1. Introduction
The past decade has witnessed significant upheavals in the brewing industry. First, a long trend of increasing beer production volumes peaked in 2013 at 1.97 billion hL [
1], but has been steadily decreasing ever since [
2]. Second, the craft beer boom made small-scale breweries serious players in beer markets and third, towards the end of the decade, the low and non-alcoholic beer category started to live up to the expectations set up for years [
3]. These trends reflect the changes in the habits of beer drinkers. Although consumers are still spending more money on beer products [
4], there is greater focus on the overall experience and the health aspects rather than volume. Hence, there is a general interest in new processes to differentiate beers and a growing appreciation of the potential of non-conventional yeast species to create novel flavor profiles, or limit alcohol content [
3,
5,
6].
The suitability of novel yeast species for the production of specialty beers is often limited by their inability to utilize maltose and maltotriose, the two most abundant sugars in wort. Steensels and Verstrepen [
7] reported that only 12% of the wild yeast they studied were able to ferment 50% or more of the sugars available in wort. In the work of Methner et al. [
8], 110 non-
Saccharomyces yeast strains were studied and only about 30% of the strains were able to utilize maltose and 25% maltotriose. Strain-dependent variance was notable, as most species included both maltose-positive and -negative strains, highlighting the importance of strain screening in addition to species screening. Maltotriose-positive strains (which were also maltose-positive) belonged to six different species (
Debaryomyces hansenii, Kazachstania servazzii, Saccharomycopsis fibuligera, Schizosaccharomyces pombe, Wickerhamomyces anomalus and
Zygosaccharomyces rouxii) [
8]. In a study of Nikulin et al. [
9], it was further shown that the ability to ferment maltose may also be revealed only after a long exposure to the sugar, thereby complicating the categorization of strains with respect to sugar use.
The type strain of the yeast
Zygotorulaspora florentina (VTT C-94199; CBS 746) has been isolated from must. Other strains have been isolated from grapes [
10], plant material and soft drinks [
11]. The former name of the species was
Zygosaccharomyces florentinus, but based on DNA analysis it was reclassified as a
Zygotorulaspora species, joining
Zygotorulaspora mrakii, in the genus [
12]. A difference between the type strains of the species can be found in sugar utilization:
Z. florentina is able to ferment maltose and assimilate trehalose, maltose and melezitose, whereas these traits are lacking in the sister species,
Z. mrakii [
12]. The sister clade of the genus is
Torulaspora [
12], making
Torulaspora delbrueckii the closest brewing-related species. Culture collection strains of
Z. florentina are also cited to be able to ferment maltose, but suitability of the species for brewing has only been investigated in a few studies to date [
8,
13,
14]. Although Holt et al. [
14] mentioned the possible suitability of the species for brewing on its own, due to maltose consumption, more attention has been paid to co- and sequential-fermentations with
Saccharomyces cerevisiae. In cofermentation with a commercial ale yeast, the species was found to affect positively beer flavor [
13]. The species has also been studied for winemaking [
10,
15,
16], and in those studies, the species has been found to promote the levels of higher alcohols and esters, and to reduce volatile acidity in cofermentations with a commercial wine strain of
S. cerevisiae. The positive effects on aroma, maltose-fermentation capability and the reported inability to grow at 37 °C (culture collection data, [
11]) make the species an interesting candidate for brewing trials.
Here, we present results relating to the brewing potential of a maltose- and maltotriose-positive Z. florentina strain isolated from oak in Espoo, Finland. A number of brewing-relevant features were assessed, and these included the fermentation performance under different conditions. Sugar utilization and the ability to produce aroma compounds were assessed and results were compared to those of the type strain of the species and to two ale strains. The sensorial quality of the beer produced was assessed by a professional sensory panel. The suitability of the strain for use in breweries was also assessed with regard to the ability of the species to tolerate ethanol and a number of preservatives commonly used in other beverages.
