–166. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Koelman, L.; Pivovarova-Ramich, O.; Pfeiffer, A.F.H.; Grune, T.; Aleksandrova, K. Cytokines for evaluation of chronic inflammatory status in ageing research: Reliability and phenotypic characterisation. Immun. Ageing 2019, 16, 11. [Google Scholar] [CrossRef] [Green Version]
- Aversa, A.; Duca, Y.; Condorelli, R.A.; Calogero, A.E.; La Vignera, S. Androgen deficiency and phosphodiesterase type 5 expression changes in aging Male: Therapeutic implications. Front. Endocrinol. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamad, N.V.; Wong, S.K.; Wan Hasan, W.N.; Jolly, J.J.; Nur-Farhana, M.F.; Ima-Nirwana, S.; Chin, K.Y. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 2019, 22, 129–140. [Google Scholar] [CrossRef]
- Schlick, B.; Massoner, P.; Lueking, A.; Charoentong, P.; Blattner, M.; Schaefer, G.; Marquart, K.; Theek, C.; Amersdorfer, P.; Zielinski, D.; et al. Serum autoantibodies in chronic prostate inflammation in prostate cancer patients. PLoS ONE 2016, 11, e0147739. [Google Scholar] [CrossRef] [Green Version]
- Rourke, E.; Sunnapwar, A.; Mais, D.; Kukkar, V.; Digiovanni, J.; Kaushik, D.; Liss, M.A. Inflammation appears as high prostate imaging–reporting and data system scores on prostate magnetic resonance imaging (MRI) leading to false positive mri fusion biopsy. Investig. Clin. Urol. 2019, 60, 388–395. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [Green Version]
- MacLennan, G.T.; Eisenberg, R.; Fleshman, R.L.; Taylor, J.M.; Fu, P.; Resnick, M.I.; Gupta, S. The Influence of Chronic Inflammation in Prostatic Carcinogenesis: A 5-Year Followup Study. J. Urol. 2006, 176, 1012–1016. [Google Scholar] [CrossRef]
- Chen, W.; Jia, L.; Gupta, S.; MacLennan, G.T. The Role of Chronic Inflammation in Prostate Carcinogenesis: A Follow-Up Study. Ann. Urol. Oncol. 2019, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cai, T.; Santi, R.; Tamanini, I.; Galli, I.C.; Perletti, G.; Bjerklund Johansen, T.E.; Nesi, G. Current knowledge of the potential links between inflammation and prostate cancer. Int. J. Mol. Sci. 2019, 20, 3833. [Google Scholar] [CrossRef] [Green Version]
- Pandareesh, M.D.; Kameshwar, V.H.; Byrappa, K. Prostate Carcinogenesis: Insights in Relation to Epigenetics and Inflammation. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 253–267. [Google Scholar] [CrossRef]
- Adekoya, T.O.; Richardson, R.M. Cytokines and Chemokines as Mediators of Prostate Cancer Metastasis. Int. J. Mol. Sci. 2020, 21, 4449. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.P.; Ertunc, O.; Kulac, I.; Baena-Del Valle, J.A.; De Marzo, A.M.; Sfanos, K.S. IL8 Expression Is Associated with Prostate Cancer Aggressiveness and Androgen Receptor Loss in Primary and Metastatic Prostate Cancer. Mol. Cancer Res. 2020, 18, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Cakir, S.S.; Polat, E.C.; Ozcan, L.; Besiroglu, H.; Ötunctemur, A.; Ozbek, E. The effect of prostatic inflammation on clinical outcomes in patients with benign prostate hyperplasia. Prostate Int. 2018, 6, 71–74. [Google Scholar] [CrossRef]
- Wu, D.; Shi, Z.-E.; Xu, D.; Wu, Y.; Qian, S.-B.; Qi, J. Serum interleukin 6 and acute urinary retention in elderly men with benign prostatic hyperplasia in China: A cross-sectional study. Transl. Androl. Urol. 2021, 10, 455–465. [Google Scholar] [CrossRef]
- Liu, T.T.; Thomas, S.; Mclean, D.T.; Roldan-Alzate, A.; Hernando, D.; Ricke, E.A.; Ricke, W.A. Prostate enlargement and altered urinary function are part of the aging process. Aging 2019, 11, 2653–2669. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, G.; Madersbacher, S.; Berger, P. Benign prostatic hyperplasia: Age-related tissue-remodeling. Exp. Gerontol. 2005, 40, 121–128. [Google Scholar] [CrossRef]
- Taoka, R.; Kakehi, Y. The influence of asymptomatic inflammatory prostatitis on the onset and progression of lower urinary tract symptoms in men with histologic benign prostatic hyperplasia. Asian J. Urol. 2017, 4, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, W.; Yu, F.; Wu, Y.; Fang, X.; Hao, W. Alternatively activated macrophages are associated with prostate volume and lower urinary tract symptoms severity of patients with benign prostate hyperplasia. Clin. Lab. 2017, 63, 1057–1062. [Google Scholar] [CrossRef]
- Xu, D.; Chen, P.; Xiao, H.; Wang, X.; DiSanto, M.E.; Zhang, X. Upregulated interleukin 21 receptor enhances proliferation and epithelial-mesenchymal transition process in benign prostatic hyperplasia. Front. Endocrinol. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; He, Y.; Qi, L.; Zu, X.; Wu, L.; Cao, Z.; Li, Y.; Liu, L.; Dube, D.A.; Wang, Z.; et al. Infiltrating mast cells enhance benign prostatic hyperplasia through IL-6/STAT3/Cyclin D1 signals. Oncotarget 2017, 8, 59156–59164. [Google Scholar] [CrossRef] [Green Version]
- White, C.W.; Xie, J.H.; Ventura, S. Age-related changes in the innervation of the prostate gland: Implications for prostate cancer initiation and progression. Organogenesis 2013, 9, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Gratzke, C.; Tamalunas, A.; Rutz, B.; Ciotkowska, A.; Strittmatter, F.; Herlemann, A.; Janich, S.; Waidelich, R.; Liu, C.; et al. Smooth muscle contraction and growth of stromal cells in the human prostate are both inhibited by the SRC family kinase inhibitors, AZM475271 and PP2. Br. J. Pharmacol. 2016, 173, 3342–3358. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.R.; Crowe, R.; Gilpin, S.A.; Gosling, J.; Burnstock, G. The innervation of the human prostate gland--the changes associated with benign enlargement. J. Urol. 1991, 146, 1637–1644. [Google Scholar] [CrossRef]
- Aikawa, K.; Yokota, T.; Okamura, H.; Yamaguchi, O. Endogenous nitric oxide-mediated relaxation and nitrinergic innervation in the rabbit prostate: The changes with aging. Prostate 2001, 48, 40–46. [Google Scholar] [CrossRef]
- Powell, M.S.; Li, R.; Dai, H.; Sayeeduddin, M.; Wheeler, T.M.; Ayala, G.E. Neuroanatomy of the normal prostate. Prostate 2005, 65, 52–57. [Google Scholar] [CrossRef]
- Xavier, F.E. Nitrergic perivascular innervation in health and diseases: Focus on vascular tone regulation. Acta Physiol. 2020, 230, e13484. [Google Scholar] [CrossRef]
- Kajiwara, S.; Ishii, K.; Sasaki, T.; Kato, M.; Nishikawa, K.; Kanda, H.; Arima, K.; Watanabe, M.; Sugimura, Y. Castration-induced stromal remodeling disrupts the reconstituted prostate epithelial structure. Lab. Investig. 2020, 100, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, P.; Yang, J.H.; Li, Y.; Lerner, L.B.; Azadzoi, K.M. Structural modifications of the prostate in hypoxia, oxidative stress, and chronic ischemia. Korean J. Urol. 2015, 56, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popovics, P.; Awadallah, W.N.; Kohrt, S.E.; Case, T.C.; Miller, N.L.; Ricke, E.A.; Huang, W.; Ramirez-Solano, M.; Liu, Q.; Vezina, C.M.; et al. Prostatic osteopontin expression is associated with symptomatic benign prostatic hyperplasia. Prostate 2020, 80, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Barabutis, N.; Schally, A.V.; Siejka, A. P53, GHRH, inflammation and cancer. EBioMedicine 2018, 37, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Agupitan, A.D.; Neeson, P.; Williams, S.; Howitt, J.; Haupt, S.; Haupt, Y. P53: A Guardian of Immunity Becomes Its Saboteur through Mutation. Int. J. Mol. Sci. 2020, 21, 3452. [Google Scholar] [CrossRef]
- Lacroix, M.; Riscal, R.; Arena, G.; Linares, L.K.; Le Cam, L. Metabolic functions of the tumor suppressor p53: Implications in normal physiology, metabolic disorders, and cancer. Mol. Metab. 2020, 33, 2–22. [Google Scholar] [CrossRef]
- Sabapathy, K.; Lane, D.P. Understanding p53 functions through p53 antibodies. J. Mol. Cell Biol. 2019, 11, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, F.; Collavin, L.; Del Sal, G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019, 26, 199–212. [Google Scholar] [CrossRef]
