The Quality of Mulberry Juice as Affected by Enzyme Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Juice Extraction
2.3. Analytical Methods
2.3.1. Determination of Juice Yield
- m1: weight of mulberry fruit mash, g
- m2: weight of mulberry juice, g
- C: concentration of soluble solid compounds in the obtained juice, % (w/w)
- w: moisture of initial mulberry mash, %
2.3.2. Measurement of Total Soluble Solids (°Bx) and Titratable Acidity (%)
- N of NaOH = 0.1 M
- Eq.wt. citric acid = 64.04
- mL of sample = 10 mL
2.3.3. Extraction of Total Phenolics
2.3.4. Measurement of Total Phenolic Contents
2.3.5. Determination of Antioxidant Capacities
2.3.6. Measurement of Total Anthocyanin Contents
- A = (A520nm − A700nm)pH 1.0 − (A520nm − A700nm)pH 4.5
- MW (molecular weight) = 449.2 g/mol for cyanidin-3-glucoside
- DF = dilution factor established in D
- l = 1 cm
- ε = 26,900 molar extinction coefficient, in L/mol·cm, for cyanidin-3-glucoside
2.3.7. Measurement of l-Ascorbic Acid Contents
2.4. Statistical Analysis
3. Results and Discussion
3.1. Juice Yield
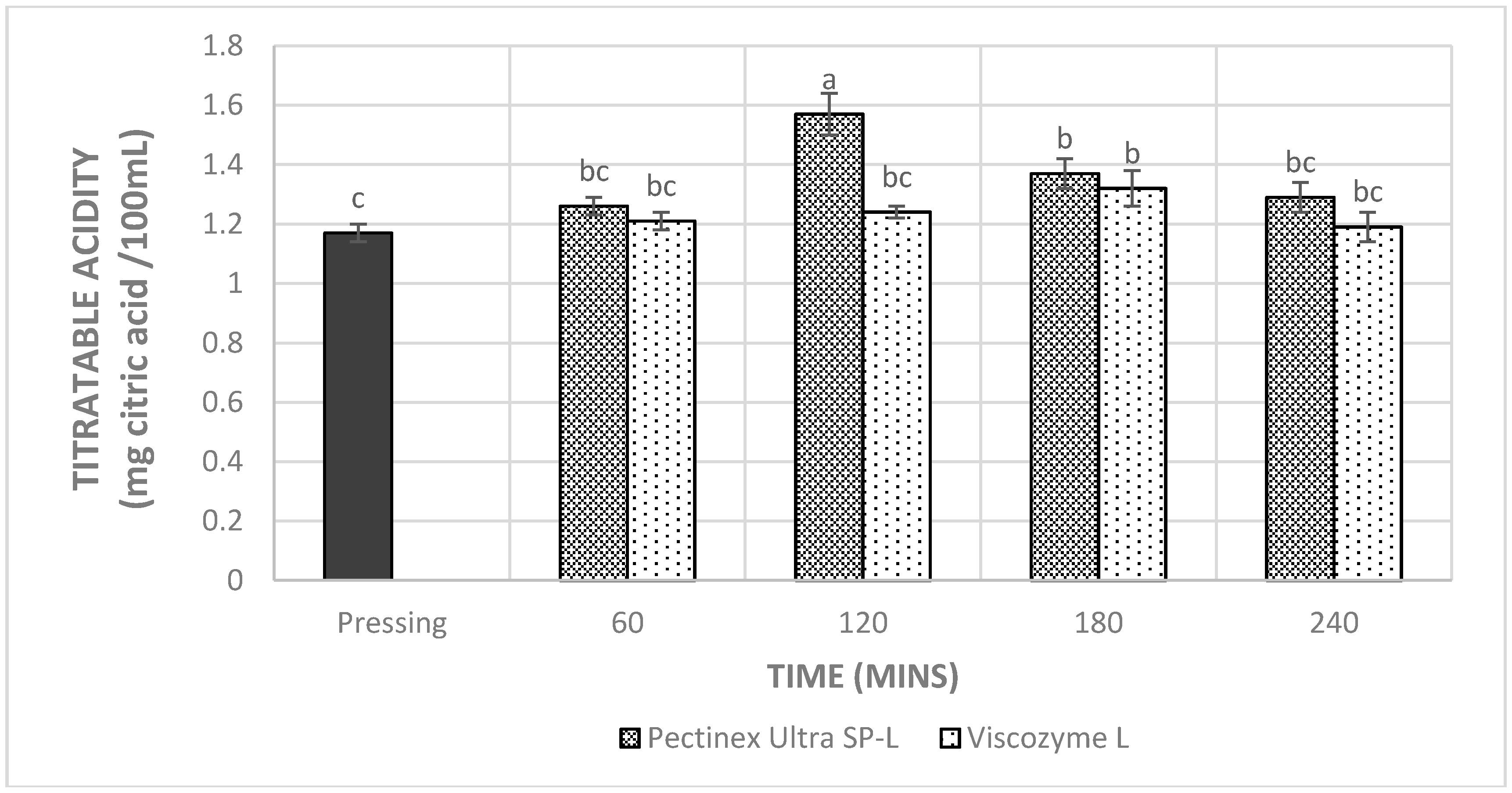
3.2. Total Soluble Solids and Titratable Acidity
3.3. l-Ascorbic Acid Contents
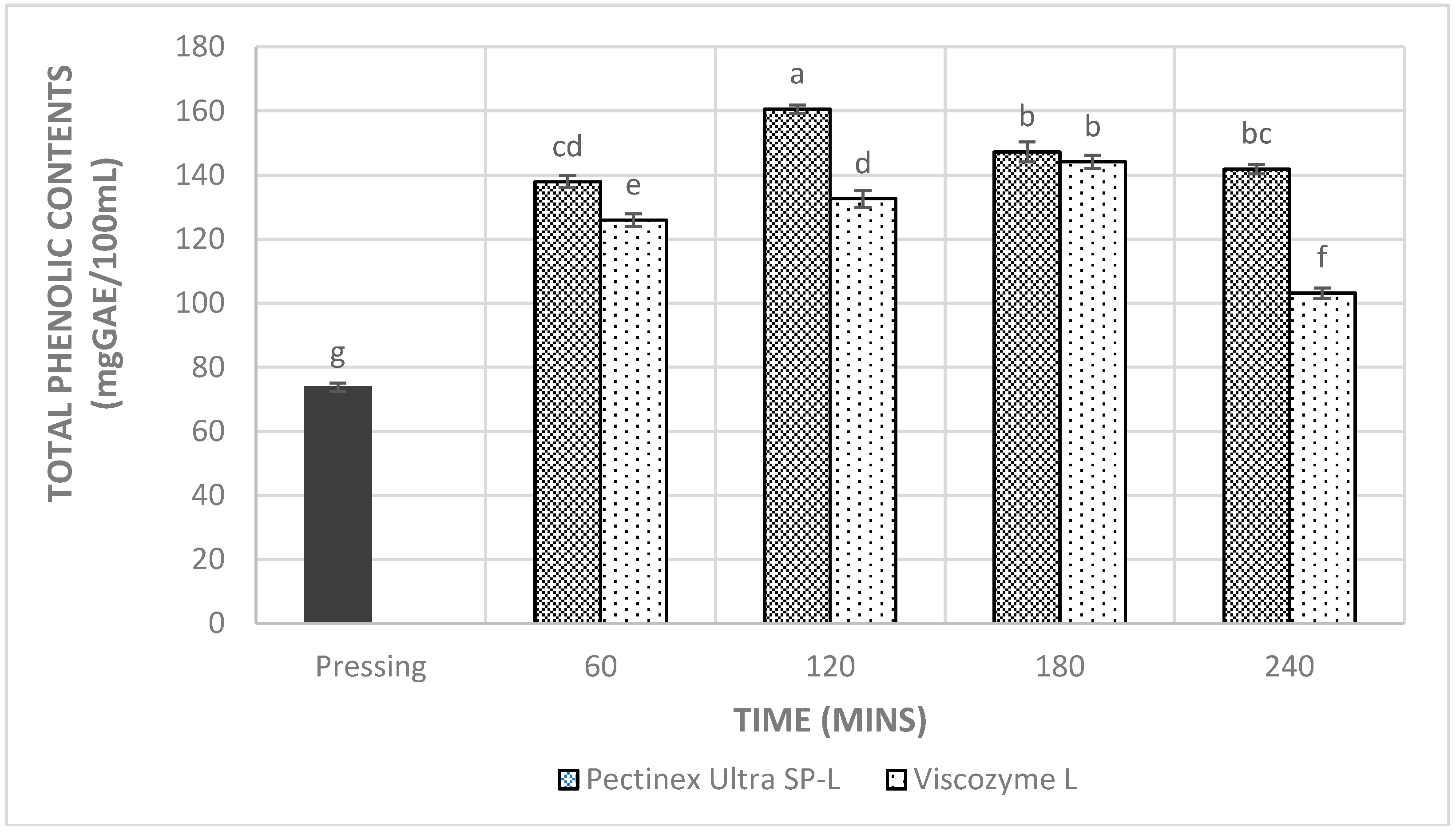
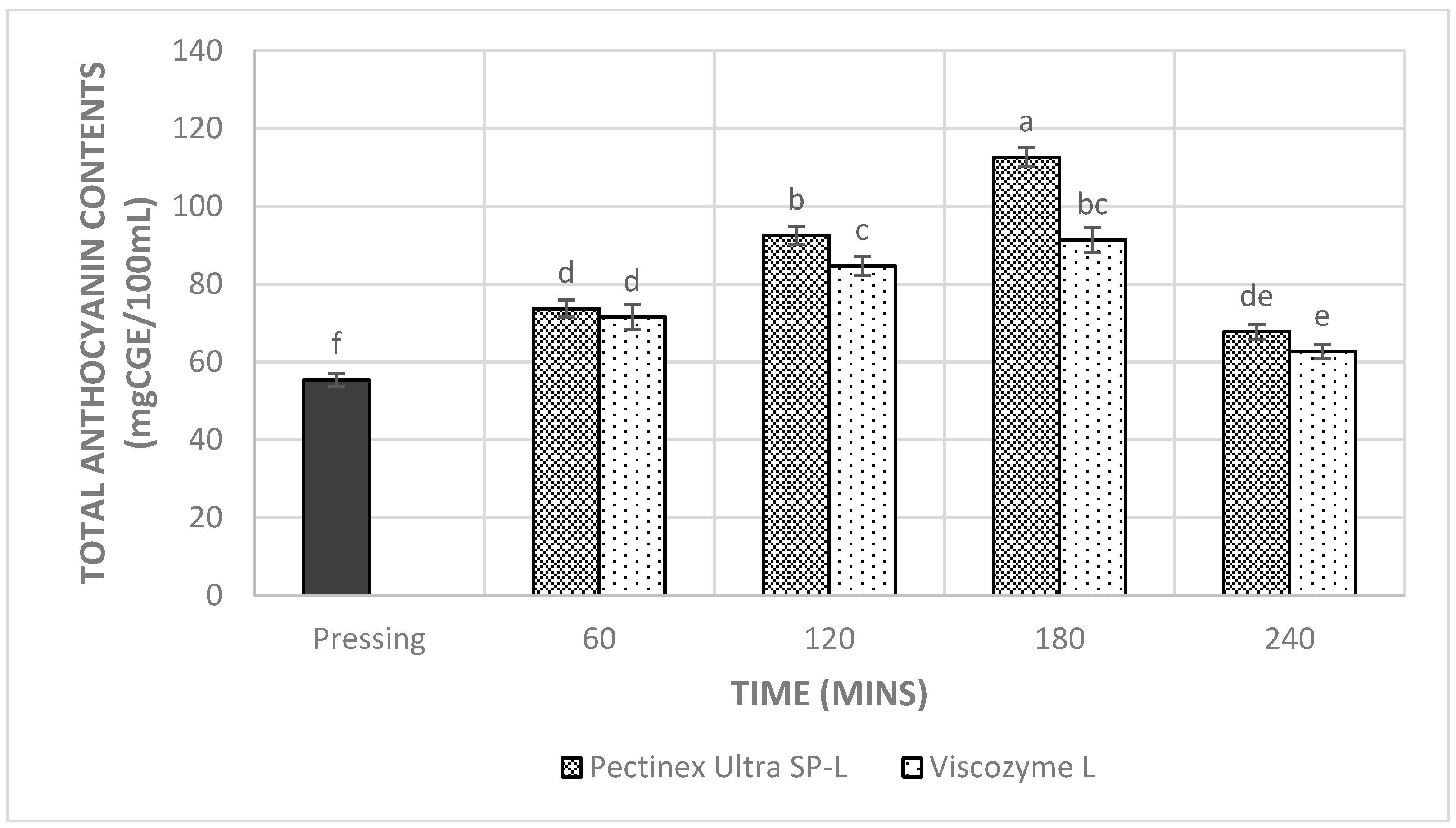
3.4. Total Phenolic Content, Total Anthocyanin Content, and Antioxidant Capacities
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Huang, H.P.; Ou, T.T.; Wang, C.J. Mulberry (Sang Shèn Zǐ) and its bioactive compounds, the chemoprevention effects and molecular mechanisms in vitro and in vivo. J. Tradit. Complement. Med. 2013, 3, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Lee, J.S.; Lee, K.R.; Kim, Y.E.; Baek, N.I.; Hong, E.K. Effects of mulberry ethanol extracts on hydrogen peroxide-induced oxidative stress in pancreatic β-cells. Int. J. Mol. Med. 2014, 33, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Salih, N.D.; Hazir, N.S.M.; Hamid, M.H.A. The effect of mulberry (Morus sp.) tea supplement on acetaminophen induced renal failure in rats. World J. Pharm. Pharm. Sci. 2015, 4, 111–125. [Google Scholar]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A review of characteristic components and health benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Eyduran, S.P.; Ercişli, S.; Akın, M.; Beyhan, Ö.; Geçer, M.K.; Eyduran, E.; Ertürk, Y.E. Organic acids, sugars, vitamin C, antioxidant capacity, and phenolic compounds in fruits of white (Morus alba L.) and black (Morus nigra L.) mulberry genotypes. J. Appl. Bot. Food Qual. 2015, 88, 134–138. [Google Scholar]
- Sreenath, H.; Frey, M.; Radola, B.; Scherz, H. Degradation of a washed carrot preparation by cellulases and pectinases. Biotechnol. Bioeng. 1984, 26, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Landbo, A.K.; Meyer, A.S. Effects of different enzymatic maceration treatments on enhancement of anthocyanins and other phenolics in black currant juice. Innov. Food Sci. Emerg. Technol. 2004, 5, 503–513. [Google Scholar] [CrossRef]
- Lee, J.; Wrolstad, R. Extraction of anthocyanins and polyphenolics from blueberry processing waste. J. Food Sci. 2004, 69, 564–573. [Google Scholar] [CrossRef]
- Wang, W.D.; Xu, S.Y.; Jin, M.K. Effects of different maceration enzymes on yield, clarity and anthocyanin and other polyphenol contents in blackberry juice. Int. J. Food Sci. Technol. 2009, 44, 2342–2349. [Google Scholar] [CrossRef]
- Sun, T.; Tang, J.; Powers, J.R. Effect of pectolytic enzyme preparations on the phenolic composition and antioxidant activity of asparagus juice. J. Agric. Food Chem. 2005, 53, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.; Dang, Q.T. Application of hydrolytic enzymes for improvement of red dragon fruit juice processing. Asia-Pac. Conf. Chem. Ecol. 2016, 4, 1–4. [Google Scholar]
- Versari, A.; Biesenbruch, S.; Barbanti, D.; Farnell, P.; Galassi, S. Effects of pectolytic enzymes on selected phenolic compounds in strawberry and raspberry juices. Food Res. Int. 1997, 30, 811–817. [Google Scholar] [CrossRef]
