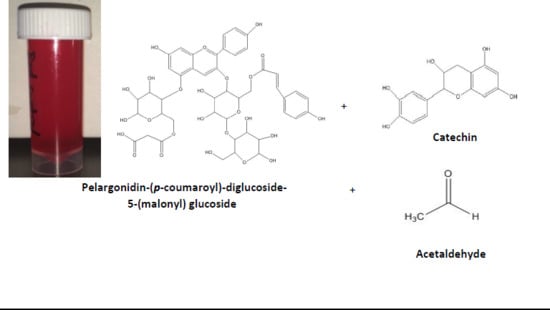
Formation, Tentative Mass Spectrometric Identification, and Color Stability of Acetaldehyde-Catalyzed Condensation of Red Radish (Raphanus sativus) Anthocyanins and (+) Catechin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Extraction of Polyphenolics and Formulation of Model Systems
2.3. Color Analysis
2.4. HPLC-PDA Analysis of Anthocyanins
2.5. HPLC-Electrospray Ionization Tandem Mass Spectrometry (HPLC-ESI-MS) Analysis of Degradation Products
2.6. Sephadex LH-20 Solid Phase Extraction and Matrix Assisted Laser Desorption Ionization Time of Flight (MALDI-TOF) Analysis
2.7. On-Fiber Derivatization Headspace Solid Phase Microextraction Gas Chromatography-Mass Spectrometry (OFD-HS-SPME-GC-MS) and OFD-HS-SPME-GC-Flame Ionization Detection (FID) Analysis of Acetaldehyde
2.8. Radish Fermentation in Bioreactor
2.9. Distillation of Zymomonas Fermentation and Distillate Model System Formation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Color Properties of Radish Samples
3.2. HPLC/HPLC-MS Identification and Quantification of Radish Anthocyanins and Anthocyanin-Catechin Ethyliden-Bridged Dimers over Storage
3.3. MALDI-TOF-MS Analysis of Radish Extracts over Storage
3.4. Zymomonas Mobilis Fermentatio
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feingold, B.F. Hyperkinesis and learning disabilities linked to the ingestion of artificial food colors and flavors. J. Learn. Disabil. 1976, 9, 551–559. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Timberlake, C.F.; Bridle, P. Interactions between anthocyanins, phenolic compounds, and acetaldehyde and their significance in red wines. Am. J. Enol. Vitic. 1976, 27, 97–105. [Google Scholar]
- Francia-Aricha, E.M.; Guerra, M.T.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. New anthocyanin pigments formed after condensation with flavanols. J. Agric. Food Chem. 1997, 45, 2262–2266. [Google Scholar] [CrossRef]
- Drinkine, J.; Lopes, P.; Kennedy, J.A.; Teissedre, P.; Saucier, C. Ethylidene-bridged flavan-3-ols in red wine and correlation with wine age. J. Agric. Food Chem. 2007, 55, 6292–6299. [Google Scholar] [CrossRef]
- Morata, A.; Calderón, F.; González, M.C.; Gómez-Cordovés, M.C.; Suárez, J.A. Formation of the highly stable pyranoanthocyanins (vitisins A and B) in red wines by the addition of pyruvic acid and acetaldehyde. Food Chem. 2007, 100, 1144–1152. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stability of anthocyanin-based aqueous extracts of Andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants. Food Chem. 2004, 86, 69–77. [Google Scholar] [CrossRef]
- Eiro, M.J.; Heinonen, M.A. Anthocyanin color behavior and stability during storage: Effect of intermolecular copigmentation. J. Agric. Food Chem. 2002, 50, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra- and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Radish anthocyanin extract as a natural colorant for maraschino cherries. J. Food Sci. 1996, 61, 688–694. [Google Scholar] [CrossRef]
- Rodriguz-Saona, L.E.; Giusti, M.M.; Wrolstad, R.E. Color and pigment stability of red radish and red-fleshed potato anthocyanins in juice model systems. J. Food Sci. 1999, 64, 451–456. [Google Scholar] [CrossRef]
- Stebbins, N.B.; Howard, L.R.; Prior, R.L.; Brownmiller, C.; Liyanage, R.; Lay, J.O.; Yang, X.; Qian, S.Y. Ascorbic acid-catalyzed degradation of cyanidin-3-O-β-glucoside: Proposed mechanism and identification of a novel hydroxylated product. J. Berry Res. 2016, 6, 175–187. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry, and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Kantz, K.; Singleton, V.L. Isolation and determination of polymeric polyphenols using Sephadex LH-20 and analysis of grape tissue extracts. Am. J. Enol. Vitic. 1990, 41, 223–228. [Google Scholar]