2. Materials and Methods
2.1. Isolation
Three natural stands of oak (
Quercus robur) in Otaniemi, Espoo, Finland, were sampled in late December 2017. Samples were collected using the method by Sniegowski et al. [
17], which has been used previously for the isolation of yeasts from oak bark [
9]: a sterile cotton swab was dipped in sterile glycerol (85%), gently rubbed against the bark and transferred to a sterile 15 mL tube. Enrichment media (yeast extract 0.3%, malt extract 0.3%, peptone 0.5%, sucrose 1%, ethanol 7.6%, 1 M HCl 0.1% and chloramphenicol 0.0001% [
17]) was added to the tubes under aseptic conditions. Samples were then incubated for 2 weeks at 12 °C prior to plating of the suspension (120 µL) on Sniegowski agar selection media (methyl-α-d-glucopyranoside 2%, Yeast Nitrogen Base (YNB)/without amino acids 0.67%, 1 M HCl 0.4% and agar 1.5% [
17]). Emerging colonies were streaked on a YPD agar plate (1% yeast extract, 2% peptone, 2% glucose and 2% agar) and single colonies from this plate were grown up in YPD (2%) and stored at −80 °C in 30% glycerol.
2.2. Identification
The isolated strain VTT C-201041, referred to here as
Z. flo OTA, was identified based on the internal transcribe spacer (ITS) sequence. The DNA was extracted as described by Pham et al. (2011). Polymerase chain reaction (PCR) of the ITS1-5.8S-ITS2 region of the ribosomal DNA (rDNA) region was performed using universal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The DNA was purified with the Qiagen MinElute PCR Purification Kit (Venio, The Netherlands) and sequenced at SeqLab (Göttingen, Germany). The obtained ITS sequence was identified using BLAST search (
https://blast.ncbi.nlm.nih.gov/Blast.cgi, National Center for Biotechnology Information, U.S. National Library of Medicine, Bethesda, MD, USA) in the NCBI nucleotide (nt) database.
2.3. Wort Preparation
At the VTT in-house pilot brewery (1 hL) 100%-malt wort of 15 °P was prepared using municipal Espoo City water. An infusion mash was carried out and involved the following profile: 30 min at 48 °C, 30 min at 63 °C, 30 min at 72 °C and 10 min at 78 °C. Wort was filtered with a Meura filter (Meura, Belgium) and boiled 60 min prior to the whirlpool. The wort was collected hot (>90 °C) in sterile kegs and stored at 0 °C. When not specified, pilsner malt (Viking Malt, Lahti, Finland) was used together with magnum hops, with a target of 45 IBU in the wort. For wheat beer, 40% of the malt bill was wheat malt (Viking Malt). Prior to fermentations, the wort was diluted to the appropriate strength using sterile, degassed Espoo City water.
2.4. Prefermentations
Screenings were carried out in the form of small-scale fermentations and confirmed in 2 L-scale fermentations. Single-cell colonies were inoculated from agar plates into Erlenmeyer flasks with 50 mL of YPD and incubated for 48 h on a shaker (120 rpm) at room temperature. For 2 L-scale fermentation, an additional propagation step was taken by adding the 50 mL into 200 mL of fresh YPD and incubated another 48 h on a shaker. The suspensions were centrifuged (4000 rpm; 5 min; 4 °C) and 20% slurries (200 mg fresh yeast/mL supernatant) were prepared. The fermentations were conducted in duplicate in 100 mL of 9 °P wort at a pitching rate of 1 g of fresh yeast/L. The fermentation vessels were 250 mL Erlenmeyer flasks, capped with airlocks filled with glycerol (85%). The fermentations were conducted on a shaker with low agitation (40 rpm) until no mass-change was observed in consecutive days. This was 10 days at 25 °C and 17 days at 15 °C. The 2 L-scale fermentations were conducted statically in stainless steel cylindrical fermentation vessels with 8 °P wort at 20 °C using the same pitching rate. The latter fermentation was monitored through regular sampling with an Anton Paar DMA 5000 M Density Meter and Alcolyzer (Anton Paar GmbH, Graz, Austria).
2.5. Ferulic Acid Usage
Ability to form the phenolic flavor compound 4-vinyl guaiacol (4-VG) was assessed as described by Mertens et al. (2017). The yeasts, Z. flo OTA and reference strains VTT A-81062 (producing 4-VG) and VTT A-63015 (not producing 4-VG), were inoculated (0.5–2 × 107 cells/mL) from YPD agar plates into 1.5 mL of YPD (2%) supplemented with ferulic acid (100 mg/L) in Eppendorf tubes and incubated five days on a shaker (120 rpm) in duplicate. The absorbances of yeast cultures and the blank YPD media, with and without the supplemented ferulic acid, were measured at ABS320. A decrease in the absorbance greater than 10% relative to the blank (uninoculated YPD supplemented with ferulic acid) was considered as an indication of the ability to form phenolic flavor compounds from ferulic acid. The production of 4-vinylguaiacol was confirmed by smelling the cultures after incubation.
