Thermodynamic Design of Organic Rankine Cycle (ORC) Based on Petroleum Coke Combustion
Abstract
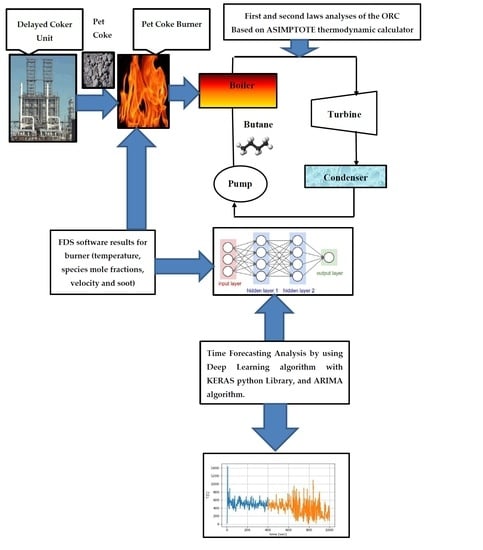
:1. Introduction
Review of the Applications of Deep Learning Analysis of Combustion
2. Materials and Methods
2.1. Fire Dynamic Simulation (FDS) Modeling of the Pet-Coke Burner
2.2. FDS Modelling of the Combustor
2.3. Time Forecasting Calculations by Applying Artificial Intelligence (AI) Algorithms
2.4. Thermodynamic Analysis of the Organic Rankine Cycle
2.5. Computation Structure Process of the Pet-Coke Burner
3. Results
3.1. Fire Dynamics Simulator Software Results for Burner
Grid Sensitivity Study Results
3.2. Time Forecasting Analyses Results for the Calculated Temperature
3.3. Thermodynamics Analysis Results of the Organic Rankine Cycle (ORC)
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| AI | Artificial Intelligence |
| ANN | Artificial Neural Network |
| ARIMA | Auto Regressive Integrated Moving Average |
| CFB | Circulating Fluidized Bed |
| CFD | Computational Fluid Dynamics |
| CNN | Convolutional Neural Networks |
| FCC | Fluid Catalytic Cracking |
| FDS | Fire Dynamics Simulation |
| FFNN | Feed Forward Neural Network |
| FVM | Finite Volume Method |
| HDS | Hydrodesulphurization |
| HSFO | High Sulfur Fuel Oil |
| HRR | Heat Release Rate |
| HRSG | Heat Recovery Steam Generation |
| IGCC | Integrated Gasification Combine Cycle |
| KERAS | Deep learning Python library |
| LES | Large Eddy Simulation |
| LSTM | Long Short Memory |
| ORC | Organic Rankine Cycle |
| SSSF | Steady State Steady Flow |
| Pet Coke | Petroleum Coke |
| RELU | Rectified Linear Unit |
| RTE | Radiation Transport Equation |
| TCNN | Transpose Convolutional Neural Networks |
| Nomenclature | |
| gravity acceleration in [m/s2] | |
| specific enthalpy of the stream in [kJ/kg] | |
| mass flow rate entering to the control volume in [kg/s] | |
| heat intecation rate invested/produced in the control volume in [kW] | |
| power in [kW] | |
| entropy change rate in [kW/K] | |
| specific entropy of the stream in [kJ/(kg K] | |
| temperature in [K] | |
| time in [K] | |
| velocity of the stream in [m/s] | |
| height of the stream in [m] | |
| Subscripts | |
| boiler | |
| condenser | |
| control volume | |
| leaving | |
| Heat Release Rate | |
| leaving | |
| net | |
| pump | |
| turbine | |
| Greek letters | |
| thermal efficency of the Organic Rankine Cycle | |
| density in [kg/m3] | |
References
- Eslami Afrooz, I.E.; Chuan Ching, D.L. A Modified Model for Kinetic Analysis of Petroleum Coke. Int. J. Chem. Eng. 2019, 2034983. [Google Scholar] [CrossRef]
- Gigilio, R. The Power Option; Digital Refining: Croydon, UK, 2019. Available online: https://www.digitalrefining.com/article/1002284/the-power-option#.XwnO7G0zbX4 (accessed on 15 July 2021).
