Hydrothermal Carbonization of Brewery’s Spent Grains for the Production of Solid Biofuels
Abstract
:1. Introduction
2. Materials and Methods
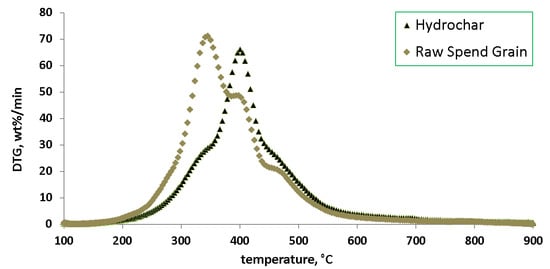
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wiśniewski, P. Piwa Historie Niezwykłe; Print Shops PREGO: Warszawa, Poland, 1993; ISBN 83-85830-00-6. [Google Scholar]
- Valamoti, S.M. Brewing beer in wine country? First archaeobotanical indications for beer making in Early and Middle Bronze Age Greece. Veg. Hist. Archaeobot. 2018, 27, 611–625. [Google Scholar] [CrossRef]
- Beer Production Worldwide from 1998 to 2017 (in Billion Hectoliters). Available online: https://0-www-statista-com.brum.beds.ac.uk/statistics/270275/worldwide-beer-production/ (accessed on 21 December 2018).
- Bentzen, J.; Smith, V. Structural Changes in the Consumption of Beer, Wine and Spirits in OECD Countries from 1961 to 2014. Beverages 2018, 4, 8. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Stojceska, V.; Ainsworth, P. The effect of different enzymes on the quality of high-fibre enriched brewer’s spent grain breads. Food Chem. 2008, 110, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Kreisz, S. Malting. In Handbook of Brewing Processes, Technology, Markets; Eßlinger, H.M., Ed.; WILEY-VCH: Weinheim, Germany, 2009; pp. 147–164. ISBN 978-3-527-31674-8. [Google Scholar]
- Dylkowski, W. Technologia Browarnictwa; Wydawnictwo przemysłu lekkiego i spożywczego: Warszawa, Poland, 1963. [Google Scholar]
- Sperandio, G.; Amoriello, T.; Carbone, K.; Fedrizzi, M.; Monteleone, A.; Tarangioli, S.; Pagano, M. Increasing the value of spent grain from craft microbreweries for energy purposes. Chem. Eng. Trans. 2017, 58, 487–492. [Google Scholar] [CrossRef]
- Enweremadu, C.C.; Waheed, M.A.; Adekunle, A.A.; Adeala, A. The Energy Potential of Brewer’s Spent Grain for Breweries in Nigeria. Eng. Appl. Sci. 2008, 3, 175–177. [Google Scholar]
- Funke, A.; Ziegler, F. Hydrothermal carbonisation of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Moscicki, K.J.; Niedzwiecki, L.; Owczarek, P.; Wnukowski, M. Commoditization of wet and high ash biomass: Wet torrefaction—A review. J. Power Technol. 2017, 97, 354–369. [Google Scholar]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Reza, M.T.; Yan, W.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J.; Vásquez, V.R. Reaction kinetics of hydrothermal carbonization of loblolly pine. Bioresour. Technol. 2013, 139, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Tungal, R.; Shende, R.V. Hydrothermal liquefaction of pinewood (Pinus ponderosa) for H2, biocrude and bio-oil generation. Appl. Energy 2014, 134, 401–412. [Google Scholar] [CrossRef]
- Nan, W.; Shende, A.R.; Shannon, J.; Shende, R.V. Insight into Catalytic Hydrothermal Liquefaction of Cardboard for Biofuels Production. Energy Fuels 2016, 30, 4933–4944. [Google Scholar] [CrossRef]
- Shende, R.; Tungal, R. Subcritical Aqueous Phase Reforming of Wastepaper for Biocrude and H2 Generation. Energy Fuels 2013, 27, 3194–3203. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Heat of reaction measurements for hydrothermal carbonization of biomass. Bioresour. Technol. 2011, 102, 7595–7598. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Effect of thermal pretreatment on equilibrium moisture content of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 4849–4854. [Google Scholar] [CrossRef] [PubMed]