- Pricelius, S.; Murkovic, M.; Souter, P.; Guebitz, G.M. Substrate specificities of glycosidases from Aspergillus species pectinase preparations on elderberry anthocyanins. J. Agric. Food Chem. 2009, 57, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; Donato, L.; Drioli, E. Ultrafiltration of kiwifruit juice: Operating parameters, juice quality and membrane fouling. J. Food Eng. 2007, 79, 613–621. [Google Scholar] [CrossRef]
- Munoz, O.; Sepulveda, M.; Schwartz, M. Effects of enzymatic treatment on anthocyanic pigments from grapes skin from Chilean wine. Food Chem. 2004, 87, 487–490. [Google Scholar] [CrossRef]
- Lee, W.; Yusof, S.; Hamid, N.; Baharin, B. Optimizing conditions for hot water extraction of banana juice using response surface methodology (RSM). J. Food Eng. 2006, 75, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.S. Standard Solutions and Titratable Acidity. In Food Analysis Laboratory Manual, 2nd ed.; Springer: New York, NY, USA, 2003; Chapter 12; p. 602. [Google Scholar]
- Vinson, J.A.; Hao, Y.; Su, X.; Zubik, L. Phenol antioxidant quantity and quality in foods: Vegetables. J. Agric. Food Chem. 1998, 46, 3630–3634. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization of red radish anthocyanins. J. Food Sci. 1996, 61, 322–326. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, M.M.R.; Hosain, M.M. Analysis of vitamin C (ascorbic acid) contents in various fruits and vegetables by UV-spectrophotometry. Bangladesh J. Sci. Ind. Res. 2007, 42, 417–424. [Google Scholar] [CrossRef]
- Huynh, N.K.; Nguyen, H.V. Effects of juice processing on oxalate contents in Carambola juice products. Plant Foods Hum. Nutr. 2017, 72, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.H.; Savage, G. Oxalate contents of kiwifruit (Actinidia deliciosa) juice extracted by pressing or enzyme extraction. J. Food Agric. Environ. 2013, 11, 228–230. [Google Scholar]