- Stein, Y.; Rotter, V.; Aloni-Grinstein, R. Gain-of-Function Mutant p53: All the Roads Lead to Tumorigenesis. Int. J. Mol. Sci. 2019, 20, 6197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minutoli, L.; Rinaldi, M.; Marini, H.; Irrera, N.; Crea, G.; Lorenzini, C.; Puzzolo, D.; Valenti, A.; Pisani, A.; Adamo, E.B.; et al. Apoptotic pathways linked to endocrine system as potential therapeutic targets for benign prostatic hyperplasia. Int. J. Mol. Sci. 2016, 17, 1311. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.Y.; Lin, P.M.; Liu, Y.C.; Hsiao, H.H.; Yang, W.C.; Hsu, J.F.; Hsu, C.M.; Lin, S.F. Induction of cellular senescence by doxorubicin is associated with upregulated miR-375 and induction of autophagy in K562 cells. PLoS ONE 2012, 7, e37205. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Crowe, D.L. Telomere DNA Damage Signaling Regulates Prostate Cancer Tumorigenesis. Mol. Cancer Res. 2020, 18, 1326–1339. [Google Scholar] [CrossRef]
- Dower, C.M.; Wills, C.A.; Frisch, S.M.; Wang, H.G. Mechanisms and context underlying the role of autophagy in cancer metastasis. Autophagy 2018, 14, 1110–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Huang, F.; Wang, B.-R.; Wang, Y.-G. Role of autophagy in tumorigenesis, metastasis, targeted therapy and drug resistance of hepatocellular carcinoma. World J. Gastroenterol. 2018, 24, 4643–4651. [Google Scholar] [CrossRef]
- Alvarez-Meythaler, J.G.; Garcia-Mayea, Y.; Mir, C.; Kondoh, H.; LLeonart, M.E. Autophagy Takes Center Stage as a Possible Cancer Hallmark. Front. Oncol. 2020, 10, 586069. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Murthy, A. Targeting Autophagy to Treat Cancer: Challenges and Opportunities. Front. Pharmacol. 2020, 11, 590344. [Google Scholar] [CrossRef] [PubMed]
- Leidal, A.M.; Levine, B.; Debnath, J. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 2018, 20, 1338–1348. [Google Scholar] [CrossRef]
- Condello, M.; Pellegrini, E.; Caraglia, M.; Meschini, S. Targeting Autophagy to Overcome Human Diseases. Int. J. Mol. Sci. 2019, 20, 725. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Qin, Z.-H. Autophagy, Aging, and Longevity. Adv. Exp. Med. Biol. 2019, 1206, 509–525. [Google Scholar] [CrossRef]
- Wong, S.Q.; Kumar, A.V.; Mills, J.; Lapierre, L.R. Autophagy in aging and longevity. Hum. Genet. 2020, 139, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Cheboub, A.; Regouat, N.; Djidjik, R.; Slimani, A.; Hadj-Bekkouche, F. Short-term aromatase inhibition induces prostatic alterations in adult wistar rat: A biochemical, histopathological and immunohistochemical study. Acta Histochem. 2019, 121, 151441. [Google Scholar] [CrossRef] [PubMed]
- Magura, C.E.; Spector, M. Scanning electron microscopy of human prostatic corpora amylacea and corpora calculi, and prostatic calculi. Scan. Electron Microsc. 1979, 3, 713–720. [Google Scholar]
- Battaglia, S.; Barbolini, G.; Botticelli, A.R.; Trentini, G.P. Apoptotic amyloid: A study on prostatic amyloidosis with particular reference to corpora amylacea. Appl. Pathol. 1985, 3, 105–114. [Google Scholar] [PubMed]
- Audas, T.E.; Audas, D.E.; Jacob, M.D.; Ho, J.J.D.; Khacho, M.; Wang, M.; Perera, J.K.; Gardiner, C.; Bennett, C.A.; Head, T.; et al. Adaptation to Stressors by Systemic Protein Amyloidogenesis. Dev. Cell 2016, 39, 155–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubel, M.S.; Fedotov, S.A.; Grizel, A.V.; Sopova, J.V.; Malikova, O.A.; Chernoff, Y.O.; Rubel, A.A. Functional Mammalian Amyloids and Amyloid-Like Proteins. Life 2020, 10, 156. [Google Scholar] [CrossRef]
- Ratovitski, E.A. Tumor Protein p63/microRNA Network in Epithelial Cancer Cells. Curr. Genom. 2013, 14, 441–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picken, M.M. The Pathology of Amyloidosis in Classification: A Review. Acta Haematol. 2020, 143, 322–334. [Google Scholar] [CrossRef]
- Sack, G.H.J. Serum amyloid A—A review. Mol. Med. 2018, 24, 46. [Google Scholar] [CrossRef]
- Sack, G.H.J. Serum Amyloid A (SAA) Proteins. Subcell. Biochem. 2020, 94, 421–436. [Google Scholar] [CrossRef]
- Nevo, A.; Muchtar, E.; Stern, K.L.; Moore, J.P.; Cheney, S.M.; Humphreys, M.R.; Grogan, M.; Stanton, M.L. The Clinical Implication of Incidental Prostatic Amyloidosis. Urology 2020, 145, 253–257. [Google Scholar] [CrossRef]
- Chuang, E.; Hori, A.M.; Hesketh, C.D.; Shorter, J. Amyloid assembly and disassembly. J. Cell Sci. 2018, 131, jcs189928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelrahman, S.; Alghrably, M.; Lachowicz, J.I.; Emwas, A.-H.; Hauser, C.A.E.; Jaremko, M. “What Doesn’t Kill You Makes You Stronger”: Future Applications of Amyloid Aggregates in Biomedicine. Molecules 2020, 25, 5245. [Google Scholar] [CrossRef] [PubMed]
- Almeida, Z.L.; Brito, R.M.M. Structure and Aggregation Mechanisms in Amyloids. Molecules 2020, 25, 1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-Y.; Hall, J.A.; Kroehling, L.; Wu, L.; Najar, T.; Nguyen, H.H.; Lin, W.-Y.; Yeung, S.T.; Silva, H.M.; Li, D.; et al. Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory Disease. Cell 2020, 180, 79–91. [Google Scholar] [CrossRef] [PubMed]
- SorićHosman, I.; Kos, I.; Lamot, L. Serum Amyloid A in Inflammatory Rheumatic Diseases: A Compendious Review of a Renowned Biomarker. Front. Immunol. 2020, 11, 631299. [Google Scholar] [CrossRef]
- Webb, N.R. High-Density Lipoproteins and Serum Amyloid A (SAA). Curr. Atheroscler. Rep. 2021, 23, 7. [Google Scholar] [CrossRef]
- Walton, C.C.; Begelman, D.; Nguyen, W.; Andersen, J.K. Senescence as an Amyloid Cascade: The Amyloid Senescence Hypothesis. Front. Cell. Neurosci. 2020, 14, 129. [Google Scholar] [CrossRef]
- Gursky, O. Structural Basis for Vital Function and Malfunction of Serum Amyloid A: An Acute-Phase Protein that Wears Hydrophobicity on Its Sleeve. Curr. Atheroscler. Rep. 2020, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Inyushin, M.; Zayas-Santiago, A.; Rojas, L.; Kucheryavykh, L. On the Role of Platelet-Generated Amyloid Beta Peptides in Certain Amyloidosis Health Complications. Front. Immunol. 2020, 11, 571083. [Google Scholar] [CrossRef]
- Mizejewski, G. Breast cancer and amyloid bodies: Is there a role for amyloidosis in cancer-cell dormancy? Breast Cancer Targets Ther. 2017, 9, 287–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavliukeviciene, B.; Zentelyte, A.; Jankunec, M.; Valiuliene, G.; Talaikis, M.; Navakauskiene, R.; Niaura, G.; Valincius, G. Amyloid β oligomers inhibit growth of human cancer cells. PLoS ONE 2019, 14, e0221563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneti, J.; Winikoff, Y.; Zimlichman, S.; Shainkin-Kestenbaum, R. Importance of serum amyloid A (SAA) level in monitoring disease activity and response to therapy in patients with prostate cancer. Urol. Res. 1984, 12, 239–241. [Google Scholar] [CrossRef]
- DuPre, N.C.; Flavin, R.; Sfanos, K.S.; Unger, R.H.; To, S.; Gazeeva, E.; Fiorentino, M.; De Marzo, A.M.; Rider, J.R.; Mucci, L.A. Corpora amylacea in prostatectomy tissue and associations with molecular, histological, and lifestyle factors. Prostate 2018, 78, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Palangmonthip, W.; Wu, R.; Tarima, S.; Bobholz, S.A.; LaViolette, P.S.; Gallan, A.J.; Iczkowski, K.A. Corpora amylacea in benign prostatic acini are associated with concurrent, predominantly low-grade cancer. Prostate 2020, 80, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Klimas, R.; Bennett, B.; Gardner, W.A.J. Prostatic calculi: A review. Prostate 1985, 7, 91–96. [Google Scholar] [CrossRef]
- Cross, P.A.; Bartley, C.J.; McClure, J. Amyloid in prostatic corpora amylacea. J. Clin. Pathol. 1992, 45, 894–897. [Google Scholar] [CrossRef] [Green Version]