- Favas, G.; Jackson, W.R.; Marshall, M. Hydrothermal dewatering of lower rank coals. 3. High-concentration slurries from hydrothermally treated lower rank coals. Fuel 2003, 82, 71–79. [Google Scholar] [CrossRef]
- Nakagawa, H.; Namba, A.; Böhlmann, M.; Miura, K. Hydrothermal dewatering of brown coal and catalytic hydrothermal gasification of the organic compounds dissolving in the water using a novel Ni/carbon catalyst. Fuel 2004, 83, 719–725. [Google Scholar] [CrossRef]
- Favas, G.; Jackson, W.R. Hydrothermal dewatering of lower rank coals. 1: Effects of process conditions on the properties of dried product. Fuel 2003, 82, 53–57. [Google Scholar] [CrossRef]
- Favas, G.; Jackson, W.R. Hydrothermal dewatering of lower rank coals. 2. Effects of coal characteristics for a range of Australian and international coals. Fuel 2003, 82, 59–69. [Google Scholar] [CrossRef]
- Rao, Z.; Zhao, Y.; Huang, C.; Duan, C.; He, J. Recent developments in drying and dewatering for low rank coals. Prog. Energy Combust. Sci. 2015, 46, 1–11. [Google Scholar] [CrossRef]
- Mursito, A.T.; Hirajima, T.; Sasaki, K. Upgrading and dewatering of raw tropical peat by hydrothermal treatment. Fuel 2010, 89, 635–641. [Google Scholar] [CrossRef]
- Wnukowski, M.; Owczarek, P.; Niedźwiecki, Ł. Wet Torrefaction of Miscanthus – Characterization of Hydrochars in View of Handling, Storage and Combustion Properties. J. Ecol. Eng. 2015, 16, 161–167. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Balasubramanian, R. Preparation and characterization of fuel pellets from woody biomass, agro-residues and their corresponding hydrochars. Appl. Energy 2014, 113, 1315–1322. [Google Scholar] [CrossRef]
- Volpe, M.; Wüst, D.; Merzari, F.; Lucian, M.; Andreottola, G.; Kruse, A.; Fiori, L. One stage olive mill waste streams valorisation via hydrothermal carbonisation. Waste Manag. 2018, 80, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Koppejan, J.; Sokhansanj, S.; Melin, S.; Madrali, S. Status Overview of Torrefaction Technologies; Bioenergy Task 32 report; International Energy Agency: Paris, France, 2015; ISBN 978-1-910154-23-6. [Google Scholar]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Svensson, K.; Kjørlaug, O.; Higgins, M.J.; Linjordet, R.; Horn, S.J. Post-anaerobic digestion thermal hydrolysis of sewage sludge and food waste: Effect on methane yields, dewaterability and solids reduction. Water Res. 2018, 132, 158–166. [Google Scholar] [CrossRef]
- Erdogan, E.; Atila, B.; Mumme, J.; Reza, M.T.; Toptas, A.; Elibol, M.; Yanik, J. Characterization of products from hydrothermal carbonization of orange pomace including anaerobic digestibility of process liquor. Bioresour. Technol. 2015, 196, 35–42. [Google Scholar] [CrossRef]
- Funke, A.; Mumme, J.; Koon, M.; Diakité, M. Cascaded production of biogas and hydrochar from wheat straw: Energetic potential and recovery of carbon and plant nutrients. Biomass Bioenergy 2013, 58, 229–237. [Google Scholar] [CrossRef]
- Wirth, B.; Mumme, J. Anaerobic Digestion of Waste Water from Hydrothermal Carbonization of Corn Silage. Appl. Bioenergy 2014, 1, 1–10. [Google Scholar] [CrossRef]
- Luz, F.C.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Anaerobic digestion of coffee grounds soluble fraction at laboratory scale: Evaluation of the biomethane potential. Appl. Energy 2017, 207, 166–175. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Ibeto, C.N.; Li, H.; Usmani, S.Q.; Semple, K.T. The challenges of anaerobic digestion and the role of biochar in optimizing anaerobic digestion. Waste Manag. 2017, 61, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Codignole Luz, F.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biochar characteristics and early applications in anaerobic digestion—A review. J. Environ. Chem. Eng. 2018, 6, 2892–2909. [Google Scholar] [CrossRef]
