Modelling Changes in Volatile Compounds in British Columbian Varietal Wines That Were Bottle Aged for Up to 120 Months
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acquisition and Evaluation of BC Wines
2.2. Identification and Quantification of Volatile Compounds Using GC-MS
2.3. Statistical Analyses
3. Results
3.1. Alcohol Compounds
3.1.1. 1-Hexanol
3.1.2. 2-Methyl-1-Butanol (Active Amyl Alcohol) and 3-Methyl-1-Butanol (Isoamyl Alcohol)
3.1.3. Isobutyl Alcohol
3.1.4. n-butanol
3.1.5. Phenylethyl Alcohol
3.1.6. Propanol
3.2. Ester/Acetate Compounds
3.2.1. Diethyl Succinate
3.2.2. Ethyl Acetate
3.2.3. Ethyl Butanoate
3.2.4. Ethyl Decanoate, Ethyl Hexanoate, and Ethyl Octanoate
3.2.5. Ethyl Formate
3.2.6. Ethyl Lactate
3.2.7. Ethyl Isovalerate
3.2.8. Isoamyl Acetate
3.2.9. Methyl Acetate
3.2.10. Isobutyl Acetate and Other Ester/Acetate Compounds
3.3. Acid Compounds
3.4. Aldehyde Compounds
3.5. Other Compounds
4. Discussion and Conclusions
5. Limitations, Precautionary Notes, Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Class of Compound | Wine Compound | Details of Detection |
|---|---|---|
| Alcohol | 1-penten-3-ol | Detected in 13% of Cabernet franc, 31% of Cabernet, 42% of Meritage, 24% of Merlot, 52% of Pinot noir and 33% of Syrah samples. |
| 2,3-butanediol | Detected in 50% of Cabernet franc, 63% of Cabernet, 50% of Chardonnay, 63% of Meritage, 34% of Merlot, 43% of Pinot gris, 71% of Pinot noir and 33% of Syrah samples. | |
| 3-ethoxy-1-propanol | Detected in 13% of Cabernet franc, 6% of Cabernet, 17% of Chardonnay, 13% of Merlot, 33% of Pinot gris, and 14% of Pinot noir samples. | |
| 3-methyl-1-pentanol | Detected in 44% of Cabernet franc, 31% of Cabernet, 33% of Chardonnay, 58% of Meritage, 29% of Merlot, 24% of Pinot gris, 24% of Pinot noir and 33% of Syrah samples. | |
| 4-methyl-1-pentanol | Detected in one Meritage sample. | |
| acetoin | Detected in 63% of Cabernet franc, 25% of Cabernet, 63% of Chardonnay, 38% of Meritage, 74% of Merlot, 19% of Pinot gris, 86% of Pinot noir and 42% of Syrah samples. | |
| cis-3-hexen-1-ol | Detected in 19% of Cabernet franc, 6% of Cabernet, 4% of Chardonnay, 29% of Meritage, 8% of Merlot, 24% of Pinot gris, 5% of Pinot noir and 50% of Syrah samples. | |
| furfuryl alcohol | Detected in 6% of Cabernet, 13% of Chardonnay, 8% of Meritage, 10% of Pinot noir and 25% of Syrah samples. | |
| trans-3-hexen-1-ol | Detected in 6% of Cabernet franc, 13% of Cabernet, 25% of Meritage, 8% of Merlot, 24% of Pinot gris, 29% of Pinot noir and 17% of Syrah samples. | |
| Ester/ Acetate | acetol acetate | Detected in 13% of Meritage, 8% of Merlot, 14% of Pinot gris, 10% of Pinot noir and 8% of Syrah samples. |
| ethyl 2-methylbutyrate | Detected in 3% of Merlot and 14% of Pinot gris samples. | |
| ethyl 3-methylbutyl butanedioate | Detected in 13% of Cabernet franc and 3% of Merlot samples. | |
| ethyl sorbate | Detected in 6% of Cabernet franc, 8% of Chardonnay and 10% of Pinot gris samples. | |
| hexyl acetate | Detected in 50% of Chardonnay, 25% of Meritage, 11% of Merlot, 48% of Pinot gris, 19% of Pinot noir and 25% of Syrah samples. | |
| phenylethyl acetate | Detected in 4% of Chardonnay, 3% of Merlot and 10% of Pinot gris samples. | |
| Acid | hexanoic acid | Detected in 25% of Chardonnay, 8% of Meritage and 33% of Pinot gris samples. |
| levulinic acid | Detected in one Pinot gris sample. | |
| octanoic acid | Detected in 25% of Chardonnay and 67% of Pinot gris samples, and one sample from each of Cabernet franc, Cabernet and Syrah samples. | |
| Aldehyde | furfural | Detected in 50% of Cabernet franc, 25% of Cabernet, 63% of Chardonnay, 29% of Meritage, 18% of Merlot, 57% of Pinot gris, 29% of Pinot noir and 50% of Syrah samples. |
Appendix B