- Lawrentschuk, N.; Pan, D.; Stillwell, R.; Bolton, D.M. Implications of amyloidosis on prostatic biopsy. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2004, 11, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Christian, J.D.; Lamm, T.C.; Morrow, J.F.; Bostwick, D.G. Corpora amylacea in adenocarcinoma of the prostate: Incidence and histology within needle core biopsies. Mod. Pathol. 2005, 18, 36–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanenawa, K.; Ueda, M.; Isoguchi, A.; Nomura, T.; Tsuda, Y.; Masuda, T.; Misumi, Y.; Yamashita, T.; Ando, Y. Histopathological and biochemical analyses of prostate corpora amylacea. Amyloid Int. J. Exp. Clin. Investig. Off. J. Int. Soc. Amyloidosis 2019, 26, 160–161. [Google Scholar] [CrossRef] [PubMed]
- Kapogiannis, F.; Fasoulakis, K.; Fragkoulis, C.; Aggelopoulos, A.; Fasoulakis, C. Total Osseous Calcification of the Prostate Gland. Cureus 2020, 12, e9239. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Wilson, B.A.; De Marzo, A.M.; Isaacs, W.B. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 3443–3448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanamandra, K.; Alexeyev, O.; Zamotin, V.; Srivastava, V.; Shchukarev, A.; Brorsson, A.C.; Tartaglia, G.G.; Vogl, T.; Kayed, R.; Wingsle, G.; et al. Amyloid formation by the pro-inflammatory S100A8/A9 proteins in the ageing prostate. PLoS ONE 2009, 4, e5562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogl, T.; Gharibyan, A.L.; Morozova-Roche, L.A. Pro-inflammatory S100A8 and S100A9 proteins: Self-assembly into multifunctional native and amyloid complexes. Int. J. Mol. Sci. 2012, 13, 2893–2917. [Google Scholar] [CrossRef] [Green Version]
- Fritz, G.; Botelho, H.M.; Morozova-Roche, L.A.; Gomes, C.M. Natural and amyloid self-assembly of S100 proteins: Structural basis of functional diversity. FEBS J. 2010, 277, 4578–4590. [Google Scholar] [CrossRef] [PubMed]
- Gharibyan, A.L.; Raveh, D.; Morozova-Roche, L.A. S100A8/A9 amyloidosis in the ageing prostate: Relating ex vivo and in vitro studies. Methods Mol. Biol. 2012, 849, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Grebhardt, S.; Veltkamp, C.; Ströbel, P.; Mayer, D. Hypoxia and HIF-1 increase S100A8 and S100A9 expression in prostate cancer. Int. J. Cancer 2012, 131, 2785–2794. [Google Scholar] [CrossRef]
- Deep, G.; Panigrahia, G.K. Hypoxia-induced signaling promotes prostate cancer progression: Exosomes role as messenger of hypoxic response in tumormicroenvironmen. Crit. Rev. Oncog. 2015, 20, 419–434. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Lin, D.; Taniguchi, C.M. Hypoxia inducible factor (HIF) in the tumor microenvironment: Friend or foe? Sci. China Life Sci. 2017, 60, 1114–1124. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, H.; Shi, Q.; Gu, M.; Wan, X.; Chen, Q.; Wang, Z. Hypoxia-inducible factor 1α (HIF-1α) mediates the epithelial-mesenchymal transition in benign prostatic hyperplasia. Int. J. Clin. Exp. Pathol. 2019, 12, 295–304. [Google Scholar] [PubMed]
- Lv, Z.; Li, W.; Wei, X. S100A9 promotes prostate cancer cell invasion by activating TLR4/NF-κB/integrin β1/FAK signaling. Onco. Targets. Ther. 2020, 13, 6443–6452. [Google Scholar] [CrossRef] [PubMed]
- Rangel, L.P.; Ferretti, G.D.S.; Costa, C.L.; Andrade, S.M.M.V.; Carvalho, R.S.; Costa, D.C.F.; Silva, J.L. p53 reactivation with induction of massive apoptosis-1 (PRIMA-1) inhibits amyloid aggregation of mutant p53 in cancer cells. J. Biol. Chem. 2019, 294, 3670–3682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.; Ryu, H.; Kim, S.; Chin, H.J.; Na, K.Y.; Chae, D.-W.; Yoon, H.-J. Comparison of cancer prevalence between patients with glomerulonephritis and the general population at the time of kidney biopsy. PLoS ONE 2019, 14, e0224024. [Google Scholar] [CrossRef]
- Zayas-Santiago, A.; Díaz-García, A.; Nuñez-Rodríguez, R.; Inyushin, M. Accumulation of amyloid beta in human glioblastomas. Clin. Exp. Immunol. 2020, 202, 325–334. [Google Scholar] [CrossRef]
- Isaacs, J.T. Prostatic structure and function in relation to the etiology of prostatic cancer. Prostate 1983, 4, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- de Bono, J.S.; Guo, C.; Gurel, B.; De Marzo, A.M.; Sfanos, K.S.; Mani, R.S.; Gil, J.; Drake, C.G.; Alimonti, A. Prostate carcinogenesis: Inflammatory storms. Nat. Rev. Cancer 2020, 20, 455–469. [Google Scholar] [CrossRef]
- Fishbein, A.; Hammock, B.D.; Serhan, C.N.; Panigrahy, D. Carcinogenesis: Failure of resolution of inflammation? Pharmacol. Ther. 2021, 218, 107670. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Platz, E.A.; Stampfer, M.J.; Willett, W.C.; Giovannucci, E. Ejaculation Frequency and Subsequent Risk of Prostate Cancer. J. Am. Med. Assoc. 2004, 291, 1578–1586. [Google Scholar] [CrossRef]
- Rider, J.R.; Wilson, K.M.; Sinnott, J.A.; Kelly, R.S.; Mucci, L.A.; Giovannucci, E.L. Ejaculation Frequency and Risk of Prostate Cancer: Updated Results with an Additional Decade of Follow-up. Eur. Urol. 2016, 70, 974–982. [Google Scholar] [CrossRef] [Green Version]
- Brendler, C.B.; Berry, S.J.; Ewing, L.L.; McCullough, A.R.; Cochran, R.C.; Strandberg, J.D.; Zirkin, B.R.; Coffey, D.S.; Wheaton, L.G.; Hiler, M.L.; et al. Spontaneous benign prostatic hyperplasia in the beagle. Age-associated changes in serum hormone levels, and the morphology and secretory function of the canine prostate. J. Clin. Investig. 1983, 71, 1114–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, S.J.; Coffey, D.S.; Ewing, L.L. Effects of aging on prostate growth in beagles. Am. J. Physiol. 1986, 250, R1039–R1046. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.S. Clinical Significance of Prostatic Calculi: A Review. World J. Mens. Health 2018, 36, 15. [Google Scholar] [CrossRef] [PubMed]
- Polacheck, J.W.; Vega, L.E. Cytologic Comparison of Semen and Expressed Prostatic Secretion from Patients with Chronic Prostatitis; Prostatitis Cent Carondelet St Joseph’s Hospital: Tucson, AZ, USA, 2010; Available online: https://forum.prostatitis.org/viewtopic.php?f=2&t=382&hilit (accessed on 8 June 2021).
- FathollahiShoorabeh, F.; Dabidiroshan, V.; Sheikh Saraf, B.; Nuri, R. Investigating the Effects of Regular Resistance Training and Prostatic Massage on Proinflammatory Markers and Serum Prostate-Specific Antigen Levels in Males with Prostate Cancer. Middle East J. Rehabil. Health 2016, 3, e33651. [Google Scholar] [CrossRef] [Green Version]
- Hennenfent, B.R.; Garcia, B.S.; Feliciano, A.E. Symptom improvement and transrectal ultrasound-documented reduction of prostate size after repetitive prostatic massage and antimicrobial therapy. J. Pelvic Surg. 2002, 8, 265–269. [Google Scholar]
- Paz, G.F.; Fainman, N.; Homonnai, Z.T.; Kraicer, P.F. The Effect of Massage Treatment of Prostatic Congestion on the Prostatic Size and Secretion of Citric Acid. Andrologia 1980, 12, 30–33. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Zeitlin, S.I. Use of prostatic massage in combination with antibiotics in the treatment of chronic prostatitis. Prostate Cancer Prostatic Dis. 1999, 2, 159–162. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.L.; He, D.L.; Luo, Y. Clinical trials of combined therapy of an oral Chinese medicine with massage for chronic nonbacterial prostatitis. Zhonghua Nan Ke Xue 2006, 12, 851–853. [Google Scholar]
- Hennenfent, B.R.; Lazarte, A.R.; Feliciano, A.E. Repetitive prostatic massage and drug therapy as an alternative to transurethral resection of the prostate. Medscape Gen. Med. 2006, 8, 19. [Google Scholar]
- Capodice, J.L.; Stone, B.A.; Katz, A.E. Evaluation of an At-Home-Use Prostate Massage Device for Men with Lower Urinary Tract Symptoms. Open Urol. Nephrol. J. 2014, 2, 20–23. [Google Scholar] [CrossRef]
- Pidddubnyi, A.; Romaniuk, A.; Radomychelski, I.M.; Moskalenko, Y.; Moskalenko, R.A. Prostate cancer with intraluminal inclusions: The association of the immunophenotype with grade score. Iran. J. Pathol. 2019, 14, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Le, W.-D. Autophagy and Ubiquitin-Proteasome System. Adv. Exp. Med. Biol. 2019, 1206, 527–550. [Google Scholar] [CrossRef] [PubMed]