- Zhou, Y.; Engler, N.; Nelles, M. Symbiotic relationship between hydrothermal carbonization technology and anaerobic digestion for food waste in China. Bioresour. Technol. 2018, 260, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Mustafa, A.M.; Lin, H.; Choe, U.Y.; Sheng, K. Effect of hydrochar on anaerobic digestion of dead pig carcass after hydrothermal pretreatment. Waste Manag. 2018, 78, 849–856. [Google Scholar] [CrossRef]
- Codignole Luz, F.; Volpe, M.; Fiori, L.; Manni, A.; Cordiner, S.; Mulone, V.; Rocco, V. Spent coffee enhanced biomethane potential via an integrated hydrothermal carbonization-anaerobic digestion process. Bioresour. Technol. 2018, 256, 102–109. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A. Hydrothermal carbonization, torrefaction and slow pyrolysis of Miscanthus giganteus. Energy 2017, 140, 1292–1304. [Google Scholar] [CrossRef]
- Gao, L.; Volpe, M.; Lucian, M.; Fiori, L.; Goldfarb, J.L. Does Hydrothermal Carbonization as a Biomass Pretreatment Reduce Fuel Segregatuin of Coal-Biomass Blends During Oxidation? Energy Convers. Manag. 2018, 93–104. [Google Scholar] [CrossRef]
- Pearce, I.; Culbert, J.; Cass, D.; Cozzolino, D.; Wilkinson, K. Influence of Sample Storage on the Composition of Carbonated Beverages by MIR Spectroscopy. Beverages 2016, 2, 26. [Google Scholar] [CrossRef]
- Yan, W.; Hastings, J.T.; Acharjee, T.C.; Coronella, C.J.; Vásquez, V.R. Mass and energy balances of wet torrefaction of lignocellulosic biomass. Energy Fuels 2010, 24, 4738–4742. [Google Scholar] [CrossRef]
- Phyliss2 Database. Available online: https://phyllis.nl/Home/Help (accessed on 24 December 2018).
- Pawlak-Kruczek, H.; Krochmalny, K.; Mościcki, K.; Zgóra, J.; Czerep, M.; Ostrycharczyk, M.; Niedźwiecki, Ł. Torrefaction of Various Types of Biomass in Laboratory Scale, Batch-Wise Isothermal Rotary Reactor and Pilot Scale, Continuous Multi-Stage Tape Reactor. Eng. Prot. Environ. 2017, 20, 457–472. [Google Scholar] [CrossRef]
- Weber, K.; Heuer, S.; Quicker, P.; Li, T.; Løvås, T.; Scherer, V. An Alternative Approach for the Estimation of Biochar Yields. Energy Fuels 2018. [Google Scholar] [CrossRef]
- Moscicki, K.J.; Niedzwiecki, L.; Owczarek, P.; Wnukowski, M. Commoditization of biomass: Dry torrefaction and pelletization—A review. J. Power Technol. 2014, 94, 233–249. [Google Scholar]
- Pawlak-Kruczek, H.; Krochmalny, K.K.; Wnukowski, M.; Niedzwiecki, L. Slow pyrolysis of the sewage sludge with additives: Calcium oxide and lignite. J. Energy Resour. Technol. 2018, 140. [Google Scholar] [CrossRef]
- Poudel, J.; Karki, S.; Gu, J.H.; Lim, Y.; Oh, S.C. Effect of Co-Torrefaction on the Properties of Sewage Sludge and Waste Wood to Enhance Solid Fuel Qualities. J. Residuals Sci. Technol. 2017, 14, 23–36. [Google Scholar] [CrossRef]
- Pulka, J.; Wiśniewski, D.; Gołaszewski, J.; Białowiec, A. Is the biochar produced from sewage sludge a good quality solid fuel? Arch. Environ. Prot. 2016, 42, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Royal Society of Chemistry ChemSpider | Search and Share Chemistry. Available online: http://www.chemspider.com/ (accessed on 23 November 2018).
