Advanced Techniques for Skeletal Muscle Tissue Engineering and Regeneration
Abstract
:1. Introduction
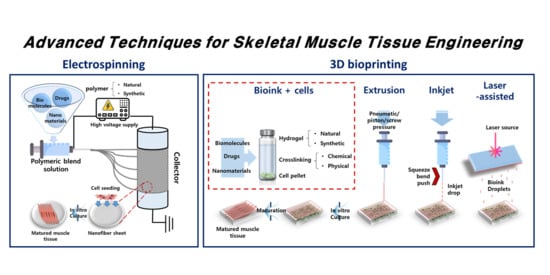
2. Electrospinning
3. Three-Dimensional Bioprinting
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Corona, B.T.; Greising, S.M. Challenges to acellular biological scaffold mediated skeletal muscle tissue regeneration. Biomaterials 2016, 104, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Bach, A. A new approach to tissue engineering of vascularized skeletal muscle. J. Cell. Mol. Med. 2006, 10, 716–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, H.; Zhou, G.-Q. Development and Progress of Engineering of Skeletal Muscle Tissue. Tissue Eng. Part B Rev. 2009, 15, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Ostrovidov, S.; Hosseini, V.; Ahadian, S.; Fujie, T.; Parthiban, S.P.; Ramalingam, M.; Bae, H.; Kaji, H.; Khademhosseini, A. Skeletal Muscle Tissue Engineering: Methods to Form Skeletal Myotubes and Their Applications. Tissue Eng. Part B Rev. 2014, 20, 403–436. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, D.; Horch, R.; Kneser, U.; Beier, J.P. Engineering skeletal muscle tissue – new perspectives in vitro and in vivo. J. Cell. Mol. Med. 2010, 14, 2622–2629. [Google Scholar] [CrossRef] [PubMed]
- Summan, M.; McKinstry, M.; Warren, G.L.; Hulderman, T.; Mishra, D.; Brumbaugh, K.; Luster, M.I.; Simeonova, P.P. Inflammatory Mediators and Skeletal Muscle Injury: A DNA Microarray Analysis. J. Interferon Cytokine Res. 2003, 23, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Gräs, S.; Klarskov, N.; Lose, G. Intraurethral Injection of Autologous Minced Skeletal Muscle: A Simple Surgical Treatment for Stress Urinary Incontinence. J. Urol. 2014, 192, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Choi, C.S.; Chin, S.; Kim, S.; Kawamori, D.; Kurpad, A.J.; Neubauer, N.; Hu, J.; Mootha, V.K.; Kim, Y.-B.; et al. Abnormal glucose homeostasis in skeletal muscle–specific PGC-1α knockout mice reveals skeletal muscle–pancreatic β cell crosstalk. J. Clin. Investig. 2007, 117, 3463–3474. [Google Scholar] [CrossRef] [Green Version]
- Sinacore, D.R.; A Gulve, E. The Role of Skeletal Muscle in Glucose Transport, Glucose Homeostasis and Insulin Resistance: Implications for Physical Therapy. Phys. Ther. 1993, 73, 878–891. [Google Scholar] [CrossRef] [Green Version]
- Sambasivan, R.; Yao, R.; Kissenpfennig, A.; Van Wittenberghe, L.; Paldi, A.; Gayraud, B.; Guenou, H.; Malissen, B.; Tajbakhsh, S.; Galy, A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 2011, 138, 3647–3656. [Google Scholar] [CrossRef] [Green Version]
- Riboldi, S.A.; Sampaolesi, M.; Neuenschwander, P.; Cossu, G.; Mantero, S. Electrospun degradable polyesterurethane membranes: Potential scaffolds for skeletal muscle tissue engineering. Biomaterials 2005, 26, 4606–4615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, Y.C.; Kim, C.; Song, S.-J.; Jun, S.; Kim, C.-S.; Hong, S.W.; Hyon, S.-H.; Han, D.-W.; Oh, J.-W. Ternary Aligned Nanofibers of RGD Peptide-Displaying M13 Bacteriophage/PLGA/Graphene Oxide for Facilitated Myogenesis. Nanotheranostics 2018, 2, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, Y.; Shin, Y.C.; Kim, M.J.; Park, J.H.; Hong, S.W.; Kim, B.; Oh, J.-W.; Park, K.D.; Han, D.-W. In situ forming gelatin/graphene oxide hydrogels for facilitated C2C12 myoblast differentiation. Appl. Spectrosc. Rev. 2016, 51, 527–539. [Google Scholar] [CrossRef]
- Shin, Y.C.; Song, S.-J.; Shin, D.-M.; Oh, J.-W.; Hong, S.W.; Choi, Y.S.; Hyon, S.-H.; Han, D.-W. Nanocomposite scaffolds for myogenesis revisited: Functionalization with carbon nanomaterials and spectroscopic analysis. Appl. Spectrosc. Rev. 2017, 53, 129–156. [Google Scholar] [CrossRef]