| Class of Compound | Wine Compound | White Wines | Red Wines | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Char-Donnay | Pinot Gris | Cabernet Franc | Cabernet Sauvignon | Merlot | Meri-Tage | Pinot Noir | Syrah | ||
| Alcohol | 1-hexanol | −0.15 | −0.07 | −0.24 | −0.20 | −0.11 | −0.21 | ||
| 2-methyl-1-butanol | −0.06 | −0.18 | −0.34 | −0.23 | −0.25 | −0.20 | |||
| 3-methyl-1-butanol | −1.02 | −0.60 | −0.96 | −1.27 | −2.26 | −0.94 | −1.47 | −0.97 | |
| isobutyl alcohol | −1.04 | −4.58 | −4.69 | −3.58 | −6.26 | −5.22 | |||
| n-butanol | −0.008 | −0.024 | −0.020 | −0.014 | −0.020 | ||||
| phenylethyl alcohol | −0.033 | −0.010 | −0.021 | −0.044 | −0.074 | −0.040 | −0.037 | ||
| propanol | −0.41 | −0.27 | −0.24 | −0.52 | −0.50 | −0.25 | −0.62 | −0.16 | |
| acetoin | −0.29 | −0.20 | |||||||
| Ester/ Acetate | diethyl succinate | 0.02 | −0.02 | 0.06 | −0.13 | 0.05 | −0.15 | ||
| ethyl acetate | −3.91 | −0.53 | −2.39 | −3.30 | −1.76 | −2.75 | −2.01 | ||
| ethyl butanoate | −0.03 | −0.02 | −0.04 | −0.02 | −0.03 | −0.03 | |||
| ethyl decanoate | −0.0010 | −0.0004 | −0.0003 | 0.0001 | −0.0006 | ||||
| ethyl formate | −0.0092 | 0.0029 | 0.0043 | ||||||
| ethyl hexanoate | −0.005 | −0.002 | −0.006 | −0.003 | −0.004 | −0.004 | |||
| ethyl lactate | −0.29 | −2.24 | −3.89 | −3.68 | −2.23 | −3.65 | |||
| ethyl octanoate | −0.009 | −0.002 | −0.006 | −0.002 | −0.004 | −0.003 | |||
| ethyl isovalerate | −0.0005 | 0.0001 | −0.0003 | −0.0003 | |||||
| isoamyl acetate | −0.007 | −0.003 | −0.003 | −0.007 | −0.004 | −0.002 | −0.010 | ||
| methyl acetate | 0.002 | −0.009 | −0.003 | −0.005 | −0.005 | ||||
| isobutyl acetate | −0.00013 | ||||||||
| Acid/ Aldehyde/ Sulfur | acetic acid | −0.06 | −0.16 | −0.20 | 0.05 | −0.14 | |||
| acetaldehyde | 0.06 | −0.07 | −0.12 | 0.03 | −0.08 | 0.06 | |||
| dimethyl sulfide | 0.00054 | 0.00024 | |||||||
References
- Wine Folly. Wines That Age Well. Available online: https://winefolly-wpengine.netdna-ssl.com/wp-content/uploads/2012/04/wines-that-age-well-chart.jpg (accessed on 8 July 2019).
- Dharmadhikari, M. Wine Aging. Available online: https://www.extension.iastate.edu/wine/wine-aging (accessed on 8 July 2019).
- Ivanova, V.; Vojnoski, B.; Stefova, M. Effect of winemaking treatment and wine aging on phenolic content in Vranec wines. J. Food Sci. Technol. 2012, 49, 161–172. [Google Scholar] [CrossRef]
- Frivik, S.K.; Ebeler, S.E. Influence of sulfur dioxide on the formation of aldehydes in white wine. Am. J. Enol. Vitic. 2003, 54, 31–38. [Google Scholar]
- Bueno, M.; Marrufo-Curtido, A.; Carrascón, V.; Fernández-Zurbano, P.; Escudero, A.; Ferreira, V. Formation and accumulation of acetaldehyde and Strecker aldehydes during red wine oxidation. Front. Chem. 2018, 6, 20. [Google Scholar] [CrossRef]
- McRae, J.M.; Mierczynska-Vasilev, A.; Soden, A.; Barker, A.M.; Day, M.P.; Smith, P.A. Effect of commercial-scale filtration on sensory and colloidal properties of red wines over 18 months bottle aging. Am. J. Enol. Vitic. 2017, 68, 3. [Google Scholar] [CrossRef]
- Alamo-Sanza, M.D.; Nevares, I. Wine aging technologies. Beverages 2019, 5, 24. [Google Scholar] [CrossRef]
- Picard, M.; van Leeuwen, C.; Guyon, F.; Gaillard, L.; de Revel, G.; Marchand, S. Vine water deficit impacts aging bouquet in fine red Bordeaux wine. Front. Chem. 2017, 85, 56. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Julien, P.; Coelho, C.; Roullier-Gall, C.; Ballester, J.; Schmitt-Kopplin, P.; Gougeon, R.D. Impact of glutathione on wines oxidative stability: A combined sensory and metabolomics study. Front. Chem. 2018, 6, 182. [Google Scholar] [CrossRef]
- Wines of Marked Quality Regulation. Available online: http://www.bclaws.ca/civix/document/id/complete/statreg/168_2018/search/CIVIX_DOCUMENT_ROOT_STEM:(Wines%20of%20marked%20quality)?2#hit1 (accessed on 5 August 2019).
- Gothe, J. Another Name for Meritage-Style Blends. Available online: https://www.straight.com/food/might-well-be-meritage (accessed on 5 August 2019).