- Colhado Rodrigues, B.L.; Lallo, M.A.; Perez, E.C. The Controversial Role of Autophagy in Tumor Development: A Systematic Review. Immunol. Investig. 2020, 49, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Galati, S.; Boni, C.; Gerra, M.C.; Lazzaretti, M.; Buschini, A. Autophagy: A Player in response to Oxidative Stress and DNA Damage. Oxid. Med. Cell. Longev. 2019, 2019, 5692958. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Karsli-Uzunbas, G.; Poillet-Perez, L.; Sawant, A.; Hu, Z.S.; Zhao, Y.; Moore, D.; Hu, W.; White, E. Autophagy promotes mammalian survival by suppressing oxidative stress and p53. Genes Dev. 2020, 34, 688–700. [Google Scholar] [CrossRef]
- Zhang, N.; Ji, N.; Jiang, W.M.; Li, Z.Y.; Wang, M.; Wen, J.M.; Li, Y.; Chen, X.; Chen, J.M. Hypoxia-induced autophagy promotes human prostate stromal cells survival and ER-stress. Biochem. Biophys. Res. Commun. 2015, 464, 1107–1112. [Google Scholar] [CrossRef]
- Oh, S.H.; Lee, D.W.; Choi, Y.B.; Lee, Y.H.; Ju, J. sun Measurement of autophagy flux in benign prostatic hyperplasia in vitro. Prostate Int. 2020, 8, 70–77. [Google Scholar] [CrossRef]
- Deretic, V.; Levine, B. Autophagy balances inflammation in innate immunity. Autophagy 2018, 14, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.K.; Hasanali, S.L.; Wang, J.; Kallifatidis, G.; Morera, D.S.; Jordan, A.R.; Terris, M.K.; Klaassen, Z.; Bollag, R.; Lokeshwar, V.B.; et al. Promotion of epithelial hyperplasia by interleukin-8—CXCR axis in human prostate. Prostate 2020, 80, 938–949. [Google Scholar] [CrossRef]
- De Nunzio, C.; Giglio, S.; Stoppacciaro, A.; Gacci, M.; Cirombella, R.; Luciani, E.; Tubaro, A.; Vecchione, A. Autophagy deactivation is associated with severe prostatic inflammation in patients with lower urinary tract symptoms and benign prostatic hyperplasia. Oncotarget 2017, 8, 50904–50910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaaf, M.B.; Houbaert, D.; Meçe, O.; Agostinis, P. Autophagy in endothelial cells and tumor angiogenesis. Cell Death Differ. 2019, 26, 665–679. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Klionsky, D.J. At a glance: A history of autophagy and cancer. Semin. Cancer Biol. 2020, 66, 3–11. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Zhou, G.; Mu, D.; Yan, J.; Xing, J.; Yao, Z.; Sheng, H.; Li, D.; Lv, C.; et al. Aurora-A regulates autophagy through the Akt pathway in human prostate cancer. Cancer Biomark. 2017, 19, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G. Autophagy: A druggable process that is deregulated in aging and human disease. J. Clin. Investig. 2015, 125, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Badyaev, A.V. Origin of the fittest: Link between emergentvariation and evolutionary change as acritical question in evolutionary biology. Proc. R. Soc. B Biol. Sci. 2011, 278, 1921–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Félix, M.-A. Phenotypic Evolution with and Beyond Genome Evolution. Curr. Top. Dev. Biol. 2016, 119, 291–347. [Google Scholar] [CrossRef]
- Somarelli, J.A.; Gardner, H.; Cannataro, V.L.; Gunady, E.F.; Boddy, A.M.; Johnson, N.A.; Fisk, J.N.; Gaffney, S.G.; Chuang, J.H.; Li, S.; et al. Molecular Biology and Evolution of Cancer: From Discovery to Action. Mol. Biol. Evol. 2020, 37, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Zahir, N.; Sun, R.; Gallahan, D.; Gatenby, R.A.; Curtis, C. Characterizing the ecological and evolutionary dynamics of cancer. Nat. Genet. 2020, 52, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Gerstung, M.; Jolly, C.; Leshchiner, I.; Dentro, S.C.; Gonzalez, S.; Rosebrock, D.; Mitchell, T.J.; Rubanova, Y.; Anur, P.; Yu, K.; et al. The evolutionary history of 2658 cancers. Nature 2020, 578, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Lahouel, K.; Younes, L.; Danilova, L.; Giardiello, F.M.; Hruban, R.H.; Groopman, J.; Kinzler, K.W.; Vogelstein, B.; Geman, D.; Tomasetti, C. Revisiting the tumorigenesis timeline with a data-driven generative model. Proc. Natl. Acad. Sci. USA 2020, 117, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Skvortsov, S.; Skvortsova, I.I.; Tang, D.G.; Dubrovska, A. Concise Review: Prostate Cancer Stem Cells: Current Understanding. Stem Cells 2018, 36, 1457–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampayo, R.G.; Bissell, M.J. Cancer stem cells in breast and prostate: Fact or fiction? In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2019; Volume 144, pp. 315–341. [Google Scholar]
- Gorodetska, I.; Lukiyanchuk, V.; Peitzsch, C.; Kozeretska, I.; Dubrovska, A. BRCA1 and EZH2 cooperate in regulation of prostate cancer stem cell phenotype. Int. J. Cancer 2019, 145, 2974–2985. [Google Scholar] [CrossRef] [PubMed]
- Werneck-Gomes, H.; Campolina-Silva, G.H.; Maria, B.T.; Barata, M.C.; Mahecha, G.A.B.; Hess, R.A.; Oliveira, C.A. Tumor-Associated Macrophages (TAM) are recruited to the aging prostate epithelial lesions and become intermingled with basal cells. Andrology 2020, 8, 1375–1386. [Google Scholar] [CrossRef]
- Chang, N.C. Autophagy and Stem Cells: Self-Eating for Self-Renewal. Front. Cell Dev. Biol. 2020, 8, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowell, P.D.; Fox, J.J.; Hashimoto, T.; Diaz, J.A.; Navarro, H.I.; Henry, G.H.; Feldmar, B.A.; Lowe, M.G.; Garcia, A.J.; Wu, Y.E.; et al. Expansion of Luminal Progenitor Cells in the Aging Mouse and Human Prostate. Cell Rep. 2019, 28, 1499–1510.e6. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-J.; Lo, U.-G.; Hsieh, J.-T. The regulatory pathways leading to stem-like cells underlie prostate cancer progression. Asian J. Androl. 2019, 21, 233–240. [Google Scholar] [CrossRef]
- Middleton, L.W.; Shen, Z.; Varma, S.; Pollack, A.S.; Gong, X.; Zhu, S.; Zhu, C.; Foley, J.W.; Vennam, S.; Sweeney, R.T.; et al. Genomic analysis of benign prostatic hyperplasia implicates cellular re-landscaping in disease pathogenesis. JCI Insight 2019, 5. [Google Scholar] [CrossRef]
- Vickman, R.E.; Broman, M.M.; Lanman, N.A.; Franco, O.E.; Sudyanti, P.A.G.; Ni, Y.; Ji, Y.; Helfand, B.T.; Petkewicz, J.; Paterakos, M.C.; et al. Heterogeneity of human prostate carcinoma-associated fibroblasts implicates a role for subpopulations in myeloid cell recruitment. Prostate 2020, 80, 173–185. [Google Scholar] [CrossRef]
- Chen, W.; Pascal, L.E.; Wang, K.; Dhir, R.; Sims, A.M.; Campbell, R.; Gasper, G.; DeFranco, D.B.; Yoshimura, N.; Wang, Z. Differential impact of paired patient-derived BPH and normal adjacent stromal cells on benign prostatic epithelial cell growth in 3D culture. Prostate 2020, 80, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shoag, J.E.; Poliak, D.; Goueli, R.S.; Ravikumar, V.; Redmond, D.; Vosoughi, A.; Fontugne, J.; Pan, H.; Lee, D.; et al. Integrative multiplatform molecular profiling of benign prostatic hyperplasia identifies distinct subtypes. Nat. Commun. 2020, 11, 1987. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Arneth, B. Tumor microenvironment. Medicina 2020, 56, 15. [Google Scholar] [CrossRef] [Green Version]
- Adav, S.S.; Sze, S.K. Hypoxia-Induced Degenerative Protein Modifications Associated with Aging and Age-Associated Disorders. Aging Dis. 2020, 11, 341–364. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.A.; Vissers, J.P.C.; Nanda, J.; Stewart, G.D.; Husi, H.; Habib, F.K.; Hammond, D.E.; Gethings, L.A. The influence of hypoxia on the prostate cancer proteome. Clin. Chem. Lab. Med. 2020, 58, 980–993. [Google Scholar] [CrossRef]
- Bhandari, V.; Hoey, C.; Liu, L.Y.; Lalonde, E.; Ray, J.; Livingstone, J.; Lesurf, R.; Shiah, Y.-J.; Vujcic, T.; Huang, X.; et al. Molecular landmarks of tumor hypoxia across cancer types. Nat. Genet. 2019, 51, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P.D.; Giafaglione, J.M.; Hashimoto, T.; Goldstein, A.S. Distinct cell-types in the prostate share an aging signature suggestive of metabolic reprogramming. Am. J. Clin. Exp. Urol. 2020, 8, 140–151. [Google Scholar] [PubMed]
- Peng, Z.; Shu, B.; Zhang, Y.; Wang, M. Endothelial Response to Pathophysiological Stress. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e233–e243. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.-B.; Cai, H.; Wu, Y.-P.; Lin, Y.-Z.; Li, X.-D.; Huang, J.-B.; Sun, X.-L.; Zheng, Q.-S.; Xue, X.-Y.; Wei, Y.; et al. Identification of key genes and pathways in benign prostatic hyperplasia. J. Cell. Physiol. 2019, 234, 19942–19950. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, S. Signaling pathways and effectors of aging. Front. Biosci. 2021, 26, 50–96. [Google Scholar] [CrossRef]