- Shin, Y.C.; Kang, S.H.; Lee, J.H.; Kim, B.; Hong, S.W.; Han, D.-W. Three-dimensional graphene oxide-coated polyurethane foams beneficial to myogenesis. J. Biomater. Sci. Polym. Ed. 2017, 29, 762–774. [Google Scholar] [CrossRef]
- McKeon-Fischer, K.D.; Freeman, J. Characterization of electrospun poly(L-lactide) and gold nanoparticle composite scaffolds for skeletal muscle tissue engineering. J. Tissue Eng. Regen. Med. 2010, 5, 560–568. [Google Scholar] [CrossRef]
- McKeon-Fischer, K.D.; Flagg, D.H.; Freeman, J. Coaxial electrospun poly(ε-caprolactone), multiwalled carbon nanotubes, and polyacrylic acid/polyvinyl alcohol scaffold for skeletal muscle tissue engineering. J. Biomed. Mater. Res. Part A 2011, 99, 493–499. [Google Scholar] [CrossRef]
- Mandrycky, C.J.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2015, 34, 422–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrovidov, S.; Salehi, S.; Costantini, M.; Suthiwanich, K.; Ebrahimi, M.; Sadeghian, R.B.; Fujie, T.; Shi, X.; Cannata, S.; Gargioli, C.; et al. 3D Bioprinting in Skeletal Muscle Tissue Engineering. Small 2019, 15, e1805530. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Beier, J.P.; Kneser, U.; Stern-Sträter, J.; Stark, G.B.; Bach, A.D. Y chromosome detection of three-dimensional tissue-engineered skeletal muscle constructs in a syngeneic rat animal model. Cell Transplant. 2004, 13, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, E.; Bianchi, F.; Sleigh, J.N.; George, J.H.; Cader, M.Z.; Cui, Z.; Ye, H. Engineered method for directional growth of muscle sheets on electrospun fibers. J. Biomed. Mater. Res. Part A 2018, 106, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- San Choi, J.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly (epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Zahari, N.K.; Ruszymah, B.H.I.; Chowdhury, S.R. Laminin-Coated Poly(Methyl Methacrylate) (PMMA) Nanofiber Scaffold Facilitates the Enrichment of Skeletal Muscle Myoblast Population. Int. J. Mol. Sci. 2017, 18, 2242. [Google Scholar] [CrossRef] [Green Version]
- Bloise, N.; Berardi, E.; Gualandi, C.; Zaghi, E.; Gigli, M.; Duelen, R.; Ceccarelli, G.; Cortesi, E.E.; Costamagna, D.; Bruni, G.; et al. Ether-Oxygen Containing Electrospun Microfibrous and Sub-Microfibrous Scaffolds Based on Poly(butylene 1,4-cyclohexanedicarboxylate) for Skeletal Muscle Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 3212. [Google Scholar] [CrossRef] [Green Version]
- Abarzúa-Illanes, P.N.; Padilla, C.; Ramos, A.; Isaacs, M.; Ramos-Grez, J.; Olguín, H.C.; Valenzuela, L.M. Improving myoblast differentiation on electrospun poly(ε-caprolactone) scaffolds. J. Biomed. Mater. Res. Part A 2017, 105, 2241–2251. [Google Scholar] [CrossRef]
- Shin, Y.C.; Lee, J.H.; Kim, M.J.; Hong, S.W.; Kim, B.; Hyun, J.K.; Choi, Y.S.; Park, J.-C.; Han, D.-W. Stimulating effect of graphene oxide on myogenesis of C2C12 myoblasts on RGD peptide-decorated PLGA nanofiber matrices. J. Boil. Eng. 2015, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Gilbert-Honick, J.; Ginn, B.; Zhang, Y.; Salehi, S.; Wagner, K.R.; Mao, H.-Q.; Grayson, W.L. Adipose-derived Stem/Stromal Cells on Electrospun Fibrin Microfiber Bundles Enable Moderate Muscle Reconstruction in a Volumetric Muscle Loss Model. Cell Transplant. 2018, 27, 1644–1656. [Google Scholar] [CrossRef] [Green Version]
- Manchineella, S.; Thrivikraman, G.; Khanum, K.K.; Ramamurthy, P.C.; Basu, B.; Govindaraju, T. Pigmented Silk Nanofibrous Composite for Skeletal Muscle Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 1222–1232. [Google Scholar] [CrossRef]