- Danzer, K.; Garcia, D.D.; Thiel, G.; Reichenbacher, M. Classification of wine samples according to origin and grape varieties on the basis of inorganic and organic trace analyses. Am. Lab. 1999, 31, 26–34. [Google Scholar]
- Ziółkowska, A.; Wńasowicz, E.; Jeleń, H.H. Differentiation of wines according to grape variety and geographical origin based on volatiles profiling using SPME-MS and SPME-GC/MS methods. Food Chem. 2016, 213, 714–720. [Google Scholar] [CrossRef]
- Næs, T.; Brockhoff, P.B.; Tomić, O. Statistics for Sensory and Consumer Science; John Wiley & Sons Ltd.: Chichester, UK, 2010; ISBN 978-0470518212. [Google Scholar]
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of Chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef]
- Riberéau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Alcohols and Other Volatile Compounds. In Handbook of Enology: The Chemistry of Wine, Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; Volume 2, pp. 51–64. ISBN 978-0470010372. [Google Scholar]
- Bakker, J.; Clarke, R.J. Volatile Components. In Wine Flavour Chemistry, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 155–238. ISBN 978-1444330427. [Google Scholar]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. 2013, 5, 202–218. [Google Scholar] [CrossRef]
- Panighel, A.; Flamini, R. Applications of solid-phase microextraction and gas chromatography/mass spectrometry (SPME-GC/MS) in the study of grape and wine volatile compounds. Molecules 2014, 19, 21291–21309. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Austr. J. Grape Wine R. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Campo, E.; Ferreira, V.; Escudero, A.; Marques, J.C.; Cacho, J. Quantitative gas chromatograpy-olfactometry and chemical quantitative study of the aroma of four Madeira wines. Anal. Chim. Acta 2005, 1140, 180–187. [Google Scholar] [CrossRef]
- Vilanova, M.; Pretorius, I.S.; Henschke, P.A. Influence of Diammonium Phosphate Addition to Fermentation on Wine Biologicals. In Processing and Impact on Active Components in Food; Preedy, V.R., Ed.; Academic Press: London, UK, 2015; pp. 483–491. ISBN 978-0124046993. [Google Scholar]
- Ugliano, M.; Henschke, P.A. Yeasts and Wine Flavour. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 313–392. ISBN 978-0387741185. [Google Scholar]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principle and Practices of Winemaking; Chapman & Hall: New York, NY, USA, 1996; pp. 102–192. ISBN 978-0834212701. [Google Scholar]
- Rapp, A.; Versini, G. Influence of nitrogen on compounds in grapes on aroma compounds in wines. J. Int. Sci. Vigne Vin. 1996, 51, 193–203. [Google Scholar]
- Pretorius, N.; Engelbrecht, L.; du Toit, M. Using MLF to create a buttery wine or not. Winetech Technical. 2017. Available online: https://www.wineland.co.za/using-mlf-create-buttery-wine-not/ (accessed on 5 August 2019).
- Australian Wine Research Institute. Wine Flavour, Faults and Taints. Oxidation-Type Faults: Acetaldehyde. Available online: https://www.awri.com.au/industry_support/winemaking_resources/sensory_assessment/recognition-of-wine-faults-and-taints/wine_faults/#oxidationfaults (accessed on 5 August 2019).
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Zamora, F. Biochemistry of Alcoholic Fermentation. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 3–26. ISBN 978-0387741185. [Google Scholar]
- Rapp, A.; Güntert, M. Changes in aroma substances during the storage of white wines in bottles. In The Shelf Life of Foods and Beverages; Charalambous, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 141–167. ISBN 978-0444426116. [Google Scholar]
- Australian Wine Research Institute. Wine Flavour, Faults and Taints. Oxidation-Type Faults: Ethyl Acetate. Available online: https://www.awri.com.au/industry_support/winemaking_resources/sensory_assessment/recognition-of-wine-faults-and-taints/wine_faults/#oxidationfaults (accessed on 5 August 2019).
- Ferreira, V.; Cacho, J. Identification of Impact Odorants of Wines. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 393–416. ISBN 978-0387741185. [Google Scholar]
- American Chemical Society. Ethyl Formate. Available online: https://0-www-acs-org.brum.beds.ac.uk/content/acs/en/molecule-of-the-week/archive/e/ethyl-formate.html (accessed on 8 July 2019).
- Jackson, R.S. Wine Science: Principles and Applications, 3rd ed.; Academic Press, Elsevier: Burlington, VT, USA, 2008; ISBN 978-0123736468. [Google Scholar]
- Somers, T.C.; Evans, M.E. Spectral evaluation of young red wines: Anthocyanin equilibria, total phenolics, free and molecular SO2 “chemical age”. J. Sci. Food Agric. 1977, 28, 240–246. [Google Scholar] [CrossRef]
- Dubourdieu, D.; Tominaga, T. Polyfunctional Thiol Compounds. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 275–294. ISBN 978-0387741185. [Google Scholar]
- Australian Wine Research Institute. Wine Flavour, Faults and Taints. Reductive Wine Faults: Disulfide (DMS). Available online: https://www.awri.com.au/industry_support/winemaking_resources/sensory_assessment/recognition-of-wine-faults-and-taints/wine_faults/#reductive (accessed on 5 August 2019).