- Lee, Y.G.; Nam, Y.; Shin, K.J.; Yoon, S.; Park, W.S.; Joung, J.Y.; Seo, J.K.; Jang, J.; Lee, S.; Nam, D.; et al. Androgen-induced expression of DRP1 regulates mitochondrial metabolic reprogramming in prostate cancer. Cancer Lett. 2020, 471, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, M.M.; Li, F.; Takeda, D.Y.; Lenci, R.; Chonkar, A.; Chabot, M.; Cejas, P.; Vazquez, F.; Cook, J.; Shivdasani, R.A.; et al. The androgen receptor cistrome is extensively reprogrammed in human prostate tumorigenesis. Nat. Genet. 2015, 47, 1346–1351. [Google Scholar] [CrossRef]
- Powell, I.J.; Chinni, S.R.; Reddy, S.S.; Zaslavsky, A.; Gavande, N. Pro-inflammatory cytokines and chemokines initiate multiple prostate cancer biologic pathways of cellular proliferation, heterogeneity and metastasis in a racially diverse population and underlie the genetic/biologic mechanism of racial disparity: Update. Urol. Oncol. 2021, 39, 34–40. [Google Scholar] [CrossRef]
- Cheng, H.H.; Sokolova, A.O.; Schaeffer, E.M.; Small, E.J.; Higano, C.S. Germline and Somatic Mutations in Prostate Cancer for the Clinician. J. Natl. Compr. Canc. Netw. 2019, 17, 515–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Salami, S.S.; Spratt, D.E.; Kaffenberger, S.D.; Jacobs, M.F.; Morgan, T.M. Bringing Prostate Cancer Germline Genetics into Clinical Practice. J. Urol. 2019, 202, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Arce, S.; Athie, A.; Pritchard, C.C.; Mateo, J. Germline and Somatic Defects in DNA Repair Pathways in Prostate Cancer. Adv. Exp. Med. Biol. 2019, 1210, 279–300. [Google Scholar] [CrossRef]
- Lozano, R.; Castro, E.; Aragón, I.M.; Cendón, Y.; Cattrini, C.; López-Casas, P.P.; Olmos, D. Genetic aberrations in DNA repair pathways: A cornerstone of precision oncology in prostate cancer. Br. J. Cancer 2020. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.; Maza, M.d.l.D.F.d.l.; Rescigno, P.; Chandran, K.; de Bono, J. Targeting defective DNA repair in prostate cancer. Curr. Opin. Oncol. 2020, 32, 503–509. [Google Scholar] [CrossRef]
- Labbé, D.P.; Brown, M. Transcriptional Regulation in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Carroll, L.; Joglekar, M.V.; Januszewski, A.S.; Wong, K.K.; Hardikar, A.A.; Jenkins, A.J.; Ma, R.C.W. Diabetes, metabolic disease, and telomere length. Lancet Diabetes Endocrinol. 2021, 9, 117–126. [Google Scholar] [CrossRef]
- Yegnasubramanian, S.; De Marzo, A.M.; Nelson, W.G. Prostate cancer epigenetics: From basic mechanisms to clinical implications. Cold Spring Harb. Perspect. Med. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Tzelepi, V.; Logotheti, S.; Efstathiou, E.; Troncoso, P.; Aparicio, A.; Sakellakis, M.; Hoang, A.; Perimenis, P.; Melachrinou, M.; Logothetis, C.; et al. Epigenetics and prostate cancer: Defining the timing of DNA methyltransferase deregulation during prostate cancer progression. Pathology 2020, 52, 218–227. [Google Scholar] [CrossRef]
- Sugiura, M.; Sato, H.; Kanesaka, M.; Imamura, Y.; Sakamoto, S.; Ichikawa, T.; Kaneda, A. Epigenetic modifications in prostate cancer. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, M.M.; Qiu, X.; Zhu, Y.; Takeda, D.Y.; Pan, W.; Baca, S.C.; Gusev, A.; Korthauer, K.D.; Severson, T.M.; Ha, G.; et al. Prostate cancer reactivates developmental epigenomic programs during metastatic progression. Nat. Genet. 2020, 52, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.B.; Patel, H.; Henrique, R.; Félix, A. Can epigenetic and inflammatory biomarkers identify clinically aggressive prostate cancer? World J. Clin. Oncol. 2020, 11, 43–52. [Google Scholar] [CrossRef]
- Watson, G.W.; Wickramasekara, S.; Fang, Y.; Maier, C.S.; Williams, D.E.; Dashwood, R.H.; Perez, V.I.; Ho, E. HDAC6 activity is not required for basal autophagic flux in metastatic prostate cancer cells. Exp. Biol. Med. 2016, 241, 1177–1185. [Google Scholar] [CrossRef] [Green Version]
- Ling, Z.; Liu, D.; Zhang, G.; Liang, Q.; Xiang, P.; Xu, Y.; Han, C.; Tao, T. miR-361-5p modulates metabolism and autophagy via the Sp1-mediated regulation of PKM2 in prostate cancer. Oncol. Rep. 2017, 38, 1621–1628. [Google Scholar] [CrossRef] [Green Version]
- Mrakovcic, M.; Fröhlich, L.F. P53-mediated molecular control of autophagy in tumor cells. Biomolecules 2018, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Eberli, D.; Kranzbühler, B.; Mortezavi, A.; Sulser, T.; Salemi, S. Apalutamide in combination with autophagy inhibitors improves treatment effects in prostate cancer cells. Urol. Oncol. 2020, 38, 683.e19–683.e26. [Google Scholar] [CrossRef]
- Chavez-Dominguez, R.; Perez-Medina, M.; Lopez-Gonzalez, J.S.; Galicia-Velasco, M.; Aguilar-Cazares, D. The Double-Edge Sword of Autophagy in Cancer: From Tumor Suppression to Pro-tumor Activity. Front. Oncol. 2020, 10, 578418. [Google Scholar] [CrossRef]
- Folkerts, H.; Hilgendorf, S.; Vellenga, E.; Bremer, E.; Wiersma, V.R. The multifaceted role of autophagy in cancer and the microenvironment. Med. Res. Rev. 2019, 39, 517–560. [Google Scholar] [CrossRef]
- Ren, H.; Wang, G. Autophagy and lysosome storage disorders. Adv. Exp. Med. Biol. 2020, 1207, 87–102. [Google Scholar] [CrossRef]
- Seranova, E.; Connolly, K.J.; Zatyka, M.; Rosenstock, T.R.; Barrett, T.; Tuxworth, R.I.; Sarkar, S. Dysregulation of autophagy as a common mechanism in lysosomal storage diseases. Essays Biochem. 2017, 61, 733–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monaco, A.; Fraldi, A. Protein Aggregation and Dysfunction of Autophagy-Lysosomal Pathway: A Vicious Cycle in Lysosomal Storage Diseases. Front. Mol. Neurosci. 2020, 13, 37. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Niu, X.; Wang, G.; Huang, J.; Liu, M.; Peng, B. Chronic prostatitis/chronic pelvic pain syndrome impairs erectile function through increased endothelial dysfunction, oxidative stress, apoptosis, and corporal fibrosis in a rat model. Andrology 2016, 4, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Nanni, S.; Grasselli, A.; Benvenuti, V.; Aiello, A.; Pantisano, V.; Re, A.; Gaetano, C.; Capogrossi, M.C.; Bacchetti, S.; Pontecorvi, A.; et al. The role of nuclear endothelial nitric oxide synthase in the endothelial and prostate microenvironments. Horm. Mol. Biol. Clin. Investig. 2011, 5, 91–96. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Ballon-Landa, E.; Parsons, J.K. Nutrition, physical activity, and lifestyle factors in prostate cancer prevention. Curr. Opin. Urol. 2018, 28, 55–61. [Google Scholar] [CrossRef]
- Wilson, K.M.; Mucci, L.A. Diet and Lifestyle in Prostate Cancer. Adv. Exp. Med. Biol. 2019, 1210, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Makarem, N.; Emin, M.; Liao, M.; Jelic, S.; Aggarwal, B. Mediterranean diet components are linked to greater endothelial function and lower inflammation in a pilot study of ethnically diverse women. Nutr. Res. 2020, 75, 77–84. [Google Scholar] [CrossRef]
- Greenwald, P. A favorable view: Progress in cancer prevention and screening. Cancer Prev. 2007, 174, 3–17. [Google Scholar] [CrossRef]
- Greenwald, P.; Dunn, B.K. Do we make optimal use of the potential of cancer prevention? Cancer Prev. II 2009, 181, 3–17. [Google Scholar] [CrossRef]
- Hewitt, K.; Son, J.; Glencer, A.; Borowsky, A.D.; Cooperberg, M.R.; Esserman, L.J. The Evolution of Our Understanding of the Biology of Cancer Is the Key to Avoiding Overdiagnosis and Overtreatment. Cancer Epidemiol. Prev. Biomark. 2020, 29, 2463–2474. [Google Scholar] [CrossRef]
- Li, X.; Gu, J.; Zhang, Y.; Feng, S.; Huang, X.; Jiang, Y.; Xia, Y.; Liu, Y.; Yang, X. l-arginine alleviates doxorubicin-induced endothelium-dependent dysfunction by promoting nitric oxide generation and inhibiting apoptosis. Toxicology 2019, 423, 105–111. [Google Scholar] [CrossRef]
