Synthesis of Single-Phase Zeolite A by Coal Gasification Fine Slag from Ningdong and Its Application as a High-Efficiency Adsorbent for Cu2+ and Pb2+ in Simulated Waste Water
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
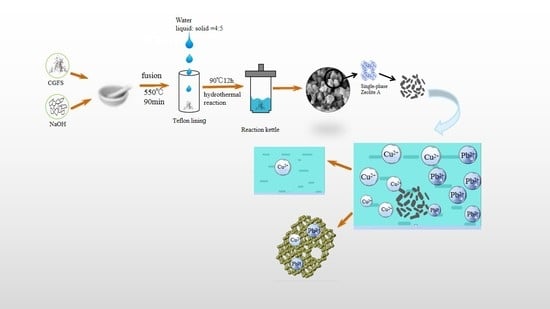
2.2. Zeolite A Synthesis
2.3. Heavy Metal Treatment Experiment
2.4. Characterization Methods
3. Results and Discussion
3.1. Types and Contents of Substances in Coal Gasification Fine Slag
3.2. Zeolite Synthesis and Optimization
3.2.1. Effect of Solid-Phase Alkali Fusion Temperature
3.2.2. Effect of Hydrothermal Reaction Temperature
3.2.3. Effect of NaOH/CGFS (Mass Ratio)
3.2.4. Effect of Hydrothermal Synthesis Time
3.2.5. The Effect of Different Liquid–Solid Ratios
3.3. Characterization of Synthetic Zeolites
3.3.1. Infrared Spectrum
3.3.2. Specific Surface Area and SEM
3.4. Treatment of Heavy Metal Ions in Simulated Waste Water
Effects of Adsorbent Dosage, Constant Temperature Oscillation Time, pH Value of the Solution, and Mixed Ions on Removal Rate of Heavy Metal Ions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xing, Y.; Guo, F.; Xu, M.; Gui, X.; Li, H.; Li, G.; Xia, Y.; Han, H. Separation of unburned carbon from coal fly ash: A review. Powder Technol. 2019, 353, 372–384. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Graham, U.M.; Ward, C.R.; Kostova, I.J.; Maroto-Valer, M.M.; Dai, S. Coal-derived unburned carbons in fly ash: A review. Int. J. Coal Geol. 2017, 179, 11–27. [Google Scholar] [CrossRef]
- Blissett, R.S.; Rowson, N.A. A review of the multi-component utilization of coal fly ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Volli, V.; Shu, C.M. Progressive utilization prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef] [PubMed]
- Höller, H.; Wirsching, U. Zeolite formation from fly ash. Fortschr. Mineral. 1985, 63, 21–43. [Google Scholar]
- Shigemoto, N.; Hayashi, H.; Miyaura, K. Selective formation of Na-X zeolite from coal fly ash by fusion with sodium hydroxide prior to hydrothermal reaction. J. Mater. Sci. 1993, 28, 4781–4786. [Google Scholar] [CrossRef]
- Molina, A.; Poole, C. A comparative study using two methods to produce zeolites from fly ash. Miner. Eng. 2004, 17, 167–173. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Wu, D.; Zhang, Z.; Kong, H. Synthesis of Zeolite/Aluminum Oxide Hydrate from Coal Fly Ash: A New Type of Adsorbent for Simultaneous Removal of Cationic and Anionic Pollutants. Ind. Eng. Chem. Res. 2013, 52, 14890–14897. [Google Scholar] [CrossRef]
- Ren, X.; Xiao, L.; Qu, R.; Liu, S.; Ye, D.; Song, H.-G.; Wu, W.; Zheng, C.; Wu, X.; Gao, X. Synthesis and characterization of a single phase zeolite A using coal fly ash. RSC Adv. 2018, 8, 42200–42209. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Qian, X.; Yuan, P.; Bai, H.; Miki, T.; Men, F.; Li, H.; Nagasaka, T. Green synthesis of zeolite 4A using fly ash fused with synergism of NaOH and Na2CO3. J. Clean. Prod. 2019, 212, 250–260. [Google Scholar] [CrossRef]
- Panitchakarn, P.; Laosiripojana, N.; Viriya-Umpikul, N.; Pavasant, P. Synthesis of high-purity Na-A and Na-X zeolite from coal fly ash. J. Air Waste Manag. Assoc. 2014, 64, 586–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, A.; Sattar, H.; Haider, R.; Munir, S. Synthesis and characterization of pure phase zeolite 4A from coal fly ash. J. Clean. Prod. 2019, 219, 258–267. [Google Scholar] [CrossRef]
- Shoumkova, A.S.; Stoyanova, V. SEM–EDX and XRD characterization of zeolite NaA, synthesized from rice husk and aluminium scrap by different procedures for preparation of the initial hydrogel. J. Porous Mater. 2012, 20, 249–255. [Google Scholar] [CrossRef]
- Tanaka, H.; Fujimoto, S.; Fujii, A.; Hino, A.R.; Kawazoe, T. Microwave Assisted Two-Step Process for Rapid Synthesis of Na−A Zeolite from Coal Fly Ash. Ind. Eng. Chem. Res. 2008, 47, 226–230. [Google Scholar] [CrossRef]
- Wu, D.; Lu, Y.; Kong, H.; Ye, A.C.; Jin, X. Synthesis of Zeolite from Thermally Treated Sediment. Ind. Eng. Chem. Res. 2008, 47, 295–302. [Google Scholar] [CrossRef]