4-vinylphenol and 4-vinylguaiacol in bottled beers were analyzed according to the MEBAK method 2.21.3.3 Wort, Beer, Beer-based Beverages [
18].
2.6. Temperature Tolerance
The ability of the yeasts to grow at different temperatures was screened using spot plates. Yeasts were propagated overnight in 20 mL of liquid YPD, centrifuged and resuspended to OD600 of 0.5 (1–5 × 106 cells per mL) using sterile Milli-Q-filtered H2O, and further-diluted to concentrations of 0.05, 0.005 and 0.0005 prior to pipetting spots (5 µL) of each dilution on YPD agar plates. The plates were incubated at 10 °C, 21 °C and 37 °C until colonies appeared. A lager yeast strain VTT A-63015 (psychrotolerant) and an ale yeast strain WLP380 (mesophilic) were used as references.
2.7. Dextrin Usage
Agar plate screening was performed using starch agar plates as described by Krogerus et al. (2019). The yeasts, Z. flo OTA and VTT A-81062, were grown overnight in YPD (2%), washed twice with sterile saline water (centrifuging 4000 rpm; 5 min; 4 °C) and resuspended to OD600 1.0 with sterile Milli-Q-filtered H2O. The suspension (100 µL) was spread on a starch agar plate (0.67% YNB/wo amino acids, 1.5% soluble starch (Merck, Darmstadt, Germany) and 40 mg/L of bromophenol blue with pH adjusted to 5.2 with 0.1 M HCl). The plates were incubated in an anaerobic jar (Anoxomat AN2CTS, Mart Microbiology, Drachten, The Netherlands) at 20 °C for 6 weeks.
2.8. Alcohol Sensitivity
Wort of 15 °P was diluted to 9 °P using autoclaved Espoo City water with and without supplemented ethanol (ABV 10%) to attain starting concentrations of 0%, 2% and 4% of ethanol. Yeasts were propagated in 50 mL of YPD 4% and in 200 mL of 9 °P wort on a shaker (120 rpm) prior to pitching at a rate of 5 g of fresh yeast mass/L into a wort with varying levels of ethanol. Static fermentations were performed in duplicate and were followed by monitoring the change in mass due to CO2 loss over time. Sensitivity was determined based on the weight-loss curves. The lager yeast strain VTT A-63015 was used as a reference.
2.9. Preservative Tolerance
The tolerance of the strain Z. flo OTA towards common food preservatives was assessed in microplate cultivations using a Bioscreen C MBR incubator and plate reader (Oy Growth Curves Ab, Helsinki, Finland). The yeast was propagated by taking a loopful of fresh yeast mass from YPD agar and inoculating into 30 mL of liquid YPD. After two days on a shaker (120 rpm), the culture was centrifuged (4000 rpm, 5 min, 1 °C) and the pellet was washed with 30 mL of sterile 0.9% NaCl. A 20%-slurry was prepared and cell density measured using the NucleoCounter YC-100. Wells were filled with YPD (1% glucose w/v) with one of the following preservatives: ethanol 5% (v/v) (AaS, Rajamäki, Finland), sorbate 250 mg/L (potassium sorbate, Sigma-Aldrich, Darmstadt, Germany), benzoate 150 mg/L (sodium benzoate, Sigma-Aldrich, Darmstadt, Germany) and sulfite 200 mg/L (potassium metabisulfite, Brown, Poland). All media were adjusted to a pH of 4 prior to pitching approximately 2000 cells per well. The final volume was 300 µL per well and the cultivations were carried out at 20 °C with moderate shaking. Cultivations were conducted in triplicate.
2.10. Fermentations
With the isolated Z. florentina strain (Z. flo OTA) 2 L-scale fermentations were conducted, together with the type strain of Z. florentina VTT C-94199 (Z. flo C199) and an ale yeast VTT A-81062 (A62; a high-attenuating ale yeast strain). The yeasts were propagated first in 25 mL of YPD and the volume was increased to 500 mL the following day. Cultures were incubated on a shaker (120 rpm) and after 48 h in 500 mL, 20%-slurries were prepared and cell densities determined with a NucleoCounter YC-100TM (Chemometec, Denmark). The total pitching rate was 1.2 × 107 cells/mL and normal pilsner type wort at a strength of 12 °P was used. The wort was aerated to 10 ppm prior to pitching and the progression of fermentation was monitored by regular sampling and determination of alcohol, wort gravity, pH and yeast mass. Samples for the HPLC analysis of sugars were collected at each sampling time.