- Chinnapaka, S.; Zheng, G.; Chen, A.; Munirathinam, G. Nitro aspirin (NCX4040) induces apoptosis in PC3 metastatic prostate cancer cells via hydrogen peroxide (H(2)O(2))-mediated oxidative stress. Free Radic. Biol. Med. 2019, 143, 494–509. [Google Scholar] [CrossRef]
- Mokbel, K.; Wazir, U.; Mokbel, K. Chemoprevention of Prostate Cancer by Natural Agents: Evidence from Molecular and Epidemiological Studies. Anticancer Res. 2019, 39, 5231–5259. [Google Scholar] [CrossRef] [Green Version]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Xie, Z.; Zhou, S.; Li, Y.; Zhou, Y.; Sun, M. Nitric Oxide (NO) and NO Synthases (NOS)-Based Targeted Therapy for Colon Cancer. Cancers 2020, 12, 1881. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Oxidative Stress, Diet and Prostate Cancer. World J. Mens. Health 2020. [Google Scholar] [CrossRef]
- Termini, D.; Den Hartogh, D.J.; Jaglanian, A.; Tsiani, E. Curcumin against Prostate Cancer: Current Evidence. Biomolecules 2020, 10, 1536. [Google Scholar] [CrossRef]
- Pascual-Geler, M.; Robles-Fernandez, I.; Monteagudo, C.; Lopez-Guarnido, O.; Rodrigo, L.; Gálvez-Ontiveros, Y.; Cozar, J.M.; Rivas, A.; Alvarez-Cubero, M.J. Impact of oxidative stress SNPs and dietary antioxidant quality score on prostate cancer. Int. J. Food Sci. Nutr. 2020, 71, 500–508. [Google Scholar] [CrossRef]
- Jahan, N.; Chowdhury, A.; Li, T.; Xu, K.; Wei, F.; Wang, S. Neferine improves oxidative stress and apoptosis in benign prostate hyperplasia via Nrf2-ARE pathway. Redox Rep. 2021, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mitsunari, K.; Miyata, Y.; Matsuo, T.; Mukae, Y.; Otsubo, A.; Harada, J.; Kondo, T.; Matsuda, T.; Ohba, K.; Sakai, H. Pharmacological Effects and Potential Clinical Usefulness of Polyphenols in Benign Prostatic Hyperplasia. Molecules 2021, 26, 450. [Google Scholar] [CrossRef]
- Hartman, W.J.; Torre, P.M.; Prior, R.L. Dietary citrulline but not ornithine counteracts dietary arginine deficiency in rats by increasing splanchnic release of citrulline. J. Nutr. 1994, 124, 1950–1960. [Google Scholar] [CrossRef] [Green Version]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Bénazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Hayashi, T.; Ochiai, M.; Maeda, M.; Yamaguchi, T.; Ina, K.; Kuzuya, M. Oral supplementation with a combination of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem. Biophys. Res. Commun. 2014, 454, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Morita, M.; Hayashi, T.; Kamimura, A. The effects on plasma L-arginine levels of combined oral L-citrulline and L-arginine supplementation in healthy males. Biosci. Biotechnol. Biochem. 2017, 81, 372–375. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.G.; Allkanjari, O.; Vitalone, A.; Busetto, G.M.; Cai, T.; Larganà, G.; Russo, G.I.; Magri, V.; Perletti, G.; della Cuna, F.S.R.; et al. Nutraceutical treatment and prevention of benign prostatic hyperplasia and prostate cancer. Arch. Ital. Urol. Androl. 2019, 91, 139–152. [Google Scholar] [CrossRef]
- Takashima, J. Prostate Massage Apparatus. U.S. Patent 8,182,503, 22 May 2012. [Google Scholar]
- Feliciano, A.E. Repetitive prostate massage. In Textbook of Prostatitis; Isis Medical Media: Oxford, UK, 1999; pp. 311–318. [Google Scholar]
- Casey, S.C.; Amedei, A.; Aquilano, K.; Azmi, A.S.; Benencia, F.; Bhakta, D.; Bilsland, A.E.; Boosani, C.S.; Chen, S.; Ciriolo, M.R.; et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin. Cancer Biol. 2015, 35, S199–S223. [Google Scholar] [CrossRef] [PubMed]
- Krakowsky, Y.; Morgentaler, A. Risk of Testosterone Flare in the Era of the Saturation Model: One More Historical Myth. Eur. Urol. Focus 2019, 5, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Morgentaler, A.; Traish, A. The History of Testosterone and the Evolution of its Therapeutic Potential. Sex. Med. Rev. 2020, 8, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Khera, M. Controversies with testosterone therapy. Can. J. Urol. 2020, 27, 20–23. [Google Scholar]
- Yassin, A.; AlRumaihi, K.; Alzubaidi, R.; Alkadhi, S.; Al Ansari, A. Testosterone, testosterone therapy and prostate cancer. Aging Male Off. J. Int. Soc. Study Aging Male 2019, 22, 219–227. [Google Scholar] [CrossRef]
- Kaplan, A.L.; Hu, J.C.; Morgentaler, A.; Mulhall, J.P.; Schulman, C.C.; Montorsi, F. Testosterone Therapy in Men with Prostate Cancer. Eur. Urol. 2016, 69, 894–903. [Google Scholar] [CrossRef] [Green Version]
- Langer, R.D. The evidence base for HRT: What can we believe? Climacteric 2017, 20, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Dehaini, H.; Fardoun, M.; Abou-Saleh, H.; El-Yazbi, A.; Eid, A.A.; Eid, A.H. Estrogen in vascular smooth muscle cells: A friend or a foe? Vascul. Pharmacol. 2018, 111, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Fardoun, M.; Dehaini, H.; Shaito, A.; Mesmar, J.; El-Yazbi, A.; Badran, A.; Beydoun, E.; Eid, A.H. The hypertensive potential of estrogen: An untold story. Vascul. Pharmacol. 2020, 124, 106600. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, K.L.; Ozemek, C.; Kohrt, W.M.; Blatchford, P.J.; Moreau, K.L. Vascular dysfunction across the stages of the menopausal transition is associated with menopausal symptoms and quality of life. Menopause 2018, 25, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Somani, Y.B.; Pawelczyk, J.A.; De Souza, M.J.; Kris-Etherton, P.M.; Proctor, D.N. Aging women and their endothelium: Probing the relative role of estrogen on vasodilator function. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H395–H404. [Google Scholar] [CrossRef]
- Novella, S.; Dantas, A.P.; Hermenegildo, C.; Hellsten, Y. Regulatory Mechanisms of Estrogen on Vascular Ageing. Oxid. Med. Cell. Longev. 2019, 2019, 4859082. [Google Scholar] [CrossRef] [PubMed]
- Hackett, G. Should All Men with Type 2 Diabetes Be Routinely Prescribed a Phosphodiesterase Type 5 Inhibitor? World J. Mens. Health 2020, 38, 271–284. [Google Scholar] [CrossRef]
- Braun, M.; Wassmer, G.; Klotz, T.; Reifenrath, B.; Mathers, M.; Engelmann, U. Epidemiology of erectile dysfunction: Results of the “Cologne Male Survey”. Int. J. Impot. Res. 2000, 12, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Peters, T.J. The Relationship between LUTS and sexual function. Prostate Cancer Prostatic Dis. 2001, 4, S2–S6. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Ioakeimidis, N.; Terentes-Printzios, D.; Stefanadis, C. The Triad: Erectile Dysfunction—Endothelial Dysfunction - Cardiovascular Disease. Curr. Pharm. Des. 2008, 14, 3700–3714. [Google Scholar] [CrossRef]
- Traish, A.M.; Miner, M.M.; Morgentaler, A.; Zitzmann, M. Testosterone deficiency. Am. J. Med. 2011, 124, 578–587. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Jackson, G.; Stefanadis, C.; Montorsi, P. Erectile dysfunction in the cardiovascular patient. Eur. Heart J. 2013, 34, 2034–2046. [Google Scholar] [CrossRef] [Green Version]
- Iacono, F.; Prezioso, D.; Ruffo, A.; Illiano, E.; Romis, L.; Di Lauro, G.; Romeo, G.; Amato, B. Testosterone deficiency causes penile fibrosis and organic erectile dysfunction in aging men. Evaluating association among Age, TDS and ED. BMC Surg. 2012, 12, S24. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, S.; Tsounapi, P.; Shimizu, T.; Honda, M.; Inoue, K.; Dimitriadis, F.; Saito, M. Lower urinary tract symptoms, benign prostatic hyperplasia/benign prostatic enlargement and erectile dysfunction: Are these conditions related to vascular dysfunction? Int. J. Urol. 2014, 21, 856–864. [Google Scholar] [CrossRef] [Green Version]
- Di Sante, S.; Mollaioli, D.; Gravina, G.L.; Ciocca, G.; Limoncin, E.; Carosa, E.; Lenzi, A.; Jannini, E.A. Epidemiology of delayed ejaculation. Transl. Androl. Urol. 2016, 5, 541–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, K.E.; Boedtkjer, D.B.; Forman, A. The link between vascular dysfunction, bladder ischemia, and aging bladder dysfunction. Ther. Adv. Urol. 2017, 9, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, A.; Hiramatsu, I.; Aoki, Y.; Shimoyama, H.; Mizuno, T.; Nozaki, T.; Shirai, M.; Kobayashi, K.; Kumamoto, Y.; Horie, S. Atherosclerosis is associated with erectile function and lower urinary tract symptoms, especially nocturia, in middle-aged men. Prostate Int. 2017, 5, 65–69. [Google Scholar] [CrossRef] [PubMed]