- Sun, T.; Powers, J.R.; Tang, J. Effect of enzymatic macerate treatment on rutin content, antioxidant activity, yield, and physical properties of asparagus juice. J. Food Sci. 2007, 72, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Vohra, P.; Chopra, S.; Tewari, R. Applications of pectinases in the commercial sector: A review. Bioresour. Technol. 2001, 77, 215–227. [Google Scholar] [CrossRef]
- Lee, W.C.; Yusof, S.A.L.M.A.H.; Hamid, N.S.A.; Baharin, B.S. Optimizing conditions for enzymatic clarification of banana juice using response surface methodology (RSM). J. Food Eng. 2006, 73, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Joshi, V.K.; Parmar, M.; Rana, N. Purification and characterization of pectinase produced from apple pomace and evaluation of its efficacy in fruit juice extraction and clarification. Indian J. Nat. Prod. Resour. 2011, 2, 189–197. [Google Scholar]
- Yusof, S.; Ibrahim, N. Quality of Soursop juice after pectinase enzyme treatment. Food Chem. 1994, 51, 83–88. [Google Scholar] [CrossRef]
- Akesowan, A.; Choonhahirun, A. Effect of enzyme treatment on guava juice production using response surface methodology. J. Anim. Plant Sci. 2013, 23, 114–120. [Google Scholar]
- Oszmiański, J.; Wojdyło, A.; Kolniak, J. Effect of pectinase treatment on extraction of antioxidant phenols from pomace, for the production of puree-enriched cloudy apple juices. Food Chem. 2011, 127, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, B.R.; Meyer, A.S. Effects of different enzymatic pre-press maceration treatments on the release of phenols into blackcurrant juice. Eur. Food Res. Technol. 2004, 219, 620–629. [Google Scholar] [CrossRef]
- Rinaldi, M.; Caligiani, A.; Borgese, R.; Palla, G.; Barbanti, D.; Massini, R. The effect of fruit processing and enzymatic treatments on pomegranate juice composition, antioxidant activity and polyphenols content. LWT Food Sci. Technol. 2013, 53, 355–359. [Google Scholar] [CrossRef]
