Comparative Study of a Cell-Based and Electrochemical Biosensor for the Rapid Detection of 2,4,6-Trichloroanisole in Barrel Water Extracts
Abstract
:1. Introduction
- To be able to detect the presence of 2,4,6-TCA below or above the cut-off concentration of 2.5 ng/L.
- To be operable in a rapid and cost-effective manner.
- To become the basis, within the framework of further development, of a high throughput device that can be used in line with the production chain.
2. Materials and Methods
2.1. Reagents
2.2. Sample Source, Composition, and Processing
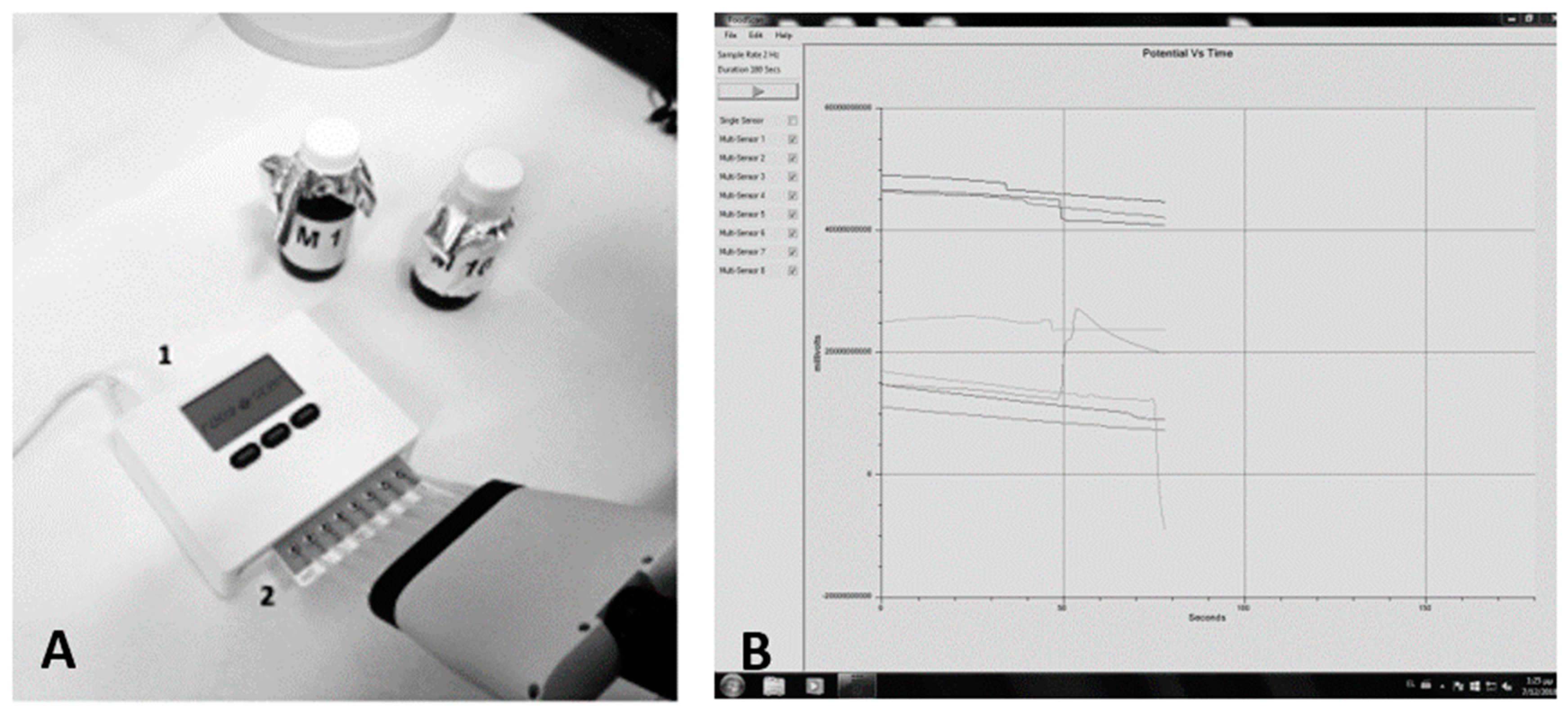
