Nutrient Addition to Low pH Base Wines (L. cv. Riesling) during Yeast Acclimatization for Sparkling Wine: Its Influence on Yeast Cell Growth, Sugar Consumption and Nitrogen Usage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strain
2.2. Chemical Analysis
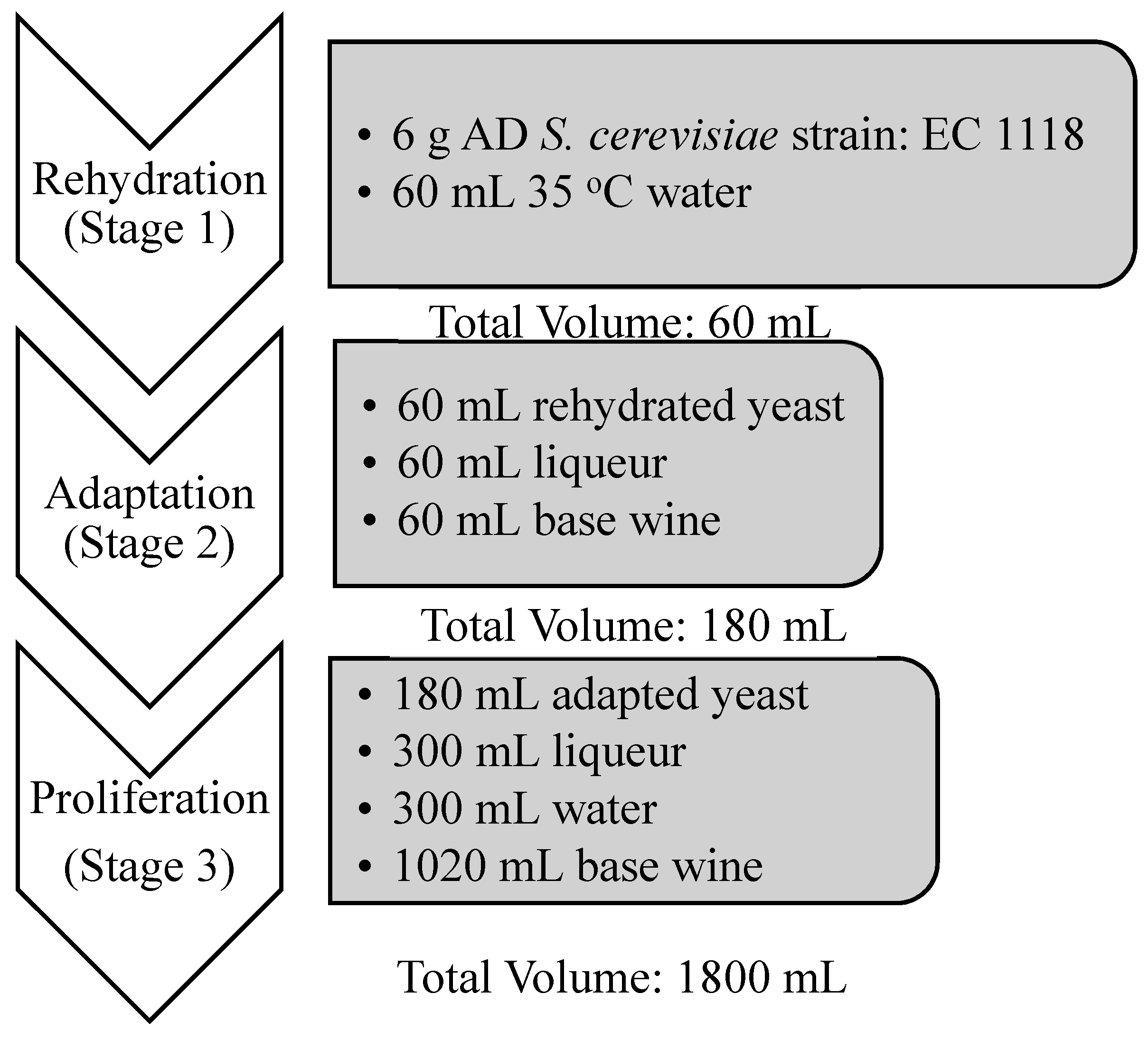
2.3. Yeast Acclimatization/Tirage
2.4. Statistical Analysis
3. Results and Discussion
3.1. Nutrient Addition Impact on Cell Growth and Viability During Yeast Acclimatization
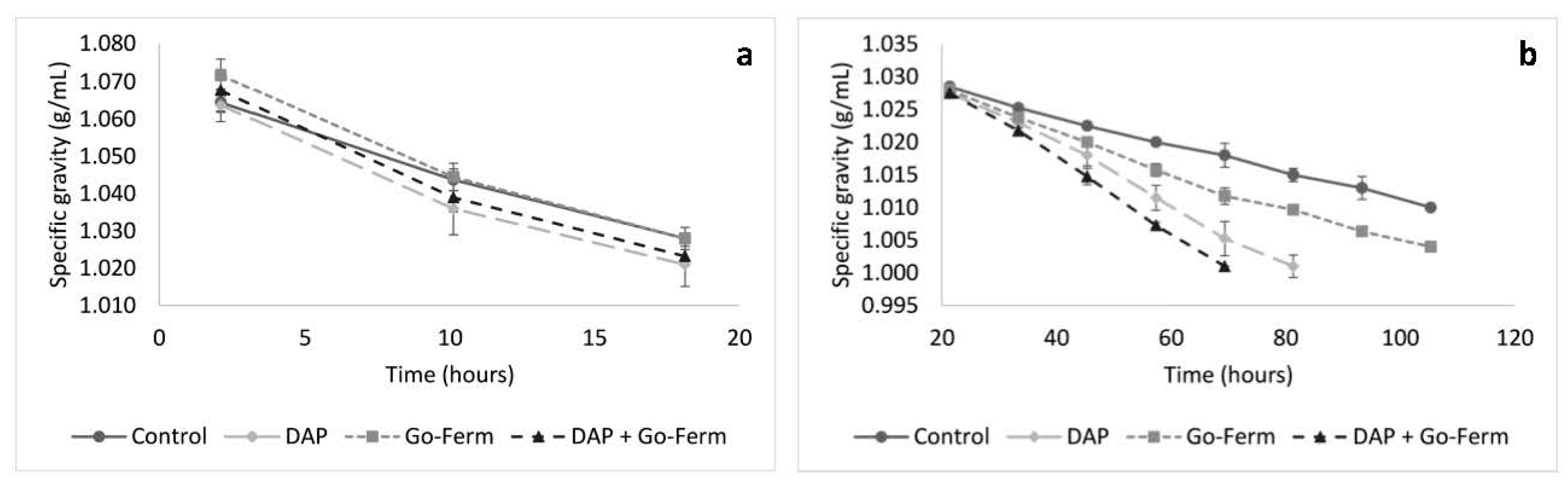
3.2. Nutrient Addition Impact on Sugar Consumption During Yeast Acclimatization
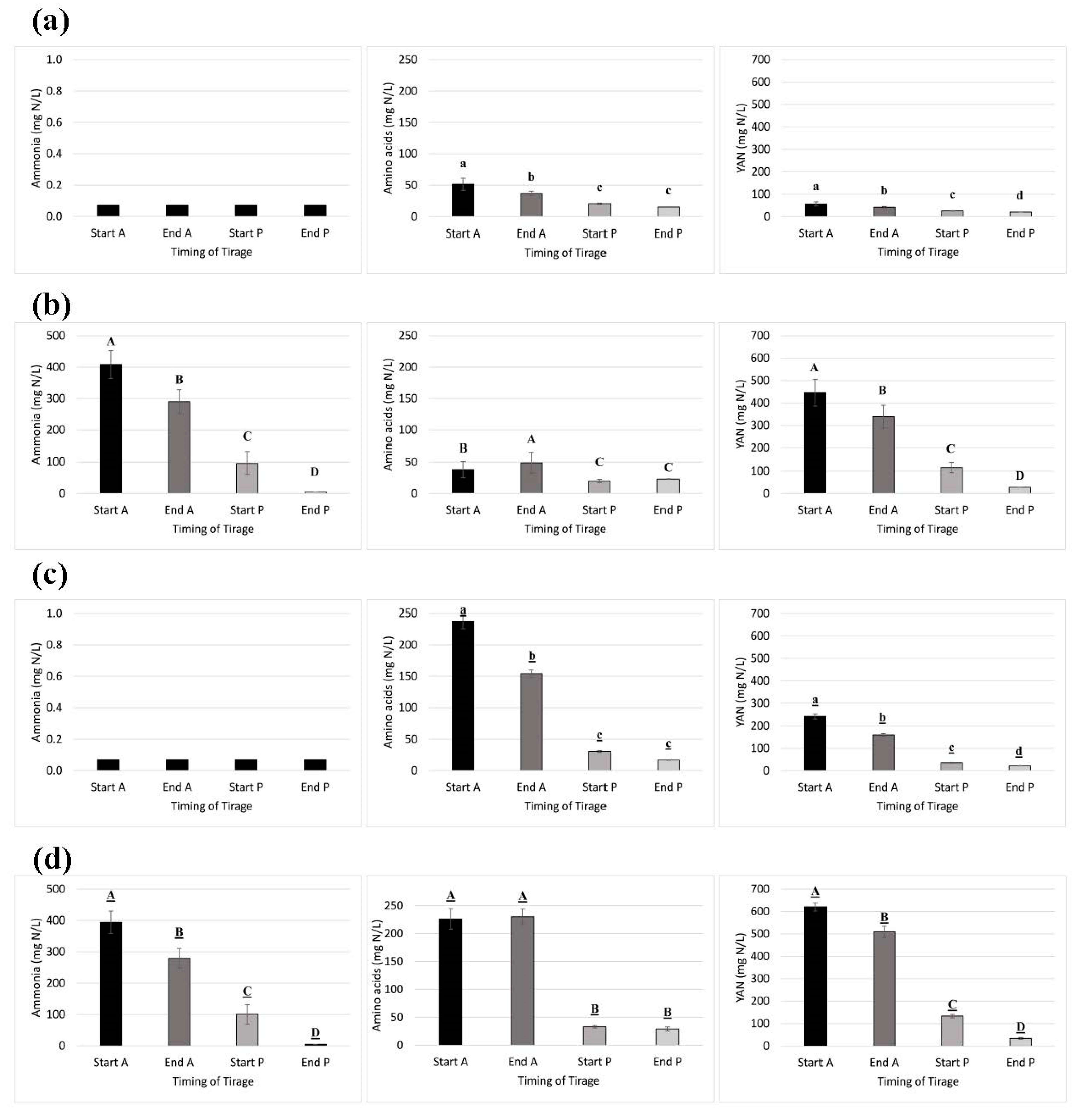
3.3. Nutrient Addition Impact on Nitrogen Consumption During Yeast Acclimatization
3.4. Overall Impact of Yeast Acclimatization with or without Nutrients Prior to Second Alcoholic Fermentation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laurent, M.; Valade, M. La Préparation du Levain a Partir de Levures Seches Actives. Le Vign Champen Mars 2007, 74–95. [Google Scholar]