- Ramesh, K. Estimating Delayed Coker Yields; Digital Refining: Croydon, UK, 2020. Available online: https://www.digitalrefining.com/article/1002409/estimating-delayed-coker-yields#.XxLFQm0zbX5 (accessed on 15 July 2021).
- Chang, J.; Wang, G.; Lan, X.; Gao, J.; Zhang, K. Computational Investigation of a Turbulent Fluidized-bed FCC Regenerator. Ind. Eng. Chem. Res. 2013, 52, 4000–4010. [Google Scholar] [CrossRef]
- Grace, J.R.; Taghipour, F. Verification and validation of CFD models and dynamic similarity for fluidized beds. Powder Technol. 2004, 139, 99–110. [Google Scholar] [CrossRef]
- Hamadeh, H.; Toor, S.Y.; Douglas, P.L.; Sarathy, S.M.; Dibble, R.W.; Croiset, E. Techno-Economic Analysis of Pressurized Oxy-Fuel Combustion of Petroleum Coke. Energies 2020, 13, 3463. [Google Scholar] [CrossRef]
- Shen, J.; Schmetz, E.; Stiegel, G.J.; Winslow, J.C.; Kornosky, R.M.; Madden, D.R.; Jain, S.C. Early Entrance Coproduction Plant—The Pathway to the Commercial CTL (Coal-to-Liquids) Fuels Production; Davis, B.H., Occelli, M.L., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2007; Volume 163, pp. 315–325. ISBN 9780444522214. ISSN 0167-2991. [Google Scholar] [CrossRef]
- Lattimer, B.Y.; Hodges, J.L.; Lattimer, A.M. Using machine learning in physics-based simulation of fire. Fire Saf. J. 2020, 114, 102991. [Google Scholar] [CrossRef]
- Hodges, J.L. Predicting Large Domain Multi-Physics Fire Behavior Using Artificial Neural Networks. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2018. [Google Scholar]
- Sun, Y.; Wang, J.; Zhu, W.; Yuan, S.; Hong, Y.; Mannan, M.S.; Wilhite, B. Development of Consequent Models for Three Categories of Fire through Artificial Neural Networks. Ind. Eng. Chem. Res. 2020, 59, 464–474. [Google Scholar] [CrossRef]
- McGrattan, K.B. Fire Dynamics Simulator (Version 5)—Technical Reference Guide Volume 1: Mathematical Model; NIST Special Publication 1018; National Institute of Standards and Technology U.S.: Washington, DC, USA, 2010. [Google Scholar]
- McGrattan, K.B.; Forney, G.P. Fire Dynamics Simulator (Version 5)—User’s Guide; NIST Special Publication 1019; National Institute of Standards and Technology U.S.: Washington, DC, USA, 2010. [Google Scholar]
- McGrattan, K.B. Numerical Simulation of the Caldecott Tunnel Fire, April 1982; NISTIR 7231; National Institute of Standards and Technology, U.S.: Washington, DC, USA, 2005. [Google Scholar]
- Davidy, A. CFD Simulation of Forced Recirculating Fired Heated Reboilers. Processes 2020, 8, 145. [Google Scholar] [CrossRef] [Green Version]
- Magee, J.S. Fluid Catalytic Cracking: Science and Technology, Studies. In Studies in Surface Science and Catalysis; Delmon, B., Yates, J.T., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Pedersen, M.N.; Nielsen, M.; Clausen, S.; Jensen, P.A.; Jensen, L.S.; Johansen, K.D. Imaging of Flames in Cement Kilns to Study the Influence of Different Fuel Types. Energy Fuels 2017, 31, 11424–11438. [Google Scholar] [CrossRef] [Green Version]
- Machine Learning Mastery. Available online: https://machinelearningmastery.com/backtest-machine-learning-models-time-series-forecasting/ (accessed on 15 July 2021).