2.3. Biosensor Device
2.4. Assay Setup
2.4.1. Potentiometry Approach
Cell Culture and Manufacturing of Biorecognition Elements
Spiking Haloanisole-Free Water Extract Samples
Assay Procedure
2.4.2. Cyclic Voltammetry Approach
Sample Preparation
Electrochemical Measurements
Calibration Plot and the Interpretation of Results
2.5. Statistical Design
3. Results
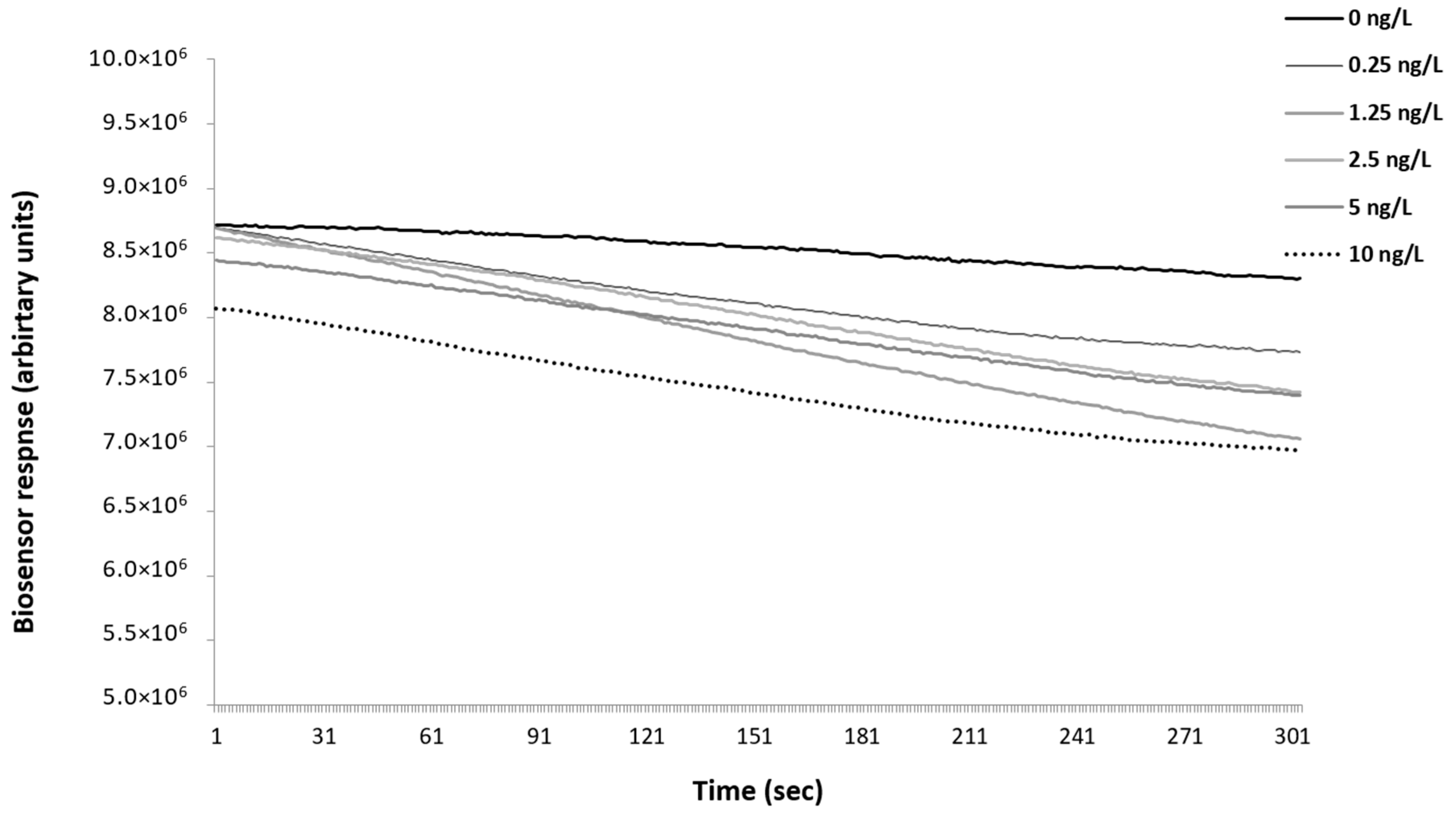
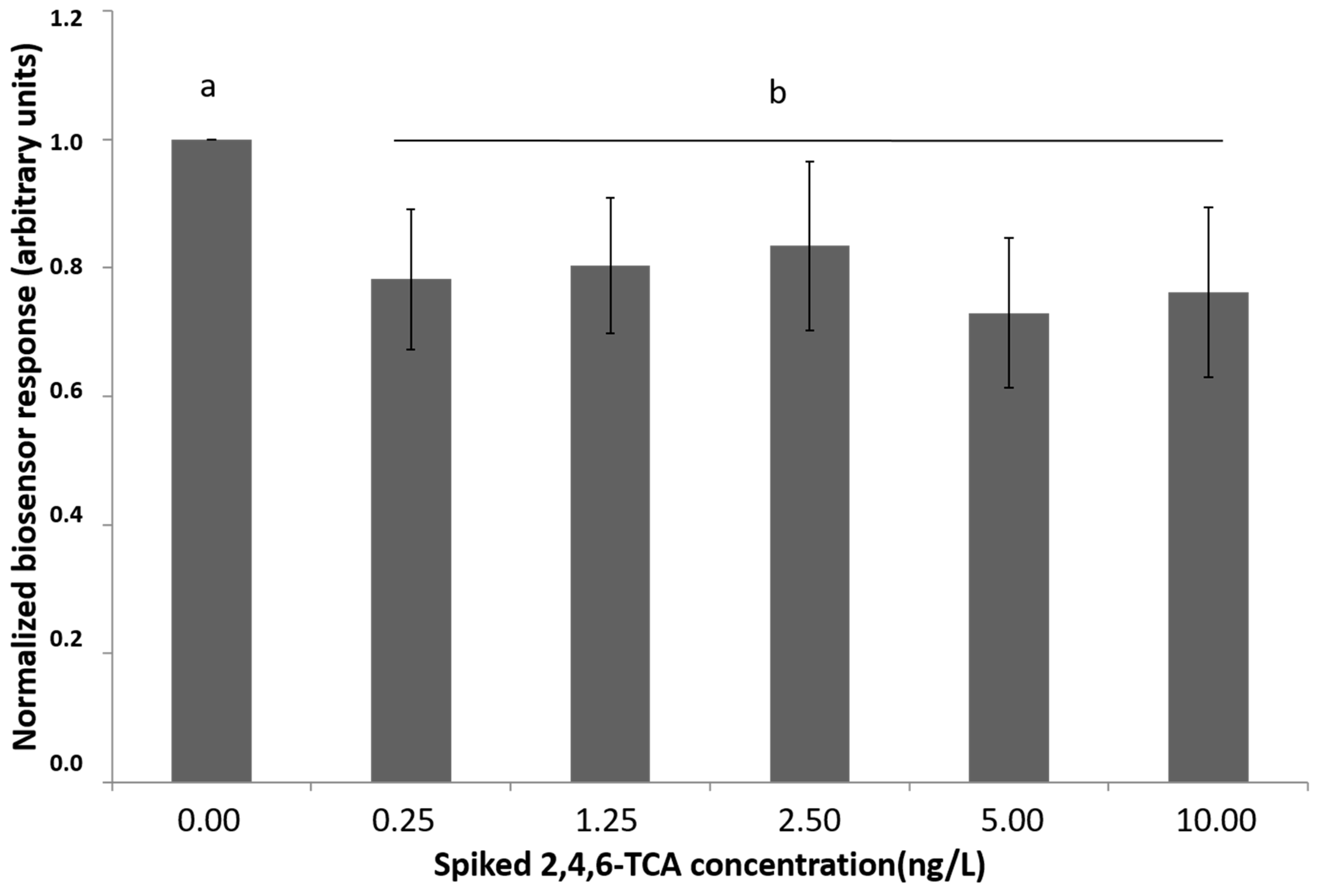
3.1. Biosensor Response to the Presence of 2,4,6-TCA in Oak Barrel Water Extracts
