Automated Bioanalyzer Based on Amperometric Enzymatic Biosensors for the Determination of Ethanol in Low-Alcohol Beers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus and Electrodes
2.2. Reagents and Solutions
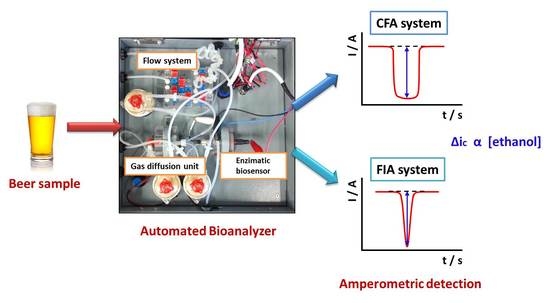
2.3. Automatic Bioanalyzer for Ethanol Determination
2.4. Procedures
2.4.1. Amperometric Detection
Detection with the Semiautomatic Bioanalyzers
Detection with the Automated Bioanalyzer
2.4.2. Analysis of Beer Samples
3. Results and Discussion
3.1. Automated Bioanalyzer Developed for Ethanol Determination
3.1.1. Optimization of Parameters Involved in the Construction of the Automated Bioanalyzer
3.1.2. Automated Bioanalyzer Working as a CFA Analyzer
3.1.3. Automated Bioanalyzer Working as a FIA Analyzer
On-line Analysis
Off-Line Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sohrabvandi, S.; Mortazavian, A.M.; Rezaei, K. Advanced analytical methods for the analysis of chemical and microbiological properties of beer. J. Food Drug Anal. 2011, 19, 202–222. [Google Scholar]
- Tonelli, D. Methods for Determining Ethanol in Beer. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; Chapter 102. [Google Scholar]
- Sohrabvandi, S.; Mousavi, S.M.; Razavi, A.M.; Mortazavian, A.M.; Rezaei, K. Alcohol-free Beer: Methods of Production, Sensorial Defects, and Healthful Effects. Food Rev. Int. 2010, 26, 335–352. [Google Scholar] [CrossRef]
- Catarino, M.; Mendes, A. Non-alcoholic beer—A new industrial process. Sep. Purif. Technol. 2011, 79, 342–351. [Google Scholar] [CrossRef]
- Pinho, O.; Ferreira, I.M.P.L.V.O. Method optimization by solid-phase microextraction in combination with gas chromatography with mass spectrometry for analysis of beer volatile fraction. J. Chromatogr. A 2006, 1121, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Vautz, W.; Baumbach, J.I.; Jung, J. Beer Fermentation Control Using Ion Mobility Spectrometry—Results of a Pilot Study. J. Inst. Brew. 2006, 112, 157–164. [Google Scholar] [CrossRef]
- Ragazzo-Sanchez, J.A.; Chalier, P.; Chevalier, D.; Calderon-Santoyo, M.; Ghommidh, C. Identification of different alcoholic beverages by electronic nose coupled to GC. Sens. Actuators B 2008, 134, 43–48. [Google Scholar] [CrossRef]
- Zapata, J.; Mateo-Vivaracho, L.; Lopez, R.; Ferreira, V. Automated and quantitative headspace in-tube extraction for the accurate determination of highly volatile compounds from wines and beers. J. Chromatogr. A 2012, 1230, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Mazlomi, M.A.; Hedström, M.; Mattiasson, B. Versatile automated continuous flow system (VersAFlo) for bioanalysis and bioprocess control. Sens. Actuators B 2012, 161, 855–861. [Google Scholar] [CrossRef]