- Valade, M.; Laurent, M. La Réussite de la Prise de Mousse 2. Le Vign Champen Mai 2015, 54–71. [Google Scholar]
- Pozo-Bayón, A.; Andújar-Ortiz, I.; Moreno-Arribas, V. Scientific evidences beyond the application of inactive dry yeast preparations in winemaking. Food Res. Int. 2009, 42, 754–761. [Google Scholar] [CrossRef]
- Marti-Raga, M.; Marullo, P.; Beltran, G.; Mas, A. Nitrogen modulation of yeast fitness and viability during sparkling wine production. Food Microbiol. 2016, 54, 106–114. [Google Scholar] [CrossRef]
- Borrull, A.; López-Martínez, G.; Miró-Abella, E.; Salvadó, Z.; Poblet, M.; Cordero-Otero, R.; Rozès, N. New insights into the physiological state of Saccharomyces cerevisiae during ethanol acclimation for producing sparkling wines. Food Microbiol. 2016, 54, 20–29. [Google Scholar] [CrossRef]
- Kemp, B.; Alexandre, H.; Robillard, B.; Marchal, R. Review: Effect of Production Phase on Bottle-Fermented Sparkling Wine Quality. J. Agric. Food Chem. 2015, 63, 19–38. [Google Scholar] [CrossRef]