3.2. Results of Potentiometric Biosensor Testing with Other Versions of the Cell Biorecognition Elements
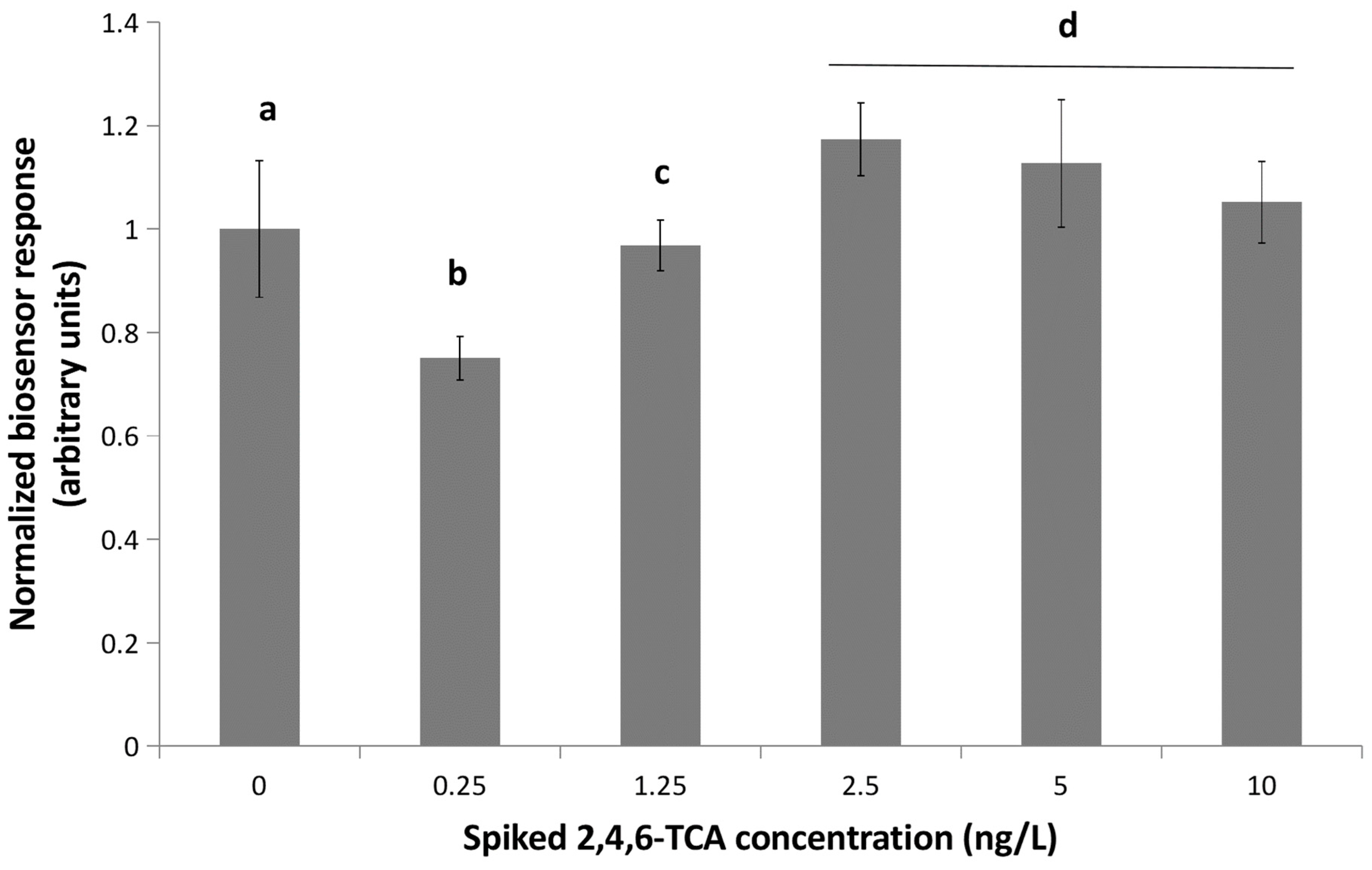
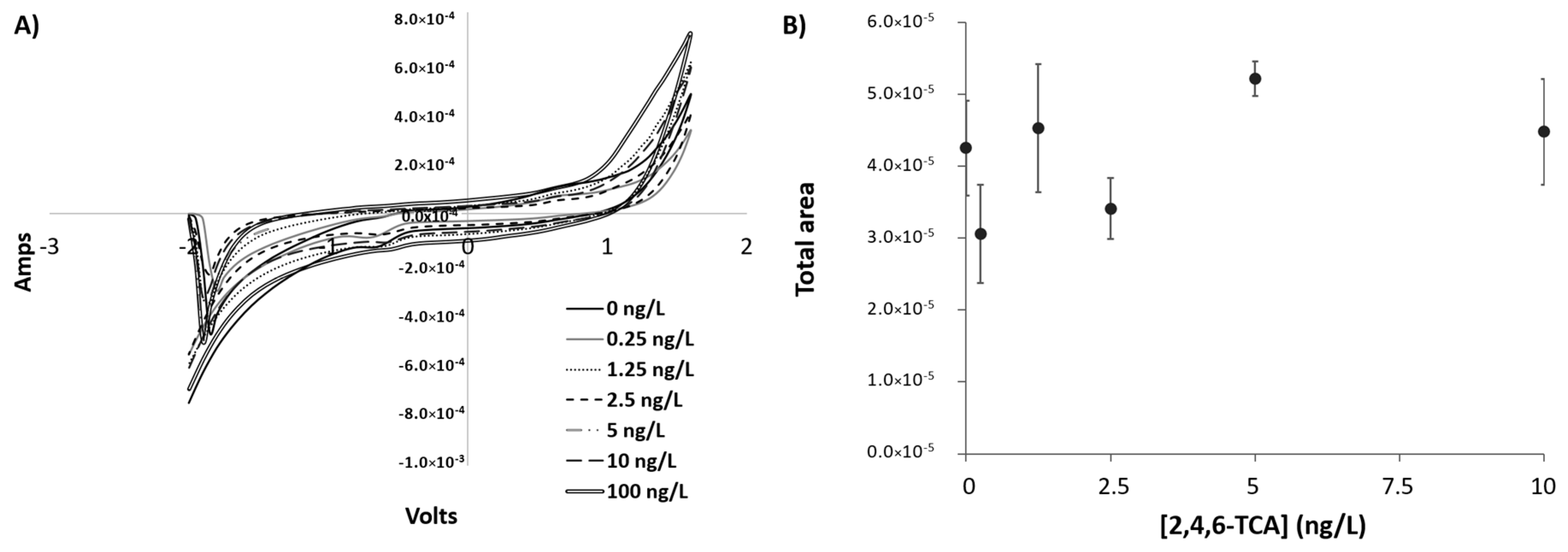
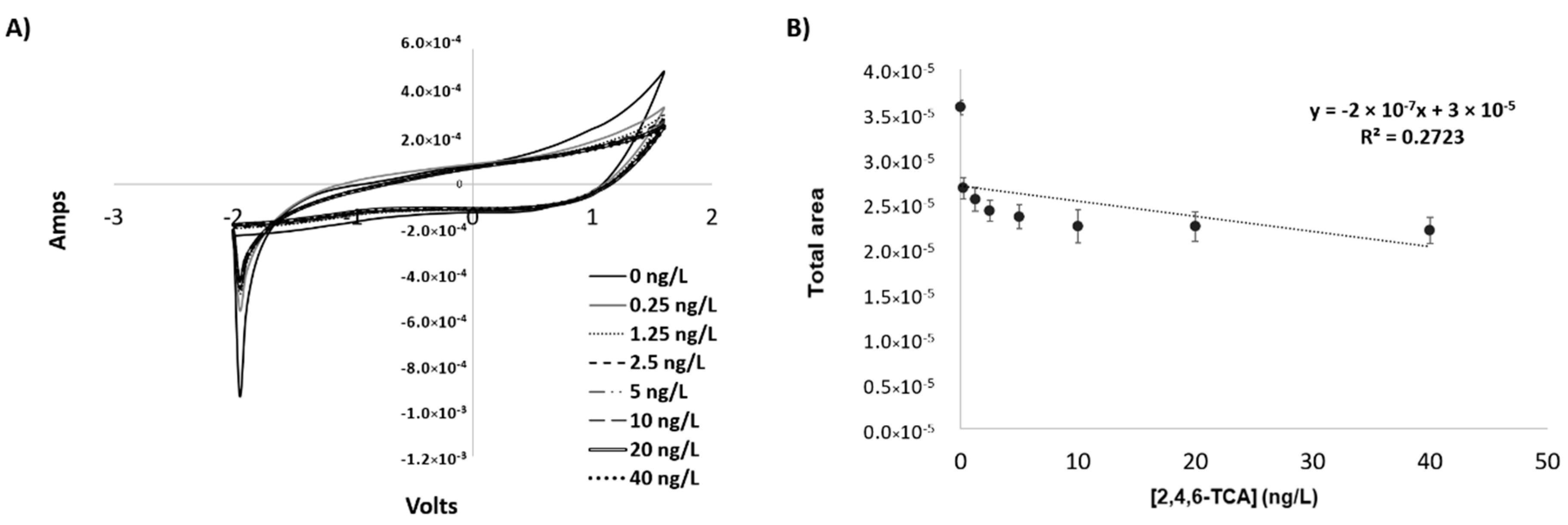
3.3. Cyclic Voltammetry Analysis
4. Discussion
5. Conclusions
- The validation of the method with actual samples in the framework of a double-blind trial
- The investigation of the extent of possible interference by compounds structurally related to 2,4,6-TCA (e.g., other haloanisoles)
- The establishment of the range of quantitative determination
Author Contributions
Funding
Conflicts of Interest