For 10 L-scale fermentations, yeasts were propagated as follows: a loopful of yeast was inoculated into YPD and incubated aerobically on a shaker (inoculation into 25 mL, continued in 500 mL on the following day). The suspension was centrifuged, 20%-slurry prepared and inoculated into 1.5 L of 12 °P wort in a 2 L Schott-bottle capped with an airlock. After five days of static fermentation, the yeast was removed by centrifugation (4000 rpm; 5 min; 4 °C) and a 20% slurry was prepared. Cell number was determined as above and a pitching rate of 7 × 106 cells/mL was used. Fermentations were carried out in 12 °P wheat wort using the wheat beer yeast strain WLP380 as a reference. The wort was aerated to 10 ppm of oxygen prior to pitching and the fermentations were monitored through regular sampling as before. The young beers, so-called green beers, were transferred from fermenters to kegs, matured for four days at 12 °C and stabilized seven days at 0 °C before depth filtration (Seitz EK, Pall Corporation, New York, NY, USA). Prior to bottling, the beers were carbonated to 5 g/L. The bottled beers were stored at 0 °C. Samples for further analysis (sugars and aroma volatiles) were collected from both green beers and the bottled beers.
2.11. Analyses
The samples collected from fermentations were centrifuged and supernatants were used in analyses after manual degassing. The specific gravity, alcohol level (% v/v) and pH of samples were determined using an Anton Paar Density Meter DMA 5000 M with Alcolyzer Beer ME and pH ME modules (Anton Paar GmbH, Graz, Austria).
The yeast mass content of the samples (i.e., yeast in suspension) was determined by washing the yeast pellets twice with 25 mL of deionized H2O in a centrifuge tube, resuspending in Milli-Q-filtered H2O and drying overnight in preweighed crucibles at 105 °C. Where necessary, the yeasts were analyzed for cell viability using the Chemometec Nucleocounter.
Sugar content of wort was analyzed by HPLC. A Waters 2695 Separation Module and Waters System Interphase Module liquid chromatograph coupled with a Waters 2414 differential refractometer (Waters Co., Milford, MA, USA) was used. An Aminex HPX-87H Organic Acid Analysis Column (300 mm × 7.8 mm; Bio-Rad, Hercules, CA, USA) was equilibrated with 5 mM H2SO4 (Titrisol, Merck, Germany) in water at 55 °C, and samples were eluted with 5 mM H2SO4 in water at a 0.3 mL/min flow rate.
Yeast-derived volatile aroma compounds (acetaldehyde, higher alcohols and esters) were determined by headspace gas chromatography with a flame ionization detector (HS-GC-FID). Samples of 4 mL were filtered (0.45 µm), incubated at 60 °C for 30 min and then 1 mL of the gas phase was injected (split mode; 225 °C; split flow of 30 mL/min) into a gas chromatograph equipped with an FID detector and headspace autosampler (Agilent 7890 Series; Palo Alto, CA, USA). Analytes were separated on a HP-5 capillary column (50 m × 320 µm × 1.05 µm column, Agilent, Santa Clara, CA, USA). The carrier gas was helium (constant flow of 1.4 mL/min). The temperature program was 50 °C for 3 min, 10 °C/min to 100 °C, 5 °C/min to 140 °C, 15 °C/min to 260 °C and then isothermal for 1 min. Compounds were identified by comparison with authentic standards and were quantified using standard curves. 1-Butanol was used as the internal standard.
Biogenic amine levels in bottled beer were determined by Eurofins Food Testing Belgium NV (Venecoweg 5, 9810 Nazareth, Belgium) using a liquid chromatograph with a method described by Smělá et al. [
19].
2.12. Sensory Analysis
Bottled beer samples (matured as described in
Section 2.10) were tasted and judged by a trained sensory panel of seven panelists certified by the Deutsche Landwirtschafts-Gesellschaft (DLG, Frankfurt, Germany). Tasting was performed in a dedicated tasting room (individual tasting chambers, white-colored room, no distracting influences and brown glasses with three-digit number labels) to exclude all external misleading factors. The main flavor impressions were determined at a range from 1 (almost no perception) to 5 (very high perception). Flavor impressions were chosen according to Meier-Dörnberg et al. [
20] (for a list of descriptors used, please see the
Supplementary Material Figure S1). In addition, a tasting was performed under the same circumstances with the DLG scheme, in which the beer is judged by its aroma, taste, carbonation, body and bitterness in a range of 1–5, 1 being the lowest value (negative) and 5 being the highest value (positive).