- EL-Naggar, M.R.; EL-Kamash, A.M.; EL-Dessouky, M.I.; Ghonaim, A.K. Two-step method for preparation of NaA-X zeolite blend from fly ash for removal of cesium ions. J. Hazard. Mater. 2008, 154, 963–972. [Google Scholar] [CrossRef]
- Chang, H.-L.; Shih, W.-H. A General Method for the Conversion of Fly Ash into Zeolites as Ion Exchangers for Cesium. Ind. Eng. Chem. Res. 1998, 37, 71–78. [Google Scholar] [CrossRef]
- Murayama, N.; Yamamoto, H.; Shibata, J. Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. Int. J. Miner. Process. 2002, 64, 1–17. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Ojumu, T.V.; Du Plessis, P.W.; Petrik, L.F. Synthesis of zeolite A from coal fly ash using ultrasonic treatment—A replacement for fusion step. Ultrason. Sonochem. 2016, 31, 342–349. [Google Scholar] [CrossRef]
- Ding, L.; Yang, H.; Rahimi, P.; Omotoso, O.; Friesen, W.; Fairbridge, C.; Shi, Y.; Ng, S. Solid transformation of zeolite NaA to sodalite. Microporous Mesoporous Mater. 2010, 130, 303–308. [Google Scholar] [CrossRef]
- Reyes, C.A.R.; Williams, C.D.; Alarcón, O.M.C. Nucleation and growth process of sodalite and cancrinite from kaolinite-rich clay under low-temperature hydrothermal conditions. Mater. Res. 2013, 16, 424–438. [Google Scholar] [CrossRef] [Green Version]
- Rios, C.A.; Williams, C.D.; Fullen, M.A. Nucleation and growth history of zeolite LTA synthesized from kaolinite by two different methods. Appl. Clay Sci. 2009, 42, 446–454. [Google Scholar] [CrossRef]
- Hong, J.L.X.; Maneerung, T.; Koh, S.N.; Kawi, S.; Wang, C.-H. Conversion of Coal Fly Ash into Zeolite Materials: Synthesis and Characterizations, Process Design, and Its Cost-Benefit Analysis. Ind. Eng. Chem. Res. 2017, 56, 11565–11574. [Google Scholar] [CrossRef]
- Barnes, M.C.; Addai-Mensah, J.; Gerson, A.R. The mechanism of the sodalite-to-cancrinite phase transformation in synthetic spent Bayer liquor. Microporous Mesoporous Mater. 1999, 31, 287–302. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Salim, C.; Hinode, H. Synthesis of pure Na-X and Na-A zeolite from bagasse fly ash. Microporous Mesoporous Mater. 2012, 162, 6–13. [Google Scholar] [CrossRef]
- Tanaka, H.; Fujii, A.; Fujimoto, S.; Tanaka, Y. Microwave-Assisted Two-Step Process for the Synthesis of a Single-Phase Na-A Zeolite from Coal Fly Ash. Adv. Powder Technol. 2008, 19, 83–94. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Petrik, L.F.; Gitari, W.M.; Balfour, G.; Hums, E. Optimization of hydrothermal synthesis of pure phase zeolite Na-P1 from South African coal fly ashes. J. Environ. Sci. Health Part A 2012, 47, 337–350. [Google Scholar] [CrossRef] [PubMed]











| Chemical Composition (wt %) | ||||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Na2O | Fe2O3 | MgO | CaO | K2O | TiO2 | Total |
| 54.5 | 15.7 | 1.77 | 7.32 | 2.76 | 10.1 | 3.43 | 1.23 | 96.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, W.; Feng, N.; Zhao, P.; Zhang, S.; Zhang, S.; Lan, L.; Huang, H.; Li, K.; Sun, Y.; Li, Y.; et al. Synthesis of Single-Phase Zeolite A by Coal Gasification Fine Slag from Ningdong and Its Application as a High-Efficiency Adsorbent for Cu2+ and Pb2+ in Simulated Waste Water. ChemEngineering 2020, 4, 65. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering4040065
Ji W, Feng N, Zhao P, Zhang S, Zhang S, Lan L, Huang H, Li K, Sun Y, Li Y, et al. Synthesis of Single-Phase Zeolite A by Coal Gasification Fine Slag from Ningdong and Its Application as a High-Efficiency Adsorbent for Cu2+ and Pb2+ in Simulated Waste Water. ChemEngineering. 2020; 4(4):65. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering4040065
Chicago/Turabian StyleJi, Wenxin, Ning Feng, Pengde Zhao, Shiyue Zhang, Shasha Zhang, Liping Lan, Honglan Huang, Kangning Li, Yonggang Sun, Yuanyuan Li, and et al. 2020. "Synthesis of Single-Phase Zeolite A by Coal Gasification Fine Slag from Ningdong and Its Application as a High-Efficiency Adsorbent for Cu2+ and Pb2+ in Simulated Waste Water" ChemEngineering 4, no. 4: 65. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering4040065