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B. Laser-assisted cell printing: Principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine 2010, 5, 507–515. [Google Scholar] [CrossRef]
- Mozetic, P.; Giannitelli, S.M.; Gori, M.; Trombetta, M.; Rainer, A. Engineering muscle cell alignment through 3D bioprinting. J. Biomed. Mater. Res. Part A 2017, 105, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Arab, W.; Kahin, K.; Khan, Z.; Hauser, C. Exploring Nanofibrous Self-assembling Peptide Hydrogels Using Mouse Myoblast Cells for three-dimensional Bioprinting and Tissue Engineering Applications. Int. J. Bioprinting 2019, 5, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, G. 3D bioprinting of functional cell-laden bioinks and its application for cell-alignment and maturation. Appl. Mater. Today 2020, 19, 100588. [Google Scholar] [CrossRef]
- Lizarribar, A.G.; Fernández-Garibay, X.; Velasco-Mallorquí, F.; Castaño, A.G.; Samitier, J.; Ramón-Azcón, J. Composite Biomaterials as Long-Lasting Scaffolds for 3D Bioprinting of Highly Aligned Muscle Tissue. Macromol. Biosci. 2018, 18, 1800167. [Google Scholar] [CrossRef]
- Kim, W.; Lee, H.; Lee, J.; Atala, A.; Yoo, J.J.; Lee, S.J.; Kim, G. Efficient myotube formation in 3D bioprinted tissue construct by biochemical and topographical cues. Biomaterials 2019, 230, 119632. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Kim, T.G.; Jeong, J.; Yi, H.-G.; Park, J.W.; Hwang, W.; Cho, D.-W. 3D Cell Printing of Functional Skeletal Muscle Constructs Using Skeletal Muscle-Derived Bioink. Adv. Healthc. Mater. 2016, 5, 2636–2645. [Google Scholar] [CrossRef]
- Hassan, M.A.; Yeom, B.Y.; Wilkie, A.; Pourdeyhimi, B.; Khan, S.A. Fabrication of nanofiber meltblown membranes and their filtration properties. J. Membr. Sci. 2013, 427, 336–344. [Google Scholar] [CrossRef]
- Zhou, F.-L.; Gong, R.-H. Manufacturing technologies of polymeric nanofibres and nanofibre yarns. Polym. Int. 2008, 57, 837–845. [Google Scholar] [CrossRef]
- Ondarçuhu, T.; Joachim, C. Drawing a single nanofibre over hundreds of microns. EPL (Europhys. Lett) 1998, 42, 215–220. [Google Scholar] [CrossRef]
- Liu, G.; Qiao, L.; Guo, A. Diblock Copolymer Nanofibers. Macromolecules 1996, 29, 5508–5510. [Google Scholar] [CrossRef]
- Lozano, K.; Sarkar, K. Superfine Fiber Creating Spinneret and Uses Thereof. U.S. Patent 8,231,378, 31 July 2012. [Google Scholar]
- Garg, K.; Bowlin, G.L. Electrospinning jets and nanofibrous structures. Biomicrofluidics 2011, 5, 013403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fu, X.; Liu, J.; Qi, Y.; Li, S.; Wang, H. The involvement of integrin β1 signaling in the migration and myofibroblastic differentiation of skin fibroblasts on anisotropic collagen-containing nanofibers. Biomaterials 2012, 33, 1791–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayati, V.; Altomare, L.; Tanzi, M.C.; Fare’, S. Adipose-derived stem cells could sense the nano-scale cues as myogenic-differentiating factors. J. Mater. Sci. Mater. Electron. 2013, 24, 2439–2447. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Oh, J.-W.; Chung, W.-J.; Heo, K.; Jin, H.-E.; Lee, B.Y.; Wang, E.; Zueger, C.; Wong, W.; Meyer, J.; Kim, C. Biomimetic virus-based colourimetric sensors. Nat. Commun. 2014, 5, 3043. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Mao, C. Biomaterials based on phages and other viruses. Adv. Drug Deliv. Rev. 2019, 145, 1–3. [Google Scholar] [CrossRef]
- Cao, B.; Li, Y.; Yang, T.; Bao, Q.; Yang, M.; Mao, C. Bacteriophage-based biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2019, 145, 73–95. [Google Scholar] [CrossRef]