- Brownlee, J. Deep Learning with Python, Develop Deep Learning Models on Theano and Tensor Flow using KERAS; Version 1.18; Machine Learning Mastery: Vermont Victoria, Australia, 2019. [Google Scholar]
- Brownlee, J. Deep Learning for Time Series Forecasting Predict the Future with MLPs, CNNs and LSTMs in Python; Version 1.6; Machine Learning Mastery: Vermont Victoria, Ausralia, 2019. [Google Scholar]
- Brownlee, J. Machine Learning Mastery with Python: Understand Your Data, Create Accurate Models and Work Projects End-to-End; Version 1.18; Machine Learning Mastery: Vermont Victoria, Ausralia, 2020. [Google Scholar]
- Van Wylen, G.J.; Sonntag, R.E. Fundamentals of Classical Thermodynamics, 3rd ed.; SI Version; John Wiley and Sons Inc: Toronto, ON, Canada, 1985. [Google Scholar]
- ASIMPTOTE. Available online: http://www.asimptote.nl/software/fluidprop/fluidprop-calculator (accessed on 8 October 2020).
- Ahmed, D.F.; Ateya, S.K. Modelling and Simulation of Fluid Catalytic Cracking Unit. J. Chem. Eng. Process Technol. 2016, 7, 308. [Google Scholar] [CrossRef] [Green Version]
- Commandre, J.L.; Salvador, S. Lack of correlation between the properties of a petroleum coke and its behavior during combustion. Fuel Process. Technol. 2005, 86, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Sandmo, T. The Norwegian Emission Inventory 2009: Documentation of Methodologies for Estimating Emissions of Greenhouse Gases and Long-Range Transboundary Air Pollutants; Statistics Norway/Department of Economics, Energy and the Environment Statistics: Oslo, Norway, 2009. [Google Scholar]
- Castelli, A.F.; Elsido, C.; Scaccabarozzi, R.; Nord Lars, O.; Martelli, E. Optimization of Organic Rankine Cycles for Waste Heat Recovery from Aluminum Production Plants. Front. Energy Res. 2019, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Hodnebrog, Ø.; Dalsøren, S.B.; Myhre, G. Lifetimes, direct and indirect radiative forcing, and global warming potentials of ethane (C2H6), propane (C3H8), and butane (C4H10). Atmos. Sci. Lett. 2018, 19, e804. [Google Scholar] [CrossRef]
- Bronicki, L.Y. History of Organic Rankine Cycle systems, in Organic Rankine Cycle (ORC) Power Systems Technologies and Applications. In Woodhead Publishing Series in Energy; Macchi, E., Astolfi, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]














| Point | Pressure [kPa] | Temperature [°C] | Enthalpy, h [kJ/kg] | Entropy, s [kJ/(kg K)] |
|---|---|---|---|---|
| 1 | 3905 | 164.2 | 770.5 | 2.3404 |
| 2 | 238 | 58.7 | 655.1 | 2.3550 |
| 3 | 238 | 24.5 | 232.7 | 0.9476 |
| 4 | 3905 | 25.9 | 239.6 | 0.9477 |
| Heat/Power | Value [kW] |
|---|---|
| 16,161 | |
| −12,856 | |
| 3305 | |
| 3513 | |
| −207.4 | |
| 3305 |
| Component | Value [kW/K] |
|---|---|
| 0.444 | |
| 0.277 | |
| 0.003 | |
| 5.442 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidy, A. Thermodynamic Design of Organic Rankine Cycle (ORC) Based on Petroleum Coke Combustion. ChemEngineering 2021, 5, 37. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering5030037
Davidy A. Thermodynamic Design of Organic Rankine Cycle (ORC) Based on Petroleum Coke Combustion. ChemEngineering. 2021; 5(3):37. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering5030037
Chicago/Turabian StyleDavidy, Alon. 2021. "Thermodynamic Design of Organic Rankine Cycle (ORC) Based on Petroleum Coke Combustion" ChemEngineering 5, no. 3: 37. https://0-doi-org.brum.beds.ac.uk/10.3390/chemengineering5030037