- Butnariu, M.; Raba, D.; Grozea, I.; Virteiu, A.; Stef, R. The impact of physical processes and chemicals of the antioxidants (bioactivity compounds). J. Bioequiv. Availab. 2013, 5, e44. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M. Binding of polyphenols to plant cell wall analogues–Part 2: Phenolic acids. Food Chem. 2012, 135, 2287–2292. [Google Scholar] [CrossRef] [PubMed]
- Haglund, A.C. Atsorption of manosubstituted phenols on sephadex G-15. J. Chromatogr. A 1978, 156, 317–322. [Google Scholar] [CrossRef]




| Enzymes | l-Ascorbic Acid Contents (mg/100 mL) | Total Phenolic Contents (mgGAE/100 mL) | Total Anthocyanin Contents (mg Cyanidin-3-Glucoside/100 mL) | |
|---|---|---|---|---|
| Pectinex Ultra SP-L | Antioxidant capacities (%DPPH inhibition) | 0.649 * | 0.837 * | 0.682 * |
| Viscozyme L | 0.465 * | 0.632 * | 0.552 * |
| Yield (%) | Total Soluble Solid (oBrix) | Total Acidity (%) | l-Ascorbic Acid Contents (mg/100 mL) | Total Phenolic Contents (mgGAE/100 mL) | Total Anthocyanin Contents (mg Cyanidin-3-Glucoside/100 mL) | Antioxidant Capacity (%DPPH Inhibition) | |
|---|---|---|---|---|---|---|---|
| Significance | |||||||
| Enzymes | * | * | * | * | * | * | * |
| Incubation time | * | * | * | * | * | * | * |
| Interactions | * | * | * | * | * | * | * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, C.L.; Nguyen, H.V.H. The Quality of Mulberry Juice as Affected by Enzyme Treatments. Beverages 2018, 4, 41. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages4020041
Nguyen CL, Nguyen HVH. The Quality of Mulberry Juice as Affected by Enzyme Treatments. Beverages. 2018; 4(2):41. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages4020041
Chicago/Turabian StyleNguyen, Chi L., and Ha V. H. Nguyen. 2018. "The Quality of Mulberry Juice as Affected by Enzyme Treatments" Beverages 4, no. 2: 41. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages4020041