- Villamor, R.R.; Ross, C.F. Wine matrix compounds affect perception of wine aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef]
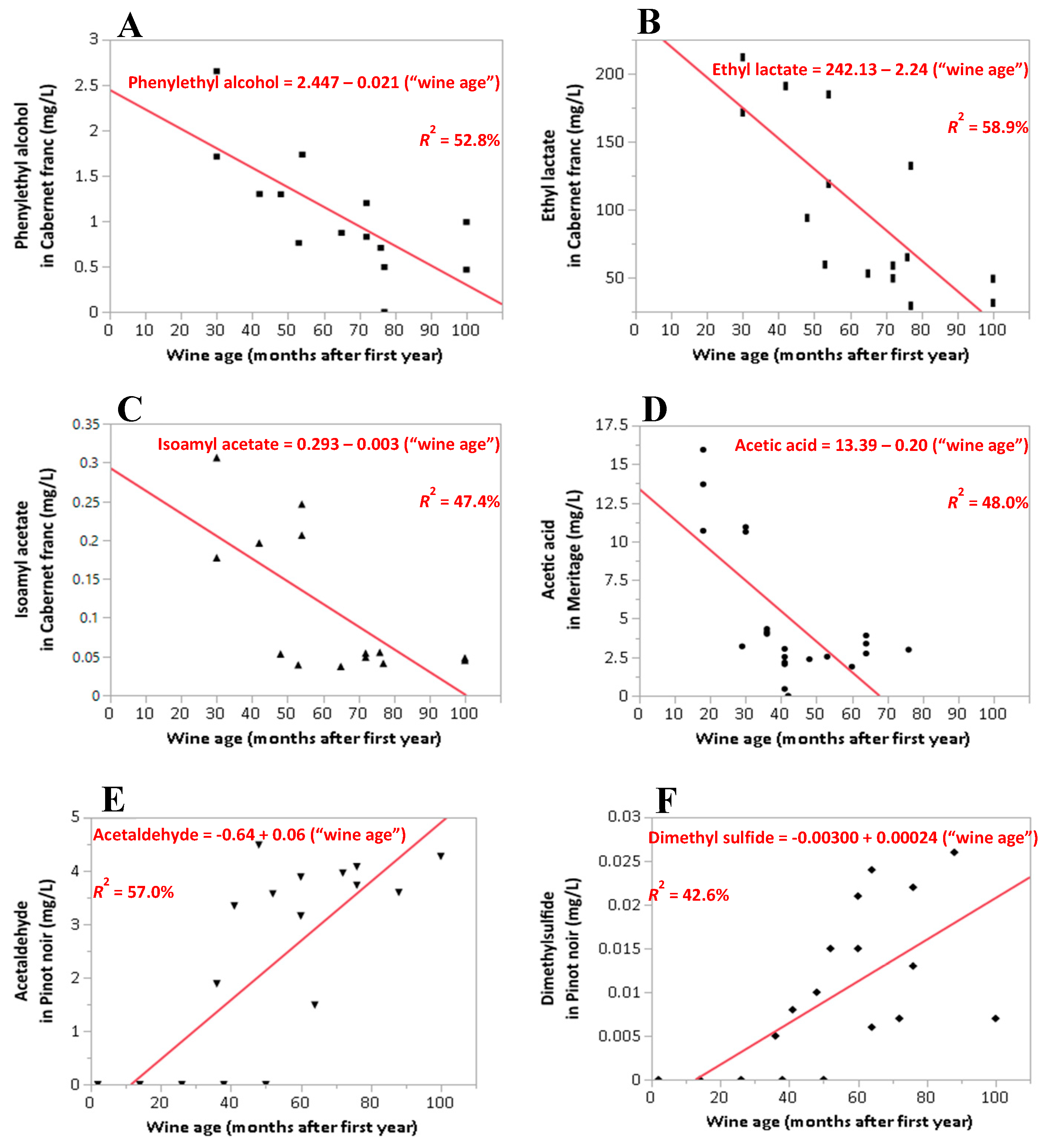
| Wine Compound a (Common Name) | Variety | Percent Detection b (%) | n Used in the Regression Model | Coefficient of Determination R2 (%) | Root Mean Square Error (RMSE) | F-Ratio | Coefficients for “Wine Age” | ||
|---|---|---|---|---|---|---|---|---|---|
| Intercept c (b0) (mg/L) | Slope (b1) (mg/L/mon) | Significance of b1 | |||||||
| 1-hexanol | Cabernet franc | 94 | 14 | 55.6 | 1.36 | 15.00 ** | 6.99 | −0.07 | ** |
| Cabernet Sauvignon | 100 | 14 | 48.9 | 4.69 | 11.48 ** | 23.27 | −0.24 | ** | |
| Chardonnay | 96 | 22 | 25.3 | 5.50 | 6.76 * | 13.90 | −0.15 | * | |
| Meritage | 100 | 21 | 25.3 | 3.00 | 6.43 * | 9.20 | −0.11 | * | |
| Merlot | 97 | 34 | 49.3 | 4.16 | 31.10 *** | 18.21 | −0.20 | *** | |
| Pinot gris | 100 | 19 | 15.4 | 3.18 | 3.10 | 6.50 | −0.05 | ||
| Pinot noir | 100 | 19 | 38.7 | 6.92 | 10.73 ** | 19.92 | −0.21 | ** | |
| Syrah | 100 | 12 | 17.5 | 6.27 | 2.12 | 15.43 | −0.13 | ||
| 2-methyl-1-butanol (active amyl alcohol) | Cabernet franc | 100 | 14 | 46.8 | 4.50 | 10.55 ** | 19.55 | −0.18 | ** |
| Cabernet Sauvignon | 94 | 14 | 12.3 | 13.71 | 1.69 | 30.79 | −0.27 | ||
| Chardonnay | 100 | 22 | 10.5 | 4.76 | 2.35 | 9.10 | −0.08 | ||
| Meritage | 96 | 23 | 31.0 | 5.76 | 9.42 ** | 16.71 | −0.23 | ** | |
| Merlot | 95 | 34 | 29.1 | 11.14 | 13.13 *** | 33.33 | −0.34 | *** | |
| Pinot gris | 100 | 20 | 34.1 | 2.10 | 9.30 ** | 5.97 | −0.06 | ** | |
| Pinot noir | 100 | 20 | 54.0 | 5.49 | 21.1 *** | 21.18 | −0.25 | *** | |
| Syrah | 100 | 11 | 45.4 | 4.83 | 7.49 * | 18.61 | −0.20 | * | |
| 3-methyl-1-butanol (isoamyl alcohol) | Cabernet franc | 100 | 14 | 46.1 | 23.95 | 10.26 ** | 100.16 | −0.96 | ** |
| Cabernet Sauvignon | 100 | 12 | 37.8 | 31.10 | 6.07 * | 124.96 | −1.27 | * | |
| Chardonnay | 100 | 22 | 22.7 | 38.35 | 5.88 * | 94.15 | −1.02 | * | |
| Meritage | 96 | 21 | 43.2 | 17.90 | 14.47 ** | 67.32 | −0.94 | ** | |
| Merlot | 97 | 32 | 40.2 | 56.18 | 20.13 *** | 207.42 | −2.26 | *** | |
| Pinot gris | 100 | 18 | 50.1 | 14.01 | 16.08 *** | 52.27 | −0.60 | *** | |
| Pinot noir | 100 | 21 | 54.1 | 33.60 | 22.37 *** | 122.52 | −1.47 | *** | |
| Syrah | 100 | 11 | 40.3 | 26.36 | 6.07 * | 90.52 | −0.97 | * | |
| isobutyl alcohol | Cabernet franc | 100 | 14 | 48.6 | 108.67 | 11.33 ** | 477.87 | −4.58 | ** |
| Cabernet Sauvignon | 94 | 12 | 24.2 | 113.58 | 3.19 | 387.86 | −3.36 | ||
| Chardonnay | 100 | 22 | 8.6 | 128.98 | 1.88 | 220.79 | −1.94 | ||
| Meritage | 100 | 22 | 37.2 | 76.31 | 11.86 ** | 302.57 | −3.58 | ** | |
| Merlot | 100 | 33 | 30.3 | 160.14 | 13.45 *** | 469.99 | −4.69 | *** | |
| Pinot gris | 100 | 18 | 46.4 | 27.51 | 13.83 ** | 104.99 | −1.04 | ** | |
| Pinot noir | 100 | 19 | 66.7 | 107.34 | 34.07 *** | 555.48 | −6.26 | *** | |
| Syrah | 100 | 11 | 68.2 | 79.65 | 19.28 ** | 457.95 | −5.22 | ** | |