- Wang, Q.; O’Reilly, J.; Pawliszyn, J. Determination of low-molecular mass aldehydes by automated headspace solid-phase microextraction with in-fibre derivatisation. J. Chromatogr. A 2005, 1071, 147–154. [Google Scholar] [CrossRef]
- Carlton, W.K.; Gump, B.; Fugelsang, K.; Hasson, A.S. Monitoring acetaldehyde concentrations during micro-oxygenation of red wine by headspace solid-phase microextraction with on-fiber derivatization. J. Agric. Food Chem. 2007, 55, 5620–5625. [Google Scholar] [CrossRef]
- Weber, F.; Winterhalter, P. Synthesis and structure elucidation of ethyliden-linked anthocyanin-flavan-3-ol oligomers. Food Res. Int. 2014, 65, 69–76. [Google Scholar] [CrossRef]
- Duenas, M.; Salas, E.; Cheynier, V.; Dangles, O.; Fulcrand, H. UV-visible spectroscopic investigation of the 8,8 methylmethine catechin-malvidin 3-glucoside pigments in aqueous solution: Structural transformations and molecular complexation with chlorogenic acid. J. Agric. Food Chem. 2006, 54, 189–196. [Google Scholar] [CrossRef]
- Harborne, J.B. Plant polyphenols IX: The glycosidic pattern of anthocyanin pigments. Phytochemistry 1963, 2, 85–97. [Google Scholar] [CrossRef]
- Fuleki, T. The anthocyanins of strawberry, rhubarb, radish, and onion. J. Food Sci. 1969, 34, 365–369. [Google Scholar] [CrossRef]
- Hanlon, P.R.; Barnes, D.M. Phytochemical composition and biological activity of 8 varieties of radish (Raphanus sativus L.) sprouts and mature taproots. J. Food Sci. 2011, 76, C185–C192. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Characterization of red radish anthocyanins. J. Food Sci. 1996, 61, 322–326. [Google Scholar] [CrossRef]
- Wu, A.; Prior, R.L. Identification and characterization of anthocyanins by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry in common foods in the United States: Vegetables, nuts and grains. J. Agric. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O.; Saito, N.; Brouillard, R. Anthocyanin intramolecular copigment effect. Phytochemistry 1993, 34, 119–124. [Google Scholar] [CrossRef]
- Brouillard, R. Origin of the exceptional colour stability of Zebrina anthocyanin. Phytochemistry 1981, 20, 143–145. [Google Scholar] [CrossRef]
- Garcia-Viguera, C.; Bridle, P.; Bakker, J. The effect of pH on the formation of coloured compounds in model solutions containing anthocyanins, catechin, and acetaldehyde. Vitis 1994, 33, 37–40. [Google Scholar]
- Roy, A.; Mukherjee, R.P.; Howard, L.; Beitle, R. Bio-based extraction and stabilization of anthocyanins. Biotechnol. Prog. 2016, 32, 601–605. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stebbins, N.B.; Howard, L.R.; Prior, R.L.; Brownmiller, C.; Liyanage, R.; Lay, J.O. Formation, Tentative Mass Spectrometric Identification, and Color Stability of Acetaldehyde-Catalyzed Condensation of Red Radish (Raphanus sativus) Anthocyanins and (+) Catechin. Beverages 2019, 5, 64. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages5040064
Stebbins NB, Howard LR, Prior RL, Brownmiller C, Liyanage R, Lay JO. Formation, Tentative Mass Spectrometric Identification, and Color Stability of Acetaldehyde-Catalyzed Condensation of Red Radish (Raphanus sativus) Anthocyanins and (+) Catechin. Beverages. 2019; 5(4):64. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages5040064
Chicago/Turabian StyleStebbins, Nathan B., Luke R. Howard, Ronald L. Prior, Cindi Brownmiller, Rohana Liyanage, and Jackson O. Lay. 2019. "Formation, Tentative Mass Spectrometric Identification, and Color Stability of Acetaldehyde-Catalyzed Condensation of Red Radish (Raphanus sativus) Anthocyanins and (+) Catechin" Beverages 5, no. 4: 64. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages5040064