- Carrascosa, A.V.; Martinez-Rodriguez, A.; Cebollero, E.; González, R. Saccharomyces Yeasts II: Secondary Fermentation. In Molecular Wine Microbiology; Elsevier: Burlington, MA, USA, 2011; pp. 33–49. [Google Scholar]
- Esteve-Zarzoso, B.; Martinez, M.; Rubires, X.; Yuste-Rojas, M.; Torres, M. Applied Wine Microbiology. In Molecular Wine Microbiology; Elsevier: Burlington, MA, USA, 2011; pp. 341–355. [Google Scholar]
- Margalit, Y. Must and Wine Composition: Nitrogen. In Concepts in Wine Chemistry, 3rd ed.; The Wine Appreciation Guild: San Francisco, CA, USA, 2012. [Google Scholar]
- Marti-Raga, M.; Sancho, M.; Marullo, P.; Guillamon, J.M.; Mas, A.; Beltran, G. Factors Affecting the Second Fermentation Development: The Role of Nitrogen on Sparkling Wine Production. In Cava Challenges:.OENOVITI INTERNATIONAL network, Proceedings of the 33rd International CAVA Congress, Sant Sadurni d’Anoia, Spain, 20 May 2015; Available online: https://en.calameo.com/books/003503752313ba0bf59de (accessed on 14 February 2020).