- Agarwala, S. A Perspective on 3D Bioprinting Technology: Present and Future. Am. J. Eng. Appl. Sci. 2016, 9, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Noguéra, R.; Lejeune, M.; Chartier, T. 3D fine scale ceramic components formed by ink-jet prototyping process. J. Eur. Ceram. Soc. 2005, 25, 2055–2059. [Google Scholar] [CrossRef]
- Nakamura, M.; Nishiyama, Y.; Henmi, C.; Iwanaga, S.; Nakagawa, H.; Yamaguchi, K.; Akita, K.; Mochizuki, S.; Takiura, K. Ink Jet Three-Dimensional Digital Fabrication for Biological Tissue Manufacturing: Analysis of Alginate Microgel Beads Produced by Ink Jet Droplets for Three Dimensional Tissue Fabrication. J. Imaging Sci. Technol. 2008, 52, 60201. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L.P. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Testa, S.; Mozetic, P.; Barbetta, A.; Fuoco, C.; Fornetti, E.; Tamiro, F.; Bernardini, S.; Jaroszewicz, J.; Święszkowski, W.; et al. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 2017, 131, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Helps, T.; Taghavi, M.; Rossiter, J. Thermoplastic electroactive gels for 3D-printable artificial muscles. Smart Mater. Struct. 2019, 28, 085001. [Google Scholar] [CrossRef]
- Merceron, T.K.; Burt, M.; Seol, Y.-J.; Kang, H.-W.; Lee, S.J.; Yoo, J.J.; Atala, A. A 3D bioprinted complex structure for engineering the muscle–tendon unit. Biofabrication 2015, 7, 035003. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.E.P.; Rathbone, C.R.; Wenke, J.C.; Guda, T. Natural polymeric hydrogel evaluation for skeletal muscle tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 672–679. [Google Scholar] [CrossRef]
- Giraud, M.-N.; Ayuni, E.; Cook, S.; Siepe, M.; Carrel, T.P.; Tevaearai, H.T. Hydrogel-based Engineered Skeletal Muscle Grafts Normalize Heart Function Early After Myocardial Infarction. Artif. Organs 2008, 32, 692–700. [Google Scholar] [CrossRef]
- Salimath, A.S.; García, A.J. Biofunctional hydrogels for skeletal muscle constructs. J. Tissue Eng. Regen. Med. 2014, 10, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, Y.; Chun, Y.W.; Crowder, S.W.; Young, P.P.; Park, K.D.; Sung, H. In Situ Crosslinkable Gelatin Hydrogels for Vasculogenic Induction and Delivery of Mesenchymal Stem Cells. Adv. Funct. Mater. 2014, 24, 6771–6781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Thi, P.; Lee, Y.; Nguyen, D.H.; Park, K.D. In situ forming gelatin hydrogels by dual-enzymatic cross-linking for enhanced tissue adhesiveness. J. Mater. Chem. B 2017, 5, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Shin, Y.C.; Lee, J.H.; Jun, S.W.; Kim, C.-S.; Lee, Y.; Park, J.-C.; Lee, S.-H.; Park, K.D.; Han, D.-W. Multiphoton imaging of myogenic differentiation in gelatin-based hydrogels as tissue engineering scaffolds. Biomater. Res. 2016, 20, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Jung, J.P.; Bhuiyan, D.B.; Ogle, B.M. Solid organ fabrication: Comparison of decellularization to 3D bioprinting. Biomater. Res. 2016, 20, 27. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, K.E.; Bang, S.; Noh, I. A desktop multi-material 3D bio-printing system with open-source hardware and software. Int. J. Precis. Eng. Manuf. 2017, 18, 605–612. [Google Scholar] [CrossRef]
- Gao, Q.; He, Y.; Fu, J.-Z.; Liu, A.; Ma, L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 2015, 61, 203–215. [Google Scholar] [CrossRef]
- Chen, S.; Hirota, N.; Okuda, M.; Takeguchi, M.; Kobayashi, H.; Hanagata, N.; Ikoma, T. Microstructures and rheological properties of tilapia fish-scale collagen hydrogels with aligned fibrils fabricated under magnetic fields. Acta Biomater. 2011, 7, 644–652. [Google Scholar] [CrossRef]