| n-butanol | Cabernet franc | 94 | 15 | 40.2 | 0.21 | 8.74 * | 0.846 | −0.008 | * |
| Cabernet Sauvignon | 94 | 14 | 51.2 | 0.11 | 12.59 ** | 2.115 | −0.024 | ** | |
| Chardonnay | 83 | 22 | 3.6 | 0.39 | 0.75 | 0.096 | 0.004 | ||
| Meritage | 100 | 22 | 55.2 | 0.20 | 24.62 *** | 0.946 | −0.014 | *** | |
| Merlot | 97 | 35 | 30.1 | 0.64 | 14.20 *** | 1.809 | −0.019 | *** | |
| Pinot gris | 86 | 18 | 2.6 | 0.04 | 0.42 | 0.058 | 0.000 | ||
| Pinot noir | 100 | 19 | 48.8 | 0.61 | 16.20 *** | 2.024 | −0.023 | *** | |
| Syrah | 100 | 10 | 34.7 | 0.26 | 4.24 | 0.994 | −0.011 | ||
| phenylethyl alcohol (phenylethanol) | Cabernet franc | 94 | 14 | 52.8 | 0.47 | 13.43 ** | 2.447 | −0.021 | ** |
| Cabernet Sauvignon | 94 | 12 | 57.2 | 0.73 | 13.36 ** | 4.043 | −0.044 | ** | |
| Chardonnay | 92 | 21 | 49.7 | 0.64 | 18.76 *** | 2.733 | −0.033 | *** | |
| Meritage | 96 | 23 | 28.0 | 1.08 | 8.16 ** | 2.907 | −0.040 | ** | |
| Merlot | 97 | 33 | 33.1 | 2.32 | 15.35 *** | 7.473 | −0.074 | *** | |
| Pinot gris | 95 | 18 | 36.6 | 0.30 | 9.23 ** | 1.005 | −0.010 | ** | |
| Pinot noir | 100 | 21 | 56.8 | 0.80 | 25.00 *** | 3.036 | −0.037 | *** | |
| Syrah | 100 | 12 | 24.5 | 0.77 | 3.24 | 2.385 | −0.020 | ||
| propanol (propyl alcohol) | Cabernet franc | 100 | 13 | 56.1 | 5.16 | 14.03 ** | 25.09 | −0.24 | ** |
| Cabernet Sauvignon | 100 | 12 | 61.5 | 7.81 | 15.94 ** | 44.85 | −0.52 | ** | |
| Chardonnay | 100 | 23 | 23.8 | 15.03 | 6.26 * | 39.14 | −0.41 | * | |
| Meritage | 100 | 22 | 23.8 | 7.79 | 6.23 * | 20.82 | −0.25 | * | |
| Merlot | 100 | 32 | 38.9 | 13.81 | 19.11 *** | 45.70 | −0.50 | *** | |
| Pinot gris | 100 | 19 | 41.4 | 7.72 | 12.01 ** | 24.28 | −0.27 | ** | |
| Pinot noir | 100 | 20 | 32.7 | 22.78 | 8.76 ** | 58.07 | −0.62 | ** | |
| Syrah | 100 | 11 | 39.4 | 4.39 | 5.86 * | 14.98 | −0.16 | * | |
| acetylmethylcarbinol (acetoin) | Merlot | 74 | 34 | 35.3 | 8.24 | 17.47 *** | 26.00 | −0.29 | *** |
| Pinot noir | 86 | 19 | 30.4 | 7.22 | 7.43 * | 18.73 | −0.20 | * | |
| Wine Compound a | Variety | Percent Detection b (%) | n Used in the Regression Model | Coefficient of Determination R2 (%) | Root Mean Square Error (RMSE) | F-Ratio | Coefficients for “Wine Age” | ||
|---|---|---|---|---|---|---|---|---|---|
| Intercept c (b0) (mg/L) | Slope (b1) (mg/L/mon) | Significance of b1 | |||||||
| diethyl succinate | Cabernet franc | 94 | 15 | 24.9 | 5.81 | 4.31 | 16.60 | −0.15 | |
| Cabernet Sauvignon | 94 | 13 | 23.5 | 5.30 | 3.38 | 17.96 | −0.16 | ||
| Chardonnay | 67 | 20 | 22.8 | 0.79 | 5.33 * | −0.10 | 0.02 | * | |
| Meritage | 88 | 22 | 33.3 | 3.11 | 9.56 ** | 9.34 | −0.13 | ** | |
| Merlot | 71 | 34 | 31.1 | 2.09 | 14.43 *** | −1.04 | 0.06 | *** | |
| Pinot gris | 100 | 19 | 21.7 | 0.82 | 4.70 * | 2.68 | −0.02 | * | |
| Pinot noir | 67 | 20 | 50.1 | 1.16 | 18.08 *** | −0.47 | 0.05 | *** | |
| Syrah | 100 | 10 | 50.2 | 3.26 | 8.07 * | 14.24 | −0.15 | * | |
| ethyl acetate | Cabernet franc | 100 | 14 | 45.6 | 60.15 | 10.06 ** | 234.07 | −2.39 | ** |
| Cabernet Sauvignon | 94 | 12 | 16.9 | 65.83 | 2.03 | 173.68 | −1.56 | ||