References
- Bhisey, R. Wine Barrel Market—Rising Demand for Wine to Be the Key Driving Factor. Available online: http://www.techyounme.com/wine-barrel-market-rising-demand-wine-key-driving-factor/ (accessed on 28 June 2018).
- Anonymous. Medium Plus Toast Segment by Toast Level Type to Witness the Fastest Revenue Growth in the Global Wine Barrel Market; Future Market Insights: Valley Cottage, NY, USA; London, UK, 2017. [Google Scholar]
- Bai, X.; Zhang, T.; Qu, Z.; Li, H.; Yang, Z. Contribution of filamentous fungi to the musty odorant 2,4,6-trichloroanisole in water supply reservoirs and associated drinking water treatment plants. Chemosphere 2017, 182, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, P.; Fleury, A.; Boutou, S. Identification of a new source of contamination of Quercus sp. oak wood by 2,4,6-trichloroanisole and its impact on the contamination of barrel-aged wines. J. Agric. Food Chem. 2010, 58, 10528–10538. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A. Analytical methods for determination of cork-taint compounds in wine. Trends Anal. Chem. 2012, 37, 135–147. [Google Scholar] [CrossRef]
- Vlachos, P.; Kampioti, A.; Kornaros, M.; Lyberatos, G. Matrix effect during the application of a rapid method using HS-SPME followed by GC–ECD for the analysis of 2,4,6-TCA in wine and cork soaks. Food Chem. 2007, 105, 681–690. [Google Scholar] [CrossRef]
- Anonymous. Cork Stoppers—Determination of Releasable 2,4,6-Trichloroanisol (TCA); ISO20752:2014(E); International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- Anonymous. Determination of releasable 2,4,6 trichloroanisole in wine by cork stoppers. Compendium of International Analysis of Methods 2009, Method OIV-MA-AS315-16.