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Louwes, A.C.; Basile, L.; Yukananto, R.; Bhagwandas, J.C.; Bramer, E.A.; Brem, G. Torrefied biomass as feed for fast pyrolysis: An experimental study and chain analysis. Biomass Bioenergy 2017, 105, 116–126. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Thompson Witrick, K.; Duncan, S.; Hurley, K.; O’Keefe, S. Acid and Volatiles of Commercially-Available Lambic Beers. Beverages 2017, 3, 51. [Google Scholar] [CrossRef]
- Stemann, J.; Putschew, A.; Ziegler, F. Hydrothermal carbonization: Process water characterization and effects of water recirculation. Bioresour. Technol. 2013, 143, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhalla, A.; Shende, R.V.; Sani, R.K. Decentralized thermophilic biohydrogen: A more efficient and cost-effective process. BioResources 2012, 7, 1–2. [Google Scholar]
- Kumar, S.; Gupta, R.B. Biocrude production from switchgrass using subcritical water. Energy Fuels 2009, 23, 5151–5159. [Google Scholar] [CrossRef]
- Braida, D.; Capurro, V.; Zani, A.; Rubino, T.; Viganò, D.; Parolaro, D.; Sala, M. Potential anxiolytic- And antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br. J. Pharmacol. 2009, 157, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Hanes, K.R. Antidepressant effects of the herb Salvia divinorum: A case report. J. Clin. Psychopharmacol. 2001, 21, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Hanes, K. Salvia divinorum: Clinical and Research Potential. Maps 2003, XIII, 18–20. [Google Scholar]


| Test | Symbol | Raw | Raw 4 (Data from [10]) | After HTC | Unit | Standard Procedure |
|---|---|---|---|---|---|---|
| Moisture content 1 | MC | 77.27 | - | 64.15 | % | EN ISO 18134-2:2015 |
| Volatile matter content | VM d | 71.20 | - | 64.42 | % | EN 15148:2009 |
| Ash content | A d | 4.30 | 4.46 | 1.91 | % | EN ISO 1822:2015 |
| Higher heating value 2 | HHV | 20,628 | 19,515 | 26,455 | kJ/kg | EN 14918:2009 |
| Lower heating value 3 | LHV | 2412 | - | 7281 | kJ/kg | EN 14918:2009 |
| Carbon content | C d | 47.18 | 48.36 | 58.57 | % | EN ISO 16948:2015 |
| Hydrogen content | H d | 8.20 | 6.02 | 8.29 | % | EN ISO 16948:2015 |
| Nitrogen content | N d | 3.32 | 4.11 | 3.75 | % | EN ISO 16948:2015 |
| Sulfur content | S d | 0.26 | 0.32 | 0.24 | % | EN ISO 16994:2016 |
| Oxygen content | O d | 41.04 | 36.73 | 29.15 | % | EN ISO 16993:2015 |
| Oxygen to carbon ratio | O/C | 0.65 | - | 0.37 | mol/mol | - |
| Hydrogen to carbon ratio | H/C | 2.07 | - | 1.68 | mol/mol | - |
| Oxygen to hydrogen ratio | O/H | 0.32 | - | 0.22 | mol/mol | - |
| Parameter | Symbol | Value | Unit |
|---|---|---|---|