| Chardonnay | 100 | 22 | 21.2 | 153.38 | 5.38 * | 357.53 | −3.91 | * | |
| Meritage | 96 | 23 | 48.4 | 30.65 | 19.68 *** | 125.92 | −1.76 | *** | |
| Merlot | 100 | 32 | 45.4 | 79.74 | 24.96 *** | 325.47 | −3.63 | *** | |
| Pinot gris | 100 | 20 | 37.3 | 17.70 | 10.69 ** | 49.58 | −0.53 | ** | |
| Pinot noir | 100 | 20 | 55.6 | 62.62 | 22.51 | 233.84 | −2.75 | *** | |
| Syrah | 100 | 11 | 58.2 | 38.12 | 12.51 ** | 166.47 | −2.01 | ** | |
| ethyl butanoate | Cabernet franc | 100 | 14 | 43.6 | 0.60 | 9.27 * | 2.41 | −0.02 | * |
| Cabernet Sauvignon | 100 | 12 | 27.7 | 0.51 | 3.83 | 1.85 | −0.02 | ||
| Chardonnay | 100 | 20 | 11.0 | 1.29 | 2.23 | 2.60 | −0.02 | ||
| Meritage | 96 | 23 | 36.7 | 0.37 | 12.18 ** | 1.35 | −0.02 | ** | |
| Merlot | 97 | 32 | 39.7 | 1.01 | 19.72 *** | 3.39 | −0.04 | *** | |
| Pinot gris | 95 | 20 | 42.1 | 0.93 | 13.08 ** | 2.91 | −0.03 | ** | |
| Pinot noir | 100 | 20 | 52.8 | 0.82 | 20.1 *** | 2.89 | −0.03 | *** | |
| Syrah | 100 | 11 | 70.7 | 0.41 | 21.68 ** | 2.25 | −0.03 | ** | |
| ethyl decanoate | Cabernet franc | 88 | 15 | 51.5 | 0.01 | 13.80 ** | 0.0346 | −0.0004 | ** |
| Cabernet Sauvignon | 94 | 14 | 6.8 | 0.02 | 0.88 | 0.0336 | −0.0003 | ||
| Chardonnay | 71 | 22 | 12.3 | 0.04 | 2.80 | −0.0040 | 0.0007 | ||
| Meritage | 88 | 22 | 33.9 | 0.01 | 10.24 ** | 0.0205 | −0.0003 | ** | |
| Merlot | 71 | 30 | 7.9 | 0.00 | 2.40 | 0.0014 | 0.0001 | ||
| Pinot gris | 95 | 20 | 27.7 | 0.04 | 6.89 * | 0.1014 | −0.0011 | * | |
| Pinot noir | 67 | 19 | 60.1 | 0.00 | 25.61 *** | −0.0006 | 0.0001 | *** | |
| Syrah | 100 | 12 | 57.5 | 0.01 | 13.55 | 0.0470 | −0.0006 | ** | |
| ethyl formate | Cabernet franc | 56 | 16 | 18.8 | 0.09 | 3.23 | −0.0512 | 0.0019 | |
| Cabernet Sauvignon | 81 | 14 | 33.8 | 0.24 | 6.11 * | 0.7799 | −0.0092 | * | |
| Chardonnay | 54 | 21 | 1.1 | 0.09 | 0.20 | 0.0465 | 0.0004 | ||
| Meritage | 88 | 22 | 0.9 | 0.20 | 0.18 | 0.2941 | −0.0012 | ||
| Merlot | 45 | 36 | 20.5 | 0.12 | 8.76 ** | −0.0831 | 0.0029 | ** | |
| Pinot gris | 48 | 20 | 0.8 | 0.05 | 0.14 | 0.0442 | −0.0002 | ||
| Pinot noir | 67 | 21 | 30.9 | 0.16 | 8.51 ** | −0.0399 | 0.0043 | ** | |
| Syrah | 75 | 10 | 0.0 | 0.12 | 0.00 | 0.0985 | −0.0001 | ||
| ethyl hexanoate | Cabernet franc | 100 | 14 | 39.5 | 0.07 | 7.83 * | 0.261 | −0.002 | * |
| Cabernet Sauvignon | 100 | 15 | 0.1 | 0.31 | 0.01 | 0.213 | 0.000 | ||
| Chardonnay | 100 | 22 | 2.4 | 0.48 | 0.50 | 0.666 | −0.003 | ||
| Meritage | 96 | 22 | 39.6 | 0.06 | 13.10 ** | 0.200 | −0.003 | ** | |
| Merlot | 100 | 36 | 22.2 | 0.25 | 9.72 ** | 0.603 | −0.006 | ** | |
| Pinot gris | 100 | 19 | 40.0 | 0.15 | 11.33 ** | 0.472 | −0.005 | ** | |
| Pinot noir | 100 | 20 | 46.9 | 0.11 | 15.92 *** | 0.346 | −0.004 | *** | |
| Syrah | 100 | 10 | 43.7 | 0.08 | 6.22 * | 0.306 | −0.004 | * | |
| ethyl lactate | Cabernet franc | 100 | 15 | 58.9 | 42.16 | 18.6 *** | 242.13 | −2.24 | *** |
| Cabernet Sauvignon | 94 | 14 | 51.6 | 71.35 | 12.81 ** | 379.44 | −3.89 | ** | |