- Gallignani, M.; Ayala, C.; Brunetto, M.R.; Burguera, J.L.; Burguera, M. A simple strategy for determining ethanol in all types of alcoholic beverages based on its on-line liquid–liquid extraction with chloroform, using a flow injection system and Fourier transform infrared spectrometric detection in the mid-IR. Talanta 2005, 68, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Iñón, F.A.; Garrigues, S.; de la Guardia, M. Development of a PLS based method for determination of the quality of beers by use of NIR: Spectral ranges and sample-introduction considerations. Anal. Bioanal. Chem. 2005, 382, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Iñón, F.A.; Garrigues, S.; de la Guardia, M. Combination of mid- and near-infrared spectroscopy for the determination of the quality properties of beers. Anal. Chim. Acta 2006, 571, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Godelmann, R.; Steiner, M.; Ansay, B.; Weigel, J.; Krieg, G. Rapid and mobile determination of alcoholic strength in wine, beer and spirits using a flow-through infrared sensor. Chem. Cent. J. 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Blum, P.; Mohr, G.J.; Matern, K.; Reichert, J.; Spichiger-Keller, U.E. Optical alcohol sensor using lipophilic Reichardt’s dyes in polymer membranes. Anal. Chim. Acta 2001, 432, 269–275. [Google Scholar] [CrossRef]
- Pérez-Ruiz, T.; Martínez-Lozano, C.; Tomás, V.; Iniesta, M.T. Flow injection determination of ethanol using online photo-oxidation and spectrophotometric detection. Microchim. Acta 2005, 149, 67–72. [Google Scholar] [CrossRef]
- Choengchan, N.; Mantim, T.; Wilairat, P.; Dasgupta, P.K.; Motomizu, S.; Nacaprocha, D. A membraneless gas diffusion unit: Design and its application to determination of ethanol in liquors by spectrophotometric flow injection. Anal. Chim. Acta 2006, 579, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Pinyou, P.; Youngvises, N.; Jakmunee, J. Flow injection colorimetric method using acidic ceric nitrate as reagent for determination of ethanol. Talanta 2011, 84, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Frank, W.; Humpfer, E.; Schäfer, H.; Keller, S.; Mörtter, M.; Spraul, M. Quality control of beer using high-resolution nuclear magnetic resonance spectroscopy and multivariate analysis. Eur. Food Res. Technol. 2005, 220, 215–221. [Google Scholar] [CrossRef]
- Tarkiainen, V.; Kotiaho, T.; Mattila, I.; Virkajärvi, I.; Aristidou, A.; Ketola, R.A. On-line monitoring of continuous beer fermentation process using automatic membrane inlet mass spectrometric system. Talanta 2005, 65, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Diamnatis, V.; Melidis, P.; Aivasidis, A. Continuous determination of volatile products in anaerobic fermenters by on-line capillary gas chromatography. Anal. Chim. Acta 2006, 573–574, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Vidigal, S.S.M.P.; Tóth, I.V.; Rangel, A.O.S.S. Sequential injection-LOV format for peak height and kinetic measurement modes in the spectrophotometric enzymatic determination of ethanol: Application to different alcoholic beverages. Talanta 2008, 77, 494–499. [Google Scholar] [CrossRef]