- Marti-Raga, M.; Sancho, M.; Guillamon, J.M.; Mas, A.; Beltran, G. The effect of nitrogen addition on the fermentative performance during sparkling wine production. Food Res. Int. 2015, 67, 126–135. [Google Scholar] [CrossRef]
- Valade, M.; Laurent, M.; Moncomble, D. La Réussite de la Prise de Mousse 1. Le Vign Champen Avril 2015, 48–63. [Google Scholar]
- Martinez-Moreno, R.; Morales, P.; Gonzalez, R.; Mas, A.; Beltran, G. Biomass production and alcoholic fermentation performance of Saccharomyces cerevisiae as a function of nitrogen source. FEMS Yeast Res. 2012, 12, 477–485. [Google Scholar] [CrossRef]
- Leao, C.; van Uden, N. Effects of ethanol and other alkanols on the ammonium transport system of Saccharomyces cerevisiae. Biotechnol. Bioeng. XXV 1983, 2085–2089. [Google Scholar] [CrossRef]
- Leao, C.; van Uden, N. Effect of ethanol and other alkanols on the general amino acid permease of Saccharomyces cerevisiae. Biotechnol. Bioeng. XXVI 1984, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Fugelsang, K.C.; Edwards, C.G. Wine Microbiology Practical Applications and Procedures; Springer: New York, NY, USA, 2010. [Google Scholar]
- Borrull, A.; Poblet, M.; Rozes, N. New insights into the capacity of commercial wine yeasts to grow on sparkling wine media. Factor screening for improving wine yeast selection. Food Microbiol. 2015, 48, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Moradas-Ferreira, P.; Costa, V.; Piper, P.; Mager, W. The molecular defences against reactive oxygen species in yeast. Mol. Microbiol. 1996, 19, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Munoz, E.; Ingledew, W.M. Effect of yeast hulls on stuck and sluggish wine fermentations: Importance of the lipid component. Appl. Environ. Microbiol. 1989, 55, 1560–1564. [Google Scholar] [CrossRef] [Green Version]
- Julien-Ortiz, A.; Dulan, L. Integrare la composizione dei mosti. Vignevini Riv. Ital. di Vitic e di Enol. 2002, 29, 65–67. [Google Scholar]
- Zhao, X.Q.; Bai, F.W. Zinc and yeast stress tolerance: Micronutrient plays a big role. J. Biotechnol. 2012, 158, 176–183. [Google Scholar] [CrossRef]
- Kudo, M.; Vagnoli, P.; Bisson, L.F. Imbalance of pH and potassium concentration as a cause of stuck fermentations. Am. J. Enol. Vitic. 1998, 49, 295–301. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology: Volume 1, The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Benucci, I.; Liburdi, K.; Cerreti, M.; Esti, M. Characterization of active dry wine yeast during starter culture (pied de cuve) preparation for sparkling wine production. J. Food Sci. 2016, 81, M2015–M2020. [Google Scholar] [CrossRef]
- Nurgel, C.; Pickering, G.; Inglis, D. Sensory and chemical characteristics of Canadian Icewines. J. Sci. Food Agric. 2004, 84, 1675–1684. [Google Scholar] [CrossRef]
- Rankine, B.C.; Pocock, K.J. Alkalimetric determination of sulfur dioxide in wine. Aust. Wine Brew Spirit Rev. 1970, 88, 40–44. [Google Scholar]