| Chardonnay | 100 | 23 | 9.6 | 97.62 | 2.23 | 183.05 | −1.45 | ||
| Meritage | 96 | 23 | 31.2 | 55.65 | 9.53 ** | 178.73 | −2.23 | ** | |
| Merlot | 100 | 37 | 38.2 | 100.62 | 21.61 *** | 373.07 | −3.68 | *** | |
| Pinot gris | 100 | 19 | 39.8 | 8.81 | 11.25 ** | 26.79 | −0.29 | ** | |
| Pinot noir | 100 | 20 | 27.3 | 150.53 | 6.75 * | 367.69 | −3.65 | * | |
| Syrah | 100 | 11 | 19.5 | 88.63 | 2.18 | 260.81 | −2.36 | ||
| ethyl octanoate | Cabernet franc | 100 | 14 | 43.0 | 0.05 | 9.05 * | 0.220 | −0.002 | * |
| Cabernet Sauvignon | 100 | 13 | 22.5 | 0.07 | 3.19 | 0.205 | −0.002 | ||
| Chardonnay | 100 | 24 | 14.2 | 0.57 | 3.63 | 1.215 | −0.011 | ||
| Meritage | 96 | 23 | 41.0 | 0.04 | 14.61 *** | 0.144 | −0.002 | *** | |
| Merlot | 100 | 36 | 50.4 | 0.12 | 34.51 *** | 0.511 | −0.006 | *** | |
| Pinot gris | 100 | 18 | 52.9 | 0.20 | 17.95 *** | 0.755 | −0.009 | *** | |
| Pinot noir | 100 | 20 | 52.8 | 0.09 | 20.15 *** | 0.297 | −0.004 | *** | |
| Syrah | 100 | 12 | 40.4 | 0.07 | 6.79 * | 0.230 | −0.003 | * | |
| ethyl isovalerate | Cabernet franc | 94 | 13 | 13.6 | 0.02 | 1.73 | 0.0374 | −0.0003 | |
| Cabernet Sauvignon | 94 | 13 | 11.9 | 0.03 | 1.49 | 0.0492 | −0.0005 | ||
| Chardonnay | 83 | 23 | 2.9 | 0.01 | 0.64 | 0.0096 | −0.0001 | ||
| Meritage | 79 | 21 | 36.4 | 0.00 | 10.85 ** | −0.0008 | 0.0001 | ** | |
| Merlot | 97 | 33 | 15.7 | 0.02 | 5.78 ** | 0.0505 | −0.0005 | * | |
| Pinot gris | 95 | 20 | 6.2 | 0.01 | 1.18 | 0.0093 | −0.0001 | ||
| Pinot noir | 95 | 19 | 48.6 | 0.01 | 16.05 *** | 0.0304 | −0.0003 | *** | |
| Syrah | 92 | 9 | 48.4 | 0.01 | 6.57 * | 0.0242 | −0.0003 | * | |
| isoamyl acetate | Cabernet franc | 100 | 14 | 47.4 | 0.07 | 10.81 ** | 0.293 | −0.003 | ** |
| Cabernet Sauvignon | 100 | 12 | 29.4 | 0.05 | 4.17 | 0.201 | −0.002 | ||
| Chardonnay | 100 | 22 | 38.8 | 0.18 | 12.67 ** | 0.579 | −0.007 | ** | |
| Meritage | 100 | 22 | 40.9 | 0.09 | 13.83 ** | 0.296 | −0.004 | ** | |
| Merlot | 100 | 36 | 16.4 | 0.35 | 6.67 * | 0.693 | −0.007 | * | |
| Pinot gris | 100 | 19 | 50.2 | 0.07 | 17.16 *** | 0.208 | −0.003 | *** | |
| Pinot noir | 100 | 19 | 39.4 | 0.06 | 11.06 ** | 0.216 | −0.002 | ** | |
| Syrah | 100 | 11 | 76.8 | 0.12 | 29.77 *** | 0.644 | −0.010 | *** | |
| methyl acetate | Cabernet franc | 63 | 15 | 35.3 | 0.05 | 7.10 * | −0.055 | 0.002 | * |
| Cabernet Sauvignon | 94 | 12 | 4.2 | 0.08 | 0.43 | 0.094 | 0.001 | ||
| Meritage | 88 | 21 | 20.3 | 0.10 | 4.85 * | 0.270 | −0.003 | * | |
| Merlot | 95 | 31 | 40.9 | 0.23 | 20.04 *** | 0.793 | −0.009 | *** | |
| Pinot noir | 100 | 18 | 54.3 | 0.10 | 19.01 *** | 0.437 | −0.005 | *** | |
| Syrah | 92 | 10 | 65.6 | 0.06 | 15.27 ** | 0.381 | −0.005 | ** | |
| isobutyl acetate | Cabernet franc | 50 | 13 | 6.2 | 0.001 | 0.72 | 0.00005 | 0.00001 | |
| Cabernet Sauvignon | 69 | 13 | 14.4 | 0.001 | 1.85 | −0.00004 | 0.00002 | ||
| Meritage | 83 | 18 | 20.8 | 0.001 | 4.21 | 0.00014 | 0.00003 | ||
| Merlot | 47 | 35 | 5.5 | 0.003 | 1.91 | 0.00367 | −0.00004 | ||