- Mohns, J.; Künnecke, W. Flow analysis with membrane separation and time based sampling for ethanol determination in beer and wine. Anal. Chim. Acta 1995, 305, 241–247. [Google Scholar] [CrossRef]
- Peña, N.; Tárrega, R.; Reviejo, A.J.; Pingarrón, J.M. reticulated vitreous carbon-based composite bienzyme electrodes for the determination of alcohols in beer samples. Anal. Lett. 2002, 35, 1931–1944. [Google Scholar] [CrossRef]
- Lapa, R.A.S.; Lima, J.L.F.C.; Pinto, I.V.O.S. Development of a sequential injection analysis system for the simultaneous biosensing of glucose and ethanol in bioreactor fermentation. Food Chem. 2003, 81, 141–146. [Google Scholar] [CrossRef]
- Guzmán-Vázquez de Prada, A.; Peña, N.; Mena, M.L.; Reviejo, A.J.; Pingarrón, J.M. Graphite-Teflon composite bienzyme amperometric biosensors for monitoring of alcohols. Biosens. Bioelectron. 2003, 18, 1279–1288. [Google Scholar] [CrossRef]
- Valach, M.; Katrlík, J.; Strurdík, E.; Gemeiner, P. Ethanol Gluconobacter biosensor designed for flow injection analysis: Application in ethanol fermentation off-line monitoring. Sens. Actuators B 2009, 138, 581–586. [Google Scholar] [CrossRef]
- Reviejo García, A.J.; Pingarrón Carrazón, J.M.; Campuzano Ruiz, S.; Gamella Caballo, M.; García-Echave, V.V.; Manso Lorenzo, J.; Guzmán Vázquez de Prada, A.; Ferrero Martín, F.J.; Campo Rodríguez, J.; Valledor Llopis, M. Biosensor amperométrico desechable, método de fabricación del mismo y método de determinación de la presencia de analitos en alimentos. PTC/ES2009/000381, 18 March 2010. [Google Scholar]
- Hansen, E.H.; Miró, M. Flow Injection Analysis in Industrial Biotechnology. Encyclopedia of Industrial Biotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Gamella, M.; Campuzano, S.; Manso, J.; González de Rivera, G.; López-Colino, F.; Reviejo, A.J.; Pingarrón, J.M. A novel non-invasive electrochemical biosensing device for in situ determination of the alcohol content in blood by monitoring ethanol in sweat. Anal. Chim. Acta 2014, 806, 1–7. [Google Scholar] [CrossRef] [PubMed]





| Flow Technique | Automatic | Detection | Flow Operation | Sample Pretreatment | LR, % (v/v) | LOD, % (v/v) | RSD, % | Samples/h | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| FIA | No | FTIR spectrometry | Liquid-liquid extraction | None | 0–15 | 0.03 | 1.3 | 25 | [10] | |
| CFA | No | NIR spectroscopy | None | Stirring, filtration | – | – | – | – | [11] | |
| CFA | No | MIR/NIR spectroscopy | None | Stirring, filtration | – | – | – | – | [12] | |
| CFA | Yes | FTIR spectrometry | None | Filtration, ultrasounds | 0–45 | 0.1 | 0.4–47 | – | [13] | |
| CFA | No | VIS reflectance spectroscopy (optical sensor) | GD | Neutralization with alkaline solution | 0 - 20 | 0.1 | 5 | 30 | [14] | |