2.13. Statistical Analysis and Graphs
Statistical analysis was performed on the fermentation data with a one-way ANOVA and Tukey’s test using the “agricolae” package in R (RStudio Inc, Boston MA, U.S.A.; R Core Team, r-project;
http://www.r-project.org/) and
t-test in Excel. The significance level analysis was performed for pairwise sensory testing of single attributes according to MEBAK Sensory 3.1.1 and DIN EN ISO 5495:2007. The graphs were constructed in Excel.
4. Discussion
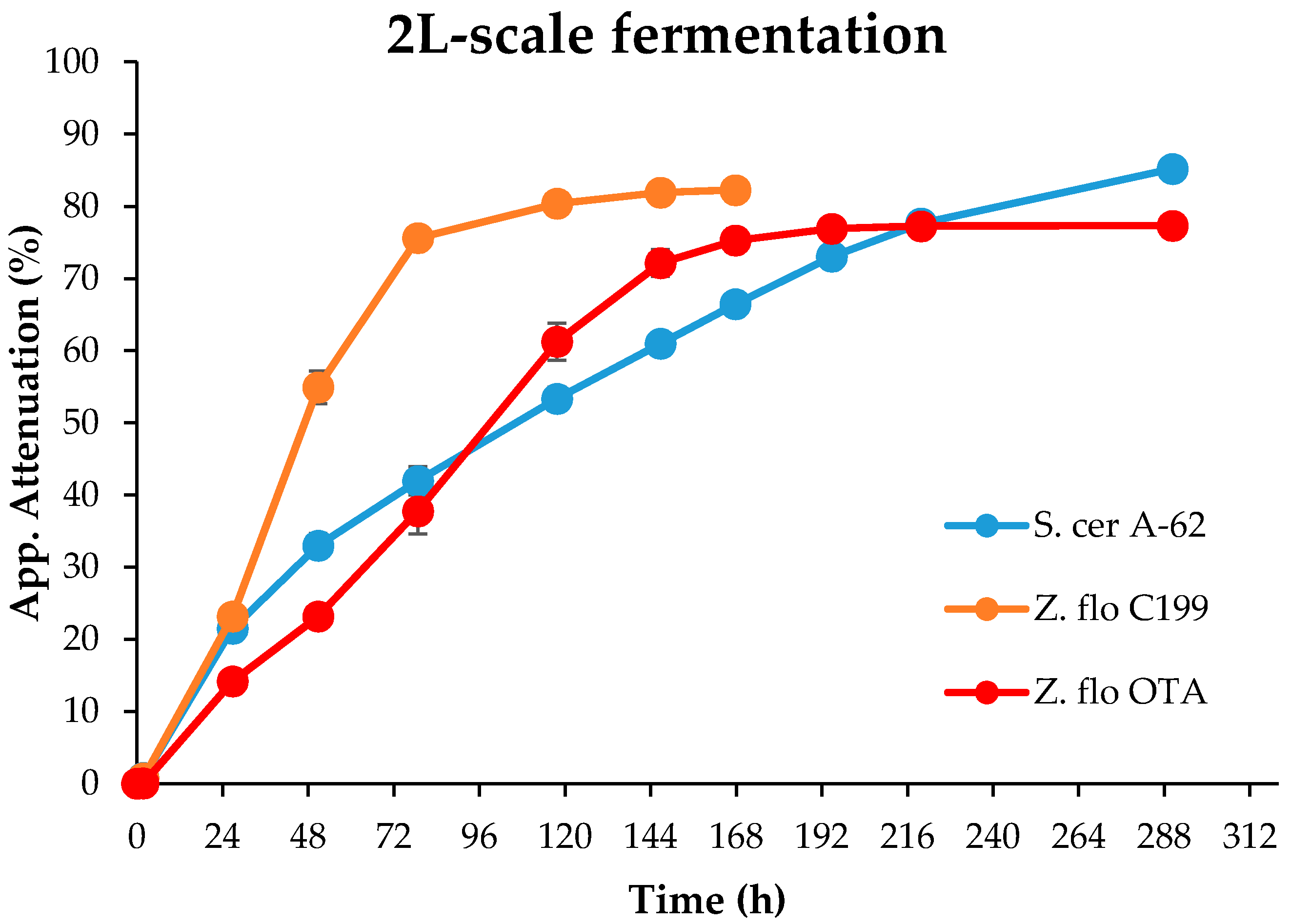