- Muysson, J.; Miller, L.; Allie, R.; Inglis, D.L. The Use of CRISPR-Cas9 Genome Editing to Determine the Importance of Glycerol Uptake in Wine Yeast during Icewine Fermentation. Fermentation 2019, 5, 93. [Google Scholar] [CrossRef]
- Bisson, L.F. Stuck and Sluggish Fermentation. Am. J. Enol. Vitic. 1999, 50, 107–119. [Google Scholar]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Kontkanen, D.; Inglis, D.L.; Pickering, G.J.; Reynolds, A. Effect of Yeast inoculation rate, acclimatization, and nutrient addition on icewine fermentation. Am. J. Enol. Vitic. 2004, 55, 363–370. [Google Scholar]
- Ponte, J.G.; Glass, R.L.; Geddes, W.F. Studies on the behavior of active dry yeast in breadmaking. Cereal Chem. 1960, 37, 263–279. [Google Scholar]

| Titratable Acidity (g/L Tartaric) | 7.3 | |
| pH | 2.85 | |
| YAN * | Ammonia (mg N/L) | <7 |
| Alpha-amino acids (mg N/L) | 26.8 | |
| Sugar | Sucrose (g/L) | <0.09 |
| Fructose (g/L) | 0.25 | |
| Glucose (g/L) | <0.09 | |
| Ethanol (% (v/v)) | 10.6 | |
| SO2 | Free (ppm) | 8.4 |
| Total (ppm) | 56.5 | |
| Stage | Supplement | Control (T1) | DAP * (T2) | Go-Ferm® (T3) | DAP + Go-Ferm® (T4) |
|---|---|---|---|---|---|
| Rehydration (60 mL volume) | Go-Ferm® (g) | 0 | 0 | 7.5 | 7.5 |
| Adaptation (180 mL volume) | DAP (mg) | 0 | 360 | 0 | 360 |
| Proliferation (1800 mL volume) | DAP (mg) | 0 | 600 | 0 | 600 |
| Rate of Sugar Consumption (mg/mL/hour) | ||
|---|---|---|
| Adaptation Stage 2 | Proliferation Stage 3 | |
| Control | 2.3 ± 0.2 b | 0.23 ± 0.01 d |
| DAP | 2.7 ± 0.1 a | 0.45 ± 0.01 b |
| Go-Ferm® | 2.7 ± 0.1 a | 0.31 ± 0.01 c |
| DAP + Go-Ferm® | 2.8 ± 0.3 a | 0.55 ± 0.01 a |
| Treatment | Nitrogen Assimilated (mg N/L) | ||
|---|---|---|---|
| Adaptation | Proliferation | Total | |
| Control | 18 ± 4 c | 5 ± 1 b | 23 ± 3 c |
| DAP | 120 ± 30 a | 100 ± 20 a | 220 ± 30 a |
| Go-Ferm® | 80 ± 10 b | 14 ± 1 b | 94 ± 9 b |
| DAP + Go-Ferm® | 110 ± 20 a | 108 ± 5 a | 228 ± 20 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemp, B.; Plante, J.; L. Inglis, D. Nutrient Addition to Low pH Base Wines (L. cv. Riesling) during Yeast Acclimatization for Sparkling Wine: Its Influence on Yeast Cell Growth, Sugar Consumption and Nitrogen Usage. Beverages 2020, 6, 10. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages6010010
Kemp B, Plante J, L. Inglis D. Nutrient Addition to Low pH Base Wines (L. cv. Riesling) during Yeast Acclimatization for Sparkling Wine: Its Influence on Yeast Cell Growth, Sugar Consumption and Nitrogen Usage. Beverages. 2020; 6(1):10. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages6010010
Chicago/Turabian StyleKemp, Belinda, Jessy Plante, and Debra L. Inglis. 2020. "Nutrient Addition to Low pH Base Wines (L. cv. Riesling) during Yeast Acclimatization for Sparkling Wine: Its Influence on Yeast Cell Growth, Sugar Consumption and Nitrogen Usage" Beverages 6, no. 1: 10. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages6010010