| FIA | No | VIS spectrophotometry | Photochemical reaction | – | 3 × 10−5–6 × 10−3 | 1 × 10−5 | 1.5 | 60 | [15] | |
| FIA | No | VIS spectrophotometry | GD with membrane | None | 1.0–30 | 0.68 | 0.55 | 18 | [16] | |
| GD without membrane | None | 0.5–30 | 0.27 | 0.50 | 16 | |||||
| FIA | No | Colorimetry (optical sensor) | GD | 1:20 dilution | 0.1–10 | 0.03 | 0.3–1.2 | 20 | [17] | |
| FIA | Yes | NMR spectroscopy | None | Filtration, ultrasounds, 9:10 dilution | – | – | – | 5 | [18] | |
| FIA | Yes | Mass spectrometry | Filtration | – | – | 6 × 10−4 | 1.8–9.8 | 60 | [19] | |
| CFA | Yes | GC-FID | Filtration, dilution with a solution of reagents | None | – | – | 4 | – | [20] | |
| SIA-LOV | No | UV spectrophotometry (enzyme ADH immobilized) | None | 1:400 dilution | 0–0.04 | 3 × 10−3 | 1 | 27 | [21] | |
| CFA | No | Amperometry (enzyme AOD immobilized) | Degassing, GD | None | 0–0.6 (550 µm membrane) 0–7.0 (500 µm membrane) | 1 × 10−4 (1500 µm membrane) | 2 | 30 | [22] | |
| FIA | No | Amperometry (AOD/HRP/Fc biosensor) | None | – | 1.2 × 10−4–1.5 × 10−2 | 5.8 × 10−5 | 5.6 | 24 | [23] | |
| SIA | Yes | Amperometry (enzyme AOD immobilized) | Filtration, dialysis | None | 0.02–3.79 | – | 2.2 | 50 | [24] | |
| FIA | No | Amperometry (AOD/HRP/Fc biosensor) | None | 1:1000 dilution (beers with alcohol) 56:100 (beers without alcohol) | 6.0 × 10−4–2.4 × 10−2 | 6.0 × 10−4 | 2.6–6.0 | 50 | [25] | |
| FIA | No | Amperometry (Gluconobacter oxydans microbial biosensor) | None | Centrifugation, dilution to 6 × 10−3% (v/v) | 6 × 10−5–9 × 10−3 | 2 × 10−5 | – | 20 | [26] | |
| CFA/FIA | Yes (automated) | Amperometry (AOD/HRP/Fc biosensor) | Degassing, GD (if on-line analysis) | None (if on-line analysis) | CFA: | 20 mm GD unit: (6.00–100) × 10−3 | 1.8 × 10−3 | 4.9 | CFA: continuous sampling | This work |
| 14 mm GD unit: (7.8–10) × 10−2 | 2.3 × 10−3 | 3.4 | ||||||||
| GD (if off-line analysis) | Degassing (if off-line analysis) | FIA: | 20 mm GD unit: (2.600–1250) × 10−3 | 7.9 × 10−4 | 2.5 | FIA: 10 | ||||
| 14 mm GD unit: (3.00–125) × 10−2 | 8.9 × 10−3 | 3.1 | ||||||||
| Mode | Sampler | Slope, nA% (v/v)−1 | Intercept, nA | r | LOD, % (v/v) | LOQ, % (v/v) |
|---|---|---|---|---|---|---|
| CFA | 20 mm | (6.98 ± 0.06) × 102 | −0.4 ± 0.4 | 0.9999 | 1.8 × 10−3 | 6.0 × 10−3 |
| 14 mm | (3.00 ± 0.07) × 102 | 0 ± 6 | 0.9988 | 2.3 × 10−2 | 7.8 × 10−2 |
| Beer Type | Sample | [EtOH], % (v/v) | texp | ttab | |
|---|---|---|---|---|---|
| Bioanalyzer | InBea’s Method | ||||
| ≤0.04% | 1 | (0.0070 ± 0.0009) RSDn=3 = 5.0% | (0.0069 ± 0.0008) RSDn=3 = 4.9% | 1.999 | 2.447 |
| 2 | (0.011 ± 0.002) RSDn=3 = 6.7% | (0.0093 ± 0.0008) RSDn=3 = 3.6% | |||
| 3 | (0.030 ± 0.001) RSDn=3 = 2.0% | (0.027 ± 0.001) RSDn=3 = 2.0% | |||
| 4 | (0.015 ± 0.003) RSDn=3 = 9.0% | (0.0135 ± 0.0003) RSDn=3 = 0.8% | |||
| <1.0% | 1 | (0.82 ± 0.02) RSDn=3 = 0.9% | (0.80 ± 0.07) RSDn=3 = 3.7% | ||