- Zawko, S.A.; Schmidt, C.E. Crystal templating dendritic pore networks and fibrillar microstructure into hydrogels. Acta Biomater. 2010, 6, 2415–2421. [Google Scholar] [CrossRef]
- Mredha, T.I.; Guo, Y.Z.; Nonoyama, T.; Nakajima, T.; Kurokawa, T.; Gong, J.P. A Facile Method to Fabricate Anisotropic Hydrogels with Perfectly Aligned Hierarchical Fibrous Structures. Adv. Mater. 2018, 30, 1704937. [Google Scholar] [CrossRef] [PubMed]
- Ungerleider, J.L.; Johnson, T.; Rao, N.; Christman, K.L. Fabrication and characterization of injectable hydrogels derived from decellularized skeletal and cardiac muscle. Methods 2015, 84, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chenl, S.-E.; Gerken, E.; Zhang, Y.; Zhan, M.; Mohan, R.K.; Li, A.S.; Reid, M.B.; Li, Y.-P. Role of TNF-α signaling in regeneration of cardiotoxin-injured muscle. Am. J. Physiol. Physiol. 2005, 289, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Kwee, B.J.; Mooney, D.J. Biomaterials for skeletal muscle tissue engineering. Curr. Opin. Biotechnol. 2017, 47, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.T.; Venkatarama, R.S.; Grasman, J. Recent Trends in Injury Models to Study Skeletal Muscle Regeneration and Repair. Bioengineering 2020, 7, 76. [Google Scholar] [CrossRef]



| Fabrication Technique | Material | Cell | Experimental Method | Fundamental Novelty | Reference |
|---|---|---|---|---|---|
| Electrospinning | PLC/gelatin | NG108-15, C2C12 | In vitro | Neuromuscular interaction | [22] |
| PCL/collagen | hSkMC | In vitro | Nanofiber alignment | [23] | |
| Laminin/PMMA | Extracted human skeletal muscle cell | Ex vivo | Fibroblast contamination prevention | [24] | |
| P(BCE-TECE) | C2C12 | In vitro and in vivo | Suitable mechanical property by the mixture of polymers | [25] | |
| PCL/PLGL-decorin | C2C12 | In vitro | Myogenic inducing effect of decorin | [26] | |
| PLGA-RGD-GO | C2C12 | In vitro | Myogenic inducing effect of GO | [27] | |
| Fibrinogen/alginate | ASC, C2C12 | In vivo and ex vivo | ASC–C2C12 cell–cell interaction | [28] | |
| Silk fibrinogen, Silk/melanin | C2C12 | In vitro | Electroactive and antioxidant nanofiber | [29] | |
| 3D bioprinting | PEG-fibrinogen | C2C12 | In vitro and in vivo | Co-axial needle extruder for high fidelity | [30] |
| Pluronic/alginate | C2C12 | In vitro | Microfabrication with aligned deposition | [31] | |
| CH-01 and CH-02 tetrameric self-assembling peptides | C2C12 | In vitro | Nanofibrous self-assembling peptide for aligned microenvironment | [32] | |
| GelMA | C2C12 | In vitro | Preculture of cell-laden bioink | [33] | |
| GelMA-Alginate/CMC | C2C12 | In vitro | Mechanical stabilization of GelMA-based hydrogel | [34] | |
| Methacrylated dECM/PVA fibrillated | C2C12 | In vitro | Decelluarization, internal fibrous structure | [35] | |
| dECM | C2C12 | In vitro | Decelluarization | [36] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.S.; Lee, S.H.; Park, W.J.; Lee, J.E.; Kim, B.; Han, D.-W. Advanced Techniques for Skeletal Muscle Tissue Engineering and Regeneration. Bioengineering 2020, 7, 99. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering7030099
Kang MS, Lee SH, Park WJ, Lee JE, Kim B, Han D-W. Advanced Techniques for Skeletal Muscle Tissue Engineering and Regeneration. Bioengineering. 2020; 7(3):99. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering7030099
Chicago/Turabian StyleKang, Moon Sung, Seok Hyun Lee, Won Jung Park, Ji Eun Lee, Bongju Kim, and Dong-Wook Han. 2020. "Advanced Techniques for Skeletal Muscle Tissue Engineering and Regeneration" Bioengineering 7, no. 3: 99. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering7030099