| Temperature | THTC | 200 | °C |
| Residence time | tHTC | 150 | min |
| Water: biomass ratio | W:B | 10:1 | - |
| Mass yield | Ym | 0.809 | - |
| Energy yield | Ye | ~1 | - |
| Ash yield | Ya | 0.360 | - |
| Sample | Moisture Content 1 | Unit |
|---|---|---|
| Raw spent grain | 77.52 | % |
| Spent grain after HTC and dripping | 84.48 | % |
| Carbonized spent grain after mechanical dewatering | 64.15 | % |
| Compound | Form | Avg. Mass | Relative Area of the Peak 1 | |
|---|---|---|---|---|
| Relative Area | SD 2 | |||
| IUPAC name | Da | % | % | |
| Methanol | CH4O | 32.042 | 0.39 | 0.02 |
| Formic acid | CH2O2 | 46.025 | 0.60 | 0.11 |
| Ethanol | C2H6O | 46.068 | 2.73 | 0.12 |
| Acetone | C3H6O | 58.079 | 1.30 | 0.53 |
| Acetic acid | C2H4O2 | 60.052 | 16.00 | 0.24 |
| 1-Hydroxyacetone | C3H6O2 | 74.078 | 4.99 | 0.04 |
| 1,2-Propanediol 3 | C3H8O2 | 76.094 | 0.41 | 0.04 |
| 2-Pyrrolidinone | C4H7NO | 85.104 | 0.23 | 0.01 |
| 3-Hydroxybutan-2-one | C4H8O2 | 88.105 | 1.17 | 0.01 |
| 1-Hydroxy-2-butanone | C4H8O2 | 88.105 | 0.04 | 0.01 |
| (2S)-2-Hydroxypropanoic acid 4 | C3H6O3 | 90.078 | 25.18 | 0.48 |
| 2,3-Butanediol 3 | C4H10O | 90.121 | 17.48 | 0.19 |
| 1,3-Propanediol 5 | C3H8O3 | 92.094 | 4.95 | 0.09 |
| 3-Pyridinol | C5H5NO | 95.099 | 17.90 | 0.15 |
| 2-Furaldehyde | C5H4O2 | 96.084 | 1.05 | 0.02 |
| 6-Methyl-3-pyridinol 3 | C6H7NO | 109.126 | 2.88 | 0.06 |
| 1-(2-Furyl)ethanone | C6H6O2 | 110.111 | 0.34 | 0.03 |
| 1,2-Cyclopentanedione, 3-methyl 3 | C6H8O2 | 112.127 | 0.30 | 0.01 |
| 5-(Hydroxymethyl)-2(3H)-furanone | C5H6O3 | 114.099 | 0.36 | 0.02 |
| 4-Oxopentanoic acid | C5H8O3 | 116.115 | 1.42 | 0.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackowski, M.; Semba, D.; Trusek, A.; Wnukowski, M.; Niedzwiecki, L.; Baranowski, M.; Krochmalny, K.; Pawlak-Kruczek, H. Hydrothermal Carbonization of Brewery’s Spent Grains for the Production of Solid Biofuels. Beverages 2019, 5, 12. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages5010012
Jackowski M, Semba D, Trusek A, Wnukowski M, Niedzwiecki L, Baranowski M, Krochmalny K, Pawlak-Kruczek H. Hydrothermal Carbonization of Brewery’s Spent Grains for the Production of Solid Biofuels. Beverages. 2019; 5(1):12. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages5010012
Chicago/Turabian StyleJackowski, Mateusz, Damian Semba, Anna Trusek, Mateusz Wnukowski, Lukasz Niedzwiecki, Marcin Baranowski, Krystian Krochmalny, and Halina Pawlak-Kruczek. 2019. "Hydrothermal Carbonization of Brewery’s Spent Grains for the Production of Solid Biofuels" Beverages 5, no. 1: 12. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages5010012