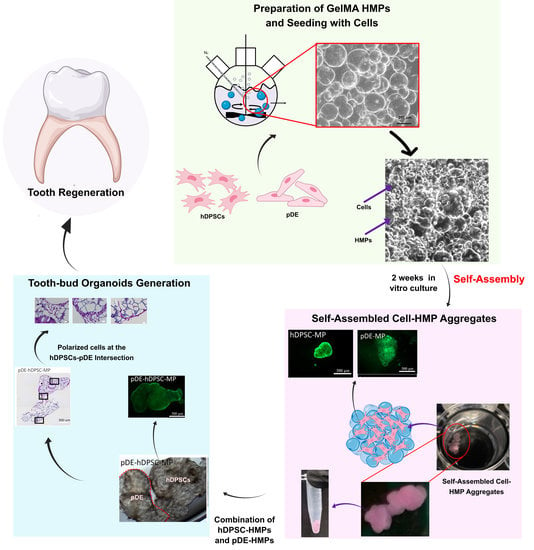
Self-Assembled Hydrogel Microparticle-Based Tooth-Germ Organoids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Methacrylated Gelatin
2.1.1. H-NMR Analysis
2.1.2. ATR-FTIR
2.1.3. Preparation of GelMA Microparticles
2.1.4. In Situ Degradation of GelMA Microparticles
2.2. In Vitro Biocompatibility Tests
2.2.1. Seeding of hDPSCs or HDFs onto GelMA Microparticles
2.2.2. Alamar Blue Cell Viability Assay
2.2.3. Live-Dead Cell Viability Assay
2.2.4. FITC Phalloidin and Hoechst Staining
2.2.5. Tooth Bud Organoid Recombination Testing
3. Results
3.1. 1H-NMR Analysis of GelMA
3.2. Characterization of Human Cell HMP Tissue Constructs
3.2.1. Characterization of Fabricated HMPs
3.2.2. Tooth-Bud Organoid Recombination Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thesleff, I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J. Cell Sci. 2003, 116, 1647–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, E.; Morita, R.; Nakao, K.; Ishida, K.; Nakamura, T.; Takano-Yamamoto, T.; Ogawa, M.; Mizuno, M.; Kasugai, S.; Tsuji, T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 13475–13480. [Google Scholar] [CrossRef] [Green Version]
- Oshima, M.; Mizuno, M.; Imamura, A.; Ogawa, M.; Yasukawa, M.; Yamazaki, H.; Morita, R.; Ikeda, E.; Nakao, K.; Takano-Yamamoto, T.; et al. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS ONE 2011, 6, e21531. [Google Scholar] [CrossRef]
- Nakao, K.; Morita, R.; Saji, Y.; Ishida, K.; Tomita, Y.; Ogawa, M.; Saitoh, M.; Tomooka, Y.; Tsuji, T. The development of a bioengineered organ germ method. Nat. Methods 2007, 4, 227–230. [Google Scholar] [CrossRef]
- Ono, M.; Oshima, M.; Ogawa, M.; Sonoyama, W.; Hara, E.S.; Oida, Y.; Shinkawa, S.; Nakajima, R.; Mine, A.; Hayano, S.; et al. Practical whole-tooth restoration utilizing autologous bioengineered tooth germ transplantation in a postnatal canine model. Sci. Rep. 2017, 7, 44522. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wu, Z.; Fan, Z.; Wu, T.; Wang, J.; Zhang, C.; Wang, S. The cell re-association-based whole-tooth regeneration strategies in large animal, Sus scrofa. Cell Prolif. 2018, 51, e12479. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.-J.; Yoon, K.-S.; Arakaki, M.; Otsu, K.; Fukumoto, S.; Harada, H.; Green, D.W.; Lee, J.-M.; Jung, H.-S. Effective Differentiation of Induced Pluripotent Stem Cells Into Dental Cells. Dev. Dyn. 2019, 248, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Ohazama, A.; Modino, S.; Miletich, I.; Sharpe, P. Stem-cell-based tissue engineering of murine teeth. J. Dent. Res. 2004, 83, 518–522. [Google Scholar] [CrossRef]
- Angelova Volponi, A.; Kawasaki, M.; Sharpe, P. Adult human gingival epithelial cells as a source for whole-tooth bioengineering. J. Dent. Res. 2013, 92, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Hasirci, N.; Kilic, C.; Kömez, A.; Bahcecioglu, G.; Hasirci, V. Hydrogels in Regenerative Medicine. In Gels Handbook Fundamentals, Properties and Applications, U.K.; Demirci, A., Ed.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2016; pp. 1–16. [Google Scholar]
- Kilic Bektas, C.; Hasirci, V. Mimicking corneal stroma using keratocyte-loaded photopolymerizable methacrylated gelatin hydrogels. J. Tissue Eng. Regen. Med. 2018, 12, E1899–E1910. [Google Scholar] [CrossRef]
- Thelu, H.V.P.; Albert, S.K.; Golla, M.; Krishnan, N.; Ram, D.; Srinivasula, S.M.; Varghese, R. Size controllable DNA nanogels from the self-assembly of DNA nanostructures through multivalent host-guest interactions. Nanoscale 2018, 10, 222–230. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, F.; Chen, Y.; Liu, J.; Wang, X.; Chen, A.T.; Deng, G.; Zhang, H.; Liu, J.; Hong, Z.; et al. Targeted Delivery of CRISPR/Cas9-Mediated Cancer Gene Therapy via Liposome-Templated Hydrogel Nanoparticles. Adv. Funct. Mater. 2017, 27, 1703036. [Google Scholar] [CrossRef]