- de la Taille, A.; Descazeaud, A.; Robert, G. How to prevent LUTS due to BPH development and progression. Prog. Urol. 2018, 28, 821–829. [Google Scholar] [CrossRef]
- Matsui, S.; Kajikawa, M.; Maruhashi, T.; Iwamoto, Y.; Oda, N.; Kishimoto, S.; Hashimoto, H.; Hidaka, T.; Kihara, Y.; Chayama, K.; et al. Endothelial dysfunction, abnormal vascular structure and lower urinary tract symptoms in men and women. Int. J. Cardiol. 2018, 261, 196–203. [Google Scholar] [CrossRef] [Green Version]
- McMahon, C.G. Current diagnosis and management of erectile dysfunction. Med. J. Aust. 2019, 210, 469–476. [Google Scholar] [CrossRef]
- Calogero, A.E.; Burgio, G.; Condorelli, R.A.; Cannarella, R.; La Vignera, S. Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male 2019, 22, 12–19. [Google Scholar] [CrossRef]
- Chung, E. Sexuality in Ageing Male: Review of Pathophysiology and Treatment Strategies for Various Male Sexual Dysfunctions. Med. Sci. 2019, 7, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, L.; Chuang, Y.-C.; Liu, S.-P.; Lee, K.-S.; Yoo, T.K.; Chu, R.; Sumarsono, B.; Wang, J.-Y. Effect of lower urinary tract symptoms on the quality of life and sexual function of males in China, Taiwan, and South Korea: Subgroup analysis of a cross-sectional, population-based study. Low. Urin. Tract Symptoms 2019, 11, O78–O84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gur, S.; Alzweri, L.; Yilmaz-Oral, D.; Kaya-Sezginer, E.; Abdel-Mageed, A.B.; Dick, B.; Sikka, S.C.; Volkan Oztekin, C.; Hellstrom, W.J.G. Testosterone positively regulates functional responses and nitric oxide expression in the isolated human corpus cavernosum. Andrology 2020. [Google Scholar] [CrossRef] [PubMed]
- Sihotang, R.C.; Alvonico, T.; Taher, A.; Birowo, P.; Rasyid, N.; Atmoko, W. Premature ejaculation in patients with lower urinary tract symptoms: A systematic review. Int. J. Impot. Res. 2020. [Google Scholar] [CrossRef]
- Leong, D.P.; Fradet, V.; Shayegan, B.; Duceppe, E.; Siemens, R.; Niazi, T.; Klotz, L.; Brown, I.; Chin, J.; Lavallee, L.; et al. Cardiovascular Risk in Men with Prostate Cancer: Insights from the RADICAL PC Study. J. Urol. 2020, 203, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Haider, K.S.; Haider, A.; Saad, F.; Doros, G.; Hanefeld, M.; Dhindsa, S.; Dandona, P.; Traish, A. Remission of type 2 diabetes following long-term treatment with injectable testosterone undecanoate in patients with hypogonadism and type 2 diabetes: 11-year data from a real-world registry study. Diabetes. Obes. Metab. 2020, 22, 2055–2068. [Google Scholar] [CrossRef]
- Diaconu, R.; Donoiu, I.; Mirea, O.; Bălşeanu, T.A. Testosterone, cardiomyopathies, and heart failure: A narrative review. Asian J. Androl. 2021. [Google Scholar] [CrossRef]
- Liang, G.; Song, Y.; Liu, L.; Zhou, K.; Tian, J.; Li, J.; Shi, H.; Zhu, Q.; Wang, J.; Zheng, J.; et al. Association of hypogonadism symptoms and serum hormones in aging males. Andrologia 2021, e14013. [Google Scholar] [CrossRef]
- Passos, G.R.; Ghezzi, A.C.; Antunes, E.; de Oliveira, M.G.; Mónica, F.Z. The Role of Periprostatic Adipose Tissue on Prostate Function in Vascular-Related Disorders. Front. Pharmacol. 2021, 12, 626155. [Google Scholar] [CrossRef]
- Pastuszak, A.W.; Kohn, T.P.; Estis, J.; Lipshultz, L.I. Low Plasma Testosterone Is Associated with Elevated Cardiovascular Disease Biomarkers. J. Sex. Med. 2017, 14, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, F.E., 3rd; Al Mheid, I.; Eapen, D.J.; Hayek, S.S.; Sher, S.; Martin, G.S.; Quyyumi, A.A. Low testosterone in men predicts impaired arterial elasticity and microvascular function. Int. J. Cardiol. 2015, 194, 94–99. [Google Scholar] [CrossRef]
- Neuzillet, Y.; Raynaud, J.-P.; Dreyfus, J.-F.; Radulescu, C.; Rouanne, M.; Schneider, M.; Krish, S.; Rouprêt, M.; Drouin, S.J.; Comperat, E.; et al. Aggressiveness of Localized Prostate Cancer: The Key Value of Testosterone Deficiency Evaluated by Both Total and Bioavailable Testosterone: AndroCan Study Results. Horm. Cancer 2019, 10, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, S.; Robuffo, I.; Caruso, M.; Giambuzzi, G.; Ferri, D.; Militello, A.; Toniato, E. Metabolic Alterations, Aggressive Hormone-Naïve Prostate Cancer and Cardiovascular Disease: A Complex Relationship. Medicina 2019, 55, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smentoch, J.; Szade, J.; Żaczek, A.J.; Eltze, E.; Semjonow, A.; Brandt, B.; Bednarz-Knoll, N. Low numbers of vascular vessels correlate to progression in Hormone-Naïve prostate carcinomas undergoing radical prostatectomy. Cancers 2019, 11, 1356. [Google Scholar] [CrossRef] [Green Version]
- Haga, N.; Akaihata, H.; Hata, J.; Aikawa, K.; Yanagida, T.; Matsuoka, K.; Koguchi, T.; Hoshi, S.; Ogawa, S.; Kataoka, M.; et al. The association between local atherosclerosis of the prostatic artery and benign prostatic enlargement in humans: Putative mechanism of chronic ischemia for prostatic enlargement. Prostate 2018, 78, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Tsounapi, P.; Oikawa, R.; Shimizu, S.; Honda, M.; Sejima, T.; Kinoshita, Y.; Tomita, S. Prostatic ischemia induces ventral prostatic hyperplasia in the SHR; possible mechanism of development of BPH. Sci. Rep. 2014, 4, 3822. [Google Scholar] [CrossRef] [Green Version]
- Gat, Y.; Goren, M. Benign Prostatic Hyperplasia: Long-term follow-up of prostate volume reduction after sclerotherapy of the internal spermatic veins. Andrologia 2018, 50. [Google Scholar] [CrossRef]
- Felix-Patrício, B.; Miranda, A.F.; Medeiros, J.L.J.; Gallo, C.B.M.; Gregório, B.M.; Souza, D.B.; Costa, W.S.; Sampaio, F.J.B. The prostate after castration and hormone replacement in a rat model: Structural and ultrastructural analysis. Int. Braz. J. Urol. 2017, 43, 957–965. [Google Scholar] [CrossRef] [Green Version]
- Angrimani, D.S.R.; Francischini, M.C.P.; Brito, M.M.; Vannucchi, C.I. Prostatic hyperplasia: Vascularization, hemodynamic and hormonal analysis of dogs treated with finasteride or orchiectomy. PLoS ONE 2020, 15, e0234714. [Google Scholar] [CrossRef]
- Yoon, S.; Alfajaro, M.M.; Cho, K.-O.; Choi, U.-S.; Je, H.; Jung, J.; Jang, Y.; Choi, J. Perfusion change in benign prostatic hyperplasia before and after castration in a canine model: Contrast enhanced ultrasonography and CT perfusion study. Theriogenology 2020, 156, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kok, V.C.; Hsiao, Y.-H.; Horng, J.-T.; Wang, K.-L. Association Between Erectile Dysfunction and Subsequent Prostate Cancer Development: A Population-Based Cohort Study with Double Concurrent Comparison Groups. Am. J. Mens. Health 2018, 12, 1492–1502. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.S.; Kim, S.W.; Sohn, D.W. Association between Nocturnal Frequency and Erectile Function in Eugonadal Men with Benign Prostatic Obstruction: A Cross Sectional Study. World J. Mens. Health 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canguven, O.; Talib, R.A.; El-Ansari, W.; Shamsoddini, A.; Salman, M.; Al-Ansari, A. RigiScan data under long-term testosterone therapy: Improving long-term blood circulation of penile arteries, penile length and girth, erectile function, and nocturnal penile tumescence and duration. Aging Male Off. J. Int. Soc. Study Aging Male 2016, 19, 215–220. [Google Scholar] [CrossRef]
- Xiong, W.; Kong, X.; Jiang, J.; Yang, Z.; Jiang, R. Low androgen status inhibits erectile function by inducing eNOS uncoupling in rat corpus cavernosum. Andrology 2020, 8, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.-H.; Park, S.-H.; Song, K.-M.; Ghatak, K.; Limanjaya, A.; Ryu, D.-S.; Ock, J.; Hong, S.-S.; Ryu, J.-K.; Suh, J.-K. Penile erection induces angiogenic, survival, and antifibrotic signals: Molecular events associated with penile erection induced by cavernous nerve stimulation in mice. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2016, 23, 614–622. [Google Scholar] [CrossRef] [Green Version]