| Pinot noir | 81 | 20 | 27.7 | 0.005 | 6.90 * | 0.01064 | −0.00013 | * | |
| Syrah | 50 | 10 | 2.5 | 0.001 | 0.20 | 0.00020 | 0.00001 | ||
| Wine Compound a | Variety | Percent Detection (%) | n Used in the Regression Model | Coefficient of Determination R2 (%) | Root Mean Square Error (RMSE) | F-Ratio | Coefficients for “Wine Age” | ||
|---|---|---|---|---|---|---|---|---|---|
| Intercept b (b0) (mg/L) | Slope (b1) (mg/L/mon) | Significance of b1 | |||||||
| acetic acid | Cabernet franc | 94 | 15 | 33.4 | 5.00 | 6.51 * | 15.99 | −0.16 | * |
| Chardonnay | 63 | 22 | 4.7 | 1.29 | 1.00 | 0.63 | 0.01 | ||
| Meritage | 96 | 23 | 48.0 | 3.46 | 19.41 *** | 13.39 | −0.20 | *** | |
| Merlot | 61 | 33 | 8.9 | 1.20 | 3.03 | 0.06 | 0.02 | ||
| Pinot gris | 81 | 20 | 37.4 | 1.85 | 10.76 ** | 4.51 | −0.06 | ** | |
| Pinot noir | 67 | 20 | 53.5 | 1.10 | 20.70 *** | −0.44 | 0.05 | *** | |
| Syrah | 100 | 11 | 43.6 | 3.61 | 6.96 * | 12.41 | −0.14 | * | |
| acetaldehyde | Cabernet franc | 100 | 14 | 34.3 | 3.89 | 6.27 * | 13.61 | −0.12 | * |
| Cabernet Sauvignon | 94 | 14 | 2.8 | 7.61 | 0.34 | 2.74 | 0.06 | ||
| Chardonnay | 71 | 20 | 26.3 | 2.12 | 6.43 * | −0.55 | 0.06 | * | |
| Meritage | 100 | 23 | 20.2 | 2.76 | 5.31 * | 8.81 | −0.08 | * | |
| Merlot | 71 | 33 | 19.3 | 1.24 | 7.42 | −0.35 | 0.03 | * | |
| Pinot gris | 100 | 20 | 35.6 | 2.57 | 9.97 ** | 7.97 | −0.07 | ** | |
| Pinot noir | 67 | 19 | 57.0 | 1.25 | 22.50 *** | −0.64 | 0.06 | *** | |
| Syrah | 92 | 10 | 38.5 | 1.58 | 5.01 | 6.74 | −0.07 | ||
| dimethylsulfide | Cabernet franc | 56 | 15 | 23.3 | 0.010 | 3.96 | −0.00601 | 0.00024 | |
| Cabernet Sauvignon | 88 | 13 | 2.7 | 0.900 | 0.31 | 0.11505 | −0.00080 | ||
| Chardonnay | 46 | 20 | 17.6 | 0.007 | 3.83 | −0.00202 | 0.00017 | ||
| Meritage | 71 | 22 | 26.1 | 0.020 | 7.05 * | −0.00065 | 0.00054 | * | |
| Merlot | 58 | 32 | 10.7 | 0.008 | 3.60 | −0.00063 | 0.00012 | ||
| Pinot gris | 43 | 18 | 1.4 | 0.005 | 0.23 | 0.00182 | 0.00002 | ||
| Pinot noir | 67 | 21 | 42.6 | 0.007 | 14.10 ** | −0.00305 | 0.00024 | ** | |
| Syrah | 50 | 10 | 14.0 | 0.007 | 1.30 | 0.00005 | 0.00011 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejaei, M.; Cliff, M.A.; Madilao, L.L.; vanVuuren, H.J.J. Modelling Changes in Volatile Compounds in British Columbian Varietal Wines That Were Bottle Aged for Up to 120 Months. Beverages 2019, 5, 57. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages5030057
Bejaei M, Cliff MA, Madilao LL, vanVuuren HJJ. Modelling Changes in Volatile Compounds in British Columbian Varietal Wines That Were Bottle Aged for Up to 120 Months. Beverages. 2019; 5(3):57. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages5030057
Chicago/Turabian StyleBejaei, Masoumeh, Margaret A. Cliff, Lufiani L. Madilao, and Hennie J. J. vanVuuren. 2019. "Modelling Changes in Volatile Compounds in British Columbian Varietal Wines That Were Bottle Aged for Up to 120 Months" Beverages 5, no. 3: 57. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages5030057