- Molinaro, G.; Leroux, J.-C.; Damas, J.; Adam, A. Biocompatibility of thermosensitive chitosan-based hydrogels: An in vivo experimental approach to injectable biomaterials. Biomaterials 2002, 23, 2717–2722. [Google Scholar] [CrossRef]
- Singh, N.; Ali, A.; Rai, P.; Ghori, I.; Sharma, A.; Malhotra, B.D.; John, R. Dual-modality microfluidic biosensor based on nanoengineered mesoporous graphene hydrogels. Lab Chip 2020, 20, 760–777. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.; Fu, J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Hasirci, V. Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater. Sci. 2020, 8, 438–449. [Google Scholar] [CrossRef]
- Khayat, A.; Monteiro, N.; Smith, E.E.; Pagni, S.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. GelMA-Encapsulated hDPSCs and HUVECs for Dental Pulp Regeneration. J. Dent. Res. 2017, 96, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.E.; Zhang, W.; Schiele, N.R.; Khademhosseini, A.; Kuo, C.K.; Yelick, P.C. Developing a biomimetic tooth bud model. J. Tissue Eng. Regen. Med. 2017, 11, 3326–3336. [Google Scholar] [CrossRef]
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The effect of BMP-mimetic peptide tethering bioinks on the differentiation of dental pulp stem cells (DPSCs) in 3D bioprinted dental constructs. Biofabrication 2020, 12, 035029. [Google Scholar] [CrossRef]
- Smith, E.; Angstadt, S.; Monteiro, N.; Zhang, W.; Khademhosseini, A.; Yelick, P. Bioengineered Tooth Buds Exhibit Features of Natural Tooth Buds. J. Dent. Res. 2018, 97, 1144–1151. [Google Scholar] [CrossRef]
- Smith, E.E.; Yelick, P.C. Bioengineering Tooth Bud Constructs Using GelMA Hydrogel. Methods Mol. Biol. 2019, 1922, 139–150. [Google Scholar] [PubMed]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Bin Sulaiman, S.; Idrus, R.B.H.; Hwei, N.M. Gelatin Microsphere for Cartilage Tissue Engineering: Current and Future Strategies. Polymers 2020, 12, 2404. [Google Scholar] [CrossRef]
- Xing, L.; Sun, J.; Tan, H.; Yuan, G.; Li, J.; Jia, Y.; Xiong, D.; Chen, G.; Lai, J.; Ling, Z.; et al. Covalently polysaccharide-based alginate/chitosan hydrogel embedded alginate microspheres for BSA encapsulation and soft tissue engineering. Int. J. Biol. Macromol. 2019, 127, 340–348. [Google Scholar] [CrossRef]
- McMillan, A.; Nguyen, M.K.; Huynh, C.T.; Sarett, S.M.; Ge, P.; Chetverikova, M.; Nguyen, K.; Grosh, D.; Duvall, C.L.; Alsberg, E. Hydrogel Microspheres For Spatiotemporally Controlled Delivery Of Sirna to stem cells. Tissue Eng. Part A 2014, 20, S107. [Google Scholar]
- Cidade, M.; Ramos, D.J.; Santos, J.; Carrelo, H.; Calero, N.; Borges, J.P. Injectable Hydrogels Based on Pluronic/Water Systems Filled with Alginate Microparticles for Biomedical Applications. Materials 2019, 12, 1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Ma, C.; Xie, X.; Sun, H.; Liu, X. Pulp regeneration in a full-length human tooth root using a hierarchical nanofibrous microsphere system. Acta Biomater. 2016, 35, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Garzón, I.; Martin-Piedra, M.A.; Carriel, V.; Alaminos, M.; Liu, X.; D’Souza, R. Bioactive injectable aggregates with nanofibrous microspheres and human dental pulp stem cells: A translational strategy in dental endodontics. J. Tissue Eng. Regen. Med. 2018, 12, 204–216. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Q.; Xie, L.; Zhang, R.; Qian, R.; Tian, Y.; Chen, G.; Tian, W. hDPSC-laden GelMA microspheres fabricated using electrostatic microdroplet method for endodontic regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111850. [Google Scholar] [CrossRef]