- DeMartino, A.W.; Kim-Shapiro, D.B.; Patel, R.P.; Gladwin, M.T. Nitrite and nitrate chemical biology and signalling. Br. J. Pharmacol. 2019, 176, 228–245. [Google Scholar] [CrossRef] [Green Version]
- Gantner, B.N.; LaFond, K.M.; Bonini, M.G. Nitric oxide in cellular adaptation and disease. Redox Biol. 2020, 34, 101550. [Google Scholar] [CrossRef]
- Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.M.; Ramasamy, R.; Arora, H. Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics. Vaccines 2021, 9, 94. [Google Scholar] [CrossRef]
- Reckelhoff, J.F.; Kellum, J.A.; Blanchard, E.J.; Bacon, E.E.; Wesley, A.J.; Kruckeberg, W.C. Changes in nitric oxide precursor, L-arginine, and metabolites, nitrate and nitrite, with aging. Life Sci. 1994, 55, 1895–1902. [Google Scholar] [CrossRef]
- Moretto, J.; Guglielmetti, A.S.; Tournier-Nappey, M.; Martin, H.; Prigent-Tessier, A.; Marie, C.; Demougeot, C. Effects of a chronic L-arginine supplementation on the arginase pathway in aged rats. Exp. Gerontol. 2017, 90, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Klawitter, J.; Hildreth, K.L.; Christians, U.; Kohrt, W.M.; Moreau, K.L. A relative L-arginine deficiency contributes to endothelial dysfunction across the stages of the menopausal transition. Physiol. Rep. 2017, 5, e13409. [Google Scholar] [CrossRef]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef]
- Hayashi, T.; Yano, K.; Matsui-Hirai, H.; Yokoo, H.; Hattori, Y.; Iguchi, A. Nitric oxide and endothelial cellular senescence. Pharmacol. Ther. 2008, 120, 333–339. [Google Scholar] [CrossRef]
- Roddam, A.W.; Allen, N.E.; Appleby, P.; Key, T.J. Endogenous sex hormones and prostate cancer: A collaborative analysis of 18 prospective studies. J. Natl. Cancer Inst. 2008, 100, 170–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melegh, Z.; Oltean, S. Targeting angiogenesis in prostate cancer. Int. J. Mol. Sci. 2019, 20, 2676. [Google Scholar] [CrossRef] [Green Version]
- Soni, Y.; Softness, K.; Arora, H.; Ramasamy, R. The Yin Yang Role of Nitric Oxide in Prostate Cancer. Am. J. Mens. Health 2020, 14, 1557988320903191. [Google Scholar] [CrossRef]
- Seabra, A.B.; Durán, N. Nitric oxide donors for prostate and bladder cancers: Current state and challenges. Eur. J. Pharmacol. 2018, 826, 158–168. [Google Scholar] [CrossRef]
- Kim, J.; Barsoum, I.B.; Loh, H.; Paré, J.-F.; Siemens, D.R.; Graham, C.H. Inhibition of hypoxia-inducible factor 1α accumulation by glyceryl trinitrate and cyclic guanosine monophosphate. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.G.; Jin, L.; Tian, Z.; Wang, J.; Yang, Y.; Liu, J.F.; Chen, Y.; Hu, C.H.; Chen, T.Y.; Zhao, Y.R.; et al. Nitric oxide inhibits autophagy and promotes apoptosis in hepatocellular carcinoma. Cancer Sci. 2019, 110, 1054–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, N.; Yamada, Y.; Takayama, K.I.; Fujimura, T.; Takahashi, S.; Kume, H.; Inoue, S. Androgen-responsive tripartite motif 36 enhances tumor-suppressive effect by regulating apoptosis-related pathway in prostate cancer. Cancer Sci. 2018, 109, 3840–3852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Wang, S.; Qin, C.; Bao, M.; Cheng, G.; Liu, B.; Shao, P.; Lv, Q.; Song, N.; Hua, L.; et al. TRIM36, a novel androgen-responsive gene, enhances anti-androgen efficacy against prostate cancer by inhibiting MAPK/ERK signaling pathways article. Cell Death Dis. 2018, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Mandell, M.A.; Saha, B.; Thompson, T.A. The Tripartite Nexus: Autophagy, Cancer, and Tripartite Motif-Containing Protein Family Members. Front. Pharmacol. 2020, 11, 308. [Google Scholar] [CrossRef]
- Markman, J.L.; Porritt, R.A.; Wakita, D.; Lane, M.E.; Martinon, D.; Noval Rivas, M.; Luu, M.; Posadas, E.M.; Crother, T.R.; Arditi, M. Loss of testosterone impairs anti-tumor neutrophil function. Nat. Commun. 2020, 11, 1613. [Google Scholar] [CrossRef]
- Malkin, C.J.; Pugh, P.J.; Morris, P.D.; Kerry, K.E.; Jones, R.D.; Jones, T.H.; Channer, K.S. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart 2004, 90, 871–876. [Google Scholar] [CrossRef]
- Mancini, A.; Leone, E.; Festa, R.; Grande, G.; Silvestrini, A.; De Marinis, L.; Pontecorvi, A.; Maira, G.; Littarru, G.P.; Meucci, E. Effects of testosterone on antioxidant systems in male secondary hypogonadism. J. Androl. 2008, 29, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Popp Marin, D.; Paola Bolin, A.; De Cassia Macedo Dos Santos, R.; Curi, R.; Otton, R. Testosterone suppresses oxidative stress in human neutrophils. Cell Biochem. Funct. 2010, 28, 394–402. [Google Scholar] [CrossRef]
- Tsikas, D.; Kinzel, M. Associations between asymmetric dimethylarginine (ADMA), nitrite-dependent renal carbonic anhydrase activity, and plasma testosterone levels in hypogonadal men. Hell. J. Cardiol. 2018, 59, 201–206. [Google Scholar] [CrossRef]
- Aminuddin, A.; Salamt, N.; Ahmad Fuad, A.F.; Chin, K.-Y.; Ugusman, A.; Soelaiman, I.N.; Wan Ngah, W.Z. Vascular Dysfunction among Malaysian Men with Increased BMI: An Indication of Synergistic Effect of Free Testosterone and Inflammation. Medicina 2019, 55, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, V.E. The Anti-Inflammatory Effects of Testosterone. J. Endocr. Soc. 2019, 3, 91–107. [Google Scholar] [CrossRef] [Green Version]
- Moreau, K.L.; Babcock, M.C.; Hildreth, K.L. Sex differences in vascular aging in response to testosterone. Biol. Sex Differ. 2020, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, V.; Scordo, M.; Yoon, R.S.; Liporace, F.A.; Greene, L.W. Revisiting the role of testosterone: Are we missing something? Rev. Urol. 2017, 19, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kohn, T.P.; Mata, D.A.; Ramasamy, R.; Lipshultz, L.I. Effects of Testosterone Replacement Therapy on Lower Urinary Tract Symptoms: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 69, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Miyake, H.; Ishida, T.; Sumii, K.; Enatsu, N.; Chiba, K.; Matsushita, K.; Fujisawa, M. Improved Lower Urinary Tract Symptoms Associated with Testosterone Replacement Therapy in Japanese Men with Late-Onset Hypogonadism. Am. J. Mens. Health 2018, 12, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, G.; Cipriani, S.; Lotti, F.; Cellai, I.; Comeglio, P.; Boddi, V.; Della, C.P.; Gacci, M.; Serni, S.; Maggi, M.; et al. Testosterone replacement therapy is able to reduce prostate inflammation in men with BPH, metabolic syndrome and hypogonadism: Preliminary results from a randomized placebo-controlled clinical trial. In Endocrine Abstracts; BioScientifica: Gifford, UK, 2019; Volume 63. [Google Scholar]
- Rastrelli, G.; Vignozzi, L.; Maggi, M. Testosterone therapy: A friend or a foe for the aging men with benign prostatic hyperplasia? Asian J. Androl. 2020, 22, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Khaltourina, D.; Matveyev, Y.; Alekseev, A.; Cortese, F.; Ioviţă, A. Aging Fits the Disease Criteria of the International Classification of Diseases. Mech. Ageing Dev. 2020, 189, 111230. [Google Scholar] [CrossRef]
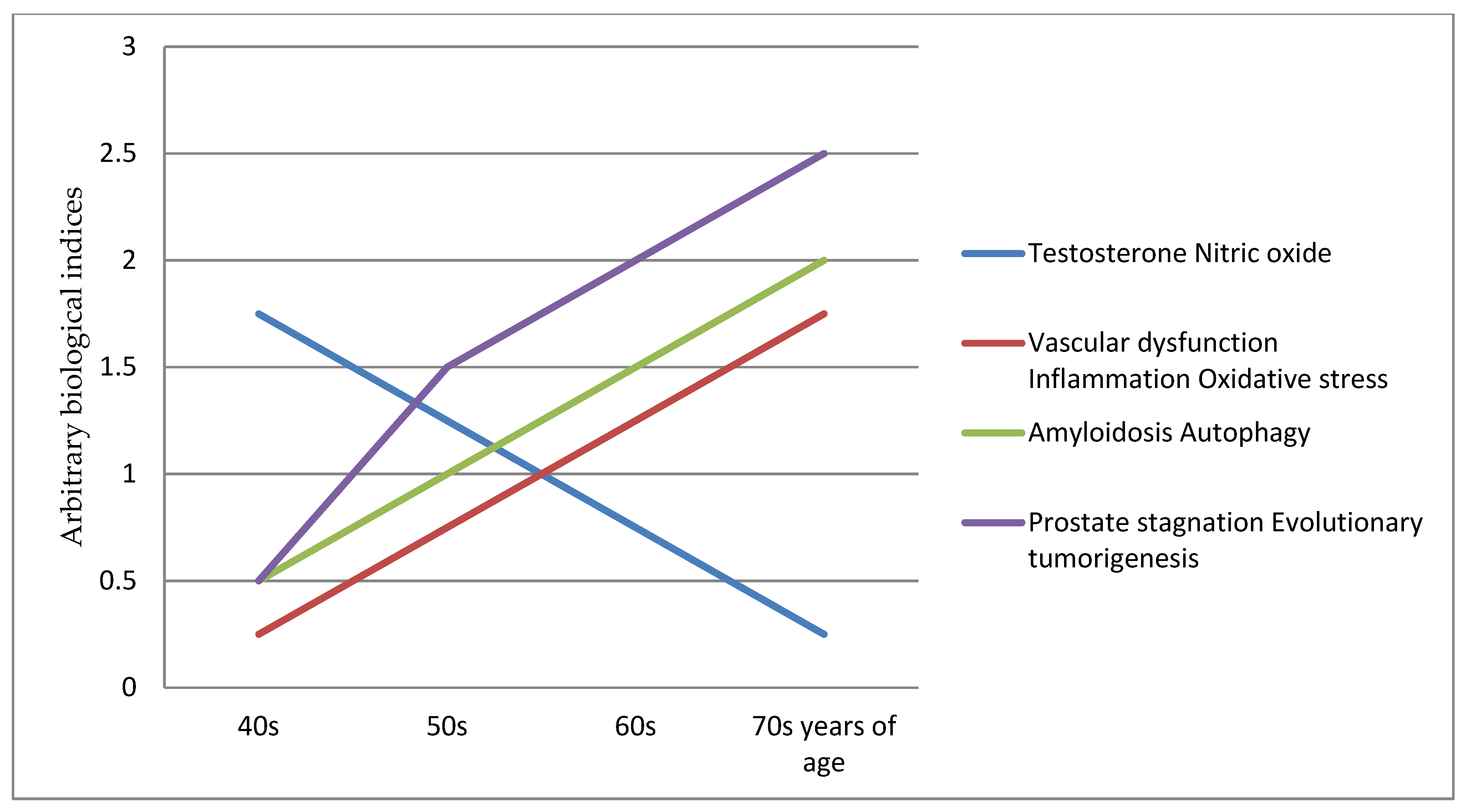
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phua, T.J. The Etiology and Pathophysiology Genesis of Benign Prostatic Hyperplasia and Prostate Cancer: A New Perspective. Medicines 2021, 8, 30. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8060030
Phua TJ. The Etiology and Pathophysiology Genesis of Benign Prostatic Hyperplasia and Prostate Cancer: A New Perspective. Medicines. 2021; 8(6):30. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8060030
Chicago/Turabian StylePhua, Teow J. 2021. "The Etiology and Pathophysiology Genesis of Benign Prostatic Hyperplasia and Prostate Cancer: A New Perspective" Medicines 8, no. 6: 30. https://0-doi-org.brum.beds.ac.uk/10.3390/medicines8060030