| 2 | (0.96 ± 0.02) RSDn=3 = 0.9% | (0.91 ± 0.08) RSDn=3 = 3.3% | |||
| 3 | (0.89 ± 0.02) RSDn=3 = 0.7% | (0.87 ± 0.05) RSDn=3 = 2.2% | |||
| Mode | Sampler | Slope, nA% (v/v)−1 | Intercept, nA | r | LOD, % (v/v) | LOQ, % (v/v) |
|---|---|---|---|---|---|---|
| FIA | 20 mm | (2.54 ± 0.06) × 103 | −3 ± 4 | 0.9987 | 7.9 × 10−4 | 2.6 × 10−3 |
| 14 mm | (7.0 ± 0.2) × 102 | −26 ± 28 | 0.9982 | 8.9 × 10−3 | 3.0 × 10−2 |
| Beer Type | Sample | [EtOH], % (v/v) | texp | ttab | |
|---|---|---|---|---|---|
| Bioanalyzer | Stirred Solutions | ||||
| ≤0.04% | 1 | (0.0109 ± 0.0006) RSDn=22 = 12.5% | (0.011 ± 0.001) RSDn=3 = 5.3% | 0.225 | 2.447 |
| 2 | (0.0133 ± 0.0006) RSDn=45 = 16.1% | (0.0135 ± 0.0007) RSDn=5 = 4.0% | 0.500 | 2.064 | |
| 3 | (0.013 ± 0.001) RSDn=19 = 16.3% | (0.0128 ± 0.0005) RSDn=3 = 3.2% | 0.385 | 2.074 | |
| 4 | (0.0122 ± 0.0009) RSDn=21 = 16.8% | (0.0122 ± 0.0003) RSDn=3 = 1.0% | 0.001 | 2.080 | |
| 5 | (0.0038 ± 0.0004) RSDn=38 = 19.1% | (0.0036 ± 0.0008) RSDn=3 = 9.1% | 0.898 | 2.776 | |
| 6 | (0.0262 ± 0.0006) RSDn=49 = 7.4% | (0.025 ± 0.002) RSDn=5 = 6.3% | 1.585 | 2.571 | |
| <1.0% | 1 | (0.88 ± 0.05) RSDn=18 = 11.8% | (0.92 ± 0.03) RSDn=5 = 2.7% | 1.488 | 2.080 |
| 2 | (0.93 ± 0.03) RSDn=72 = 13.0% | (0.91 ± 0.04) RSDn=5 = 3.9% | 0.938 | 2.160 | |
| Sample | [EtOH], % (v/v) Gas Chromatography | Sample Temperature | [EtOH], % (v/v) Automated Bioanalyzer | texp a | ttab a | texp b | ttab b |
|---|---|---|---|---|---|---|---|
| 1 | 0.005 | RT | (0.005 ± 0.001) RSDn=4 = 12.3% | 0.472 | 3.182 | 1.272 | 2.447 |
| 0 °C | (0.006 ± 0.001) RSDn=4 = 13.0% | 2.067 | |||||
| 2 | 0.004 | RT | (0.0038 ± 0.0008) RSDn=4 = 13.5% | 0.577 | 0.590 | ||
| 0 °C | (0.0037 ± 0.0004) RSDn=4 = 7.8% | 2.263 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, E.; Conzuelo, F.; Ruiz, M.A.; Campuzano, S.; Ruiz-Valdepeñas Montiel, V.; González de Rivera, G.; López-Colino, F.; Reviejo, Á.J.; Pingarrón, J.M. Automated Bioanalyzer Based on Amperometric Enzymatic Biosensors for the Determination of Ethanol in Low-Alcohol Beers. Beverages 2017, 3, 22. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages3020022
Vargas E, Conzuelo F, Ruiz MA, Campuzano S, Ruiz-Valdepeñas Montiel V, González de Rivera G, López-Colino F, Reviejo ÁJ, Pingarrón JM. Automated Bioanalyzer Based on Amperometric Enzymatic Biosensors for the Determination of Ethanol in Low-Alcohol Beers. Beverages. 2017; 3(2):22. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages3020022
Chicago/Turabian StyleVargas, Eva, Felipe Conzuelo, M. Asunción Ruiz, Susana Campuzano, Víctor Ruiz-Valdepeñas Montiel, Guillermo González de Rivera, Fernando López-Colino, Á. Julio Reviejo, and José M. Pingarrón. 2017. "Automated Bioanalyzer Based on Amperometric Enzymatic Biosensors for the Determination of Ethanol in Low-Alcohol Beers" Beverages 3, no. 2: 22. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages3020022