- Abukawa, H.; Zhang, W.; Young, C.S.; Asrican, R.; Vacanti, J.P.; Kaban, L.B.; Troulis, M.J.; Yelick, P.C. Reconstructing mandibular defects using autologous tissue-engineered tooth and bone constructs. J. Oral Maxillofac. Surg. 2009, 67, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Abukawa, H.; Troulis, M.J.; Kaban, L.B.; Vacanti, J.P.; Yelick, P.C. Tissue engineered hybrid tooth-bone constructs. Methods 2009, 47, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xie, L.; Wu, H.; Yang, T.; Zhang, Q.; Tian, Y.; Liu, Y.; Han, X.; Guo, W.; He, M.; et al. Alginate/laponite hydrogel microspheres co-encapsulating dental pulp stem cells and VEGF for endodontic regeneration. Acta Biomater. 2020, 113, 305–316. [Google Scholar] [CrossRef]
- Zhang, W.; Ahluwalia, I.P.; Literman, R.; Kaplan, D.L.; Yelick, P.C. Human dental pulp progenitor cell behavior on aqueous and hexafluoroisopropanol based silk scaffolds. J. Biomed. Mater. Res. A 2011, 97, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Young, C.; Terada, S.; Vacanti, J.; Honda, M.; Bartlett, J.; Yelick, P. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J. Dent. Res. 2002, 81, 695–700. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.-J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef] [Green Version]
- Parker, F.S. Amides and Amines. In Applications of Infrared Spectroscopy in Biochemistry, Biology, and Medicine; Parker, F.S., Ed.; Springer US: Boston, MA, USA, 1971; pp. 165–172. [Google Scholar]
- Kilic Bektas, C.; Kimiz, I.; Sendemir, A.; Hasirci, V.; Hasirci, N. A bilayer scaffold prepared from collagen and carboxymethyl cellulose for skin tissue engineering applications. J. Biomater. Sci.-Polym. Ed. 2018, 29, 1764–1784. [Google Scholar] [CrossRef] [PubMed]
- Bahcecioglu, G.; Hasirci, N.; Bilgen, B.; Hasirci, V. Hydrogels of agarose, and methacrylated gelatin and hyaluronic acid are more supportive for in vitro meniscus regeneration than three dimensional printed polycaprolactone scaffolds. Int. J. Biol. Macromol. 2019, 122, 1152–1162. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [Green Version]
- Shirahama, H.; Lee, B.H.; Tan, L.P.; Cho, N.-J. Precise Tuning of Facile One-Pot Gelatin Methacryloyl (GelMA) Synthesis. Sci. Rep. 2016, 6, 31036. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.L.; Garcia, A.J. Methods for Generating Hydrogel Particles for Protein Delivery. Ann. Biomed. Eng. 2016, 44, 1946–1958. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Wang, Y.; Gou, W.; Yuan, X.; Peng, J.; Guo, Q.; Lu, S. Past, present, and future of microcarrier-based tissue engineering. J. Orthop. Transl. 2015, 3, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neto, M.; Oliveira, M.B.; Mano, J.F. Microparticles in Contact with Cells: From Carriers to Multifunctional Tissue Modulators. Trends Biotechnol. 2019, 37, 1011–1028. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hoopen, S.T.; Zhang, W.; Yi, C.; Yang, W.; Yang, F.; Jansen, J.A.; Walboomers, X.F.; Yelick, P.C. Influence of highly porous electrospun PLGA/PCL/nHA fibrous scaffolds on the differentiation of tooth bud cells in vitro. J. Biomed. Mater. Res. A 2017, 105, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Vazquez, B.; Oreadi, D.; Yelick, P. Decellularized Tooth Bud Scaffolds for Tooth Regeneration. J. Dent. Res. 2017, 96, 516–523. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilic Bektas, C.; Zhang, W.; Mao, Y.; Wu, X.; Kohn, J.; Yelick, P.C. Self-Assembled Hydrogel Microparticle-Based Tooth-Germ Organoids. Bioengineering 2022, 9, 215. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering9050215
Kilic Bektas C, Zhang W, Mao Y, Wu X, Kohn J, Yelick PC. Self-Assembled Hydrogel Microparticle-Based Tooth-Germ Organoids. Bioengineering. 2022; 9(5):215. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering9050215
Chicago/Turabian StyleKilic Bektas, Cemile, Weibo Zhang, Yong Mao, Xiaohuan Wu, Joachim Kohn, and Pamela C. Yelick. 2022. "Self-Assembled Hydrogel Microparticle-Based Tooth-Germ Organoids" Bioengineering 9, no. 5: 215. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering9050215