- Slabizki, P.; Fischer, C.; Legrum, C.; Schmarr, H. Characterization of Atypical Off-Flavor Compounds in Natural Cork Stoppers by Multidimensional Gas Chromatographic Techniques. J. Agric. Food Chem. 2015, 63, 7840–7848. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.; Rauhut, D.; Jung, R. “Cork taint” responsible compounds. Determination of haloanisoles and halophenols in cork matrix: A review. Talanta 2017, 175, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, C.; Pérez-del-Notario, N.; González-Sáiz, J. Optimisation of a microwave-assisted extraction method for the simultaneous determination of haloanisoles and halophenols in cork stoppers. J. Chromatogr. A 2007, 1149, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Kintzios, S.; Pistola, E.; Konstas, J.; Bem, F.; Matakiadis, T.; Alexandropoulos, N.; Biselis, I.; Levin, R. Application of the Bioelectric recognition assay (BERA) for the detection of human and plant viruses: Definition of operational parameters. Biosens. Bioelectron. 2001, 16, 467–480. [Google Scholar] [CrossRef]
- Kintzios, S.; Pistola, E.; Panagiotopoulos, P.; Bomsel, M.; Alexandropoulos, N.; Bem, F.; Biselis, I.; Levin, R. Bioelectric recognition assay (BERA). Biosens. Bioelectron. 2001, 16, 325–336. [Google Scholar] [CrossRef]
- Apostolou, T.; Pascual, N.; Marco, M.P.; Moschos, A.; Petropoulos, A.; Kaltsas, G.; Kintzios, S. Extraction-less, rapid assay for the direct detection of 2,4,6-trichloroanisole (TCA) in cork samples. Talanta 2014, 125, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Varelas, V.; Sanvicens, N.; Pilar-Marco, M.; Kintzios, S. Development of a cellular biosensor for the detection of 2,4,6-trichloroanisole (TCA). Talanta 2011, 84, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, P.; Boutou, S. Process of Monitoring the Contamination of Barrel. U.S. Patent 2011/0113868 A1, 2011. [Google Scholar]
- Ferentinos, K.P.; Yialouris, C.P.; Blouchos, P.; Moschopoulou, G.; Tsourou, V.; Kintzios, S. Pesticide Residue Screening Using a Novel Artificial Neural Network Combined with a Bioelectric Cellular Biosensor. BioMed Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Moschopoulou, G.; Dourou, A.M.; Fidaki, A.; Kintzios, S. Assessment of pesticides cytoxicity by means of bioelectric profiling of mammalian cells. Environ. Nanotechnol. Monit. Manag. 2017, 8, 254–260. [Google Scholar] [CrossRef]
- Freitas, P.; Dias, L.G.; Peres, A.M.; Castro, L.M.; Veloso, A.C.A. Determination of 2,4,6-Trichloroanisole by Cyclic Voltammetry. Procedia Eng. 2012, 47, 1125–1128. [Google Scholar] [CrossRef] [Green Version]
- Peres, A.M.; Freitas, P.; Dias, L.G.; Sousa, M.E.B.C.; Castro, L.M.; Veloso, A.C.A. Cyclic voltammetry: A tool to quantify 2,4,6-trichloroanisole in aqueous samples from cork planks boiling industrial process. Talanta 2013, 117, 438–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warwick, M.J. Standardisation of immunoassay. In Immunoassay, A Practical Guide; Brian, L., Ed.; Taylor & Francis Ltd.: London, UK, 1996; p. 160. ISBN 9781482272857. [Google Scholar]
- Fry, A.J.; Britton, E. Solvent and supporting electrolytes. In Laboratory Techniques in Electroanalytical Chemistry; Kissinger, P.T., Heineman, W.R., Eds.; Marcel Dekker: New York, NY, USA; Basel, Switzerland, 1996; pp. 467–485. ISBN 9780824794453. [Google Scholar]
- Mavrikou, S.; Kintzios, S. Biosensor-based approaches for detecting ochratoxin A and 2,4,6-trichloroanisole in beverages. Beverages 2018, 4, 24. [Google Scholar] [CrossRef]
- Jeleń, H.; Dziadas, M.; Majcher, M. Different headspace solid phase microextraction--gas chromatography/mass spectrometry approaches to haloanisoles analysis in wine. J. Chromatogr. A 2013, 1313, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Pravda, M.; Guilbault, C. Development of a biosensor for the quantitative detection of 2,4,6-trichloroanisole using screen printed electrodes. Anal. Chim. Acta 2003, 484, 15–24. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moschopoulou, G.; Mavrikou, S.; Valdes, D.; Kintzios, S. Comparative Study of a Cell-Based and Electrochemical Biosensor for the Rapid Detection of 2,4,6-Trichloroanisole in Barrel Water Extracts. Beverages 2019, 5, 1. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages5010001
Moschopoulou G, Mavrikou S, Valdes D, Kintzios S. Comparative Study of a Cell-Based and Electrochemical Biosensor for the Rapid Detection of 2,4,6-Trichloroanisole in Barrel Water Extracts. Beverages. 2019; 5(1):1. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages5010001
Chicago/Turabian StyleMoschopoulou, Georgia, Sophie Mavrikou, Diego Valdes, and Spyridon Kintzios. 2019. "Comparative Study of a Cell-Based and Electrochemical Biosensor for the Rapid Detection of 2,4,6-Trichloroanisole in Barrel Water Extracts" Beverages 5, no. 1: 1. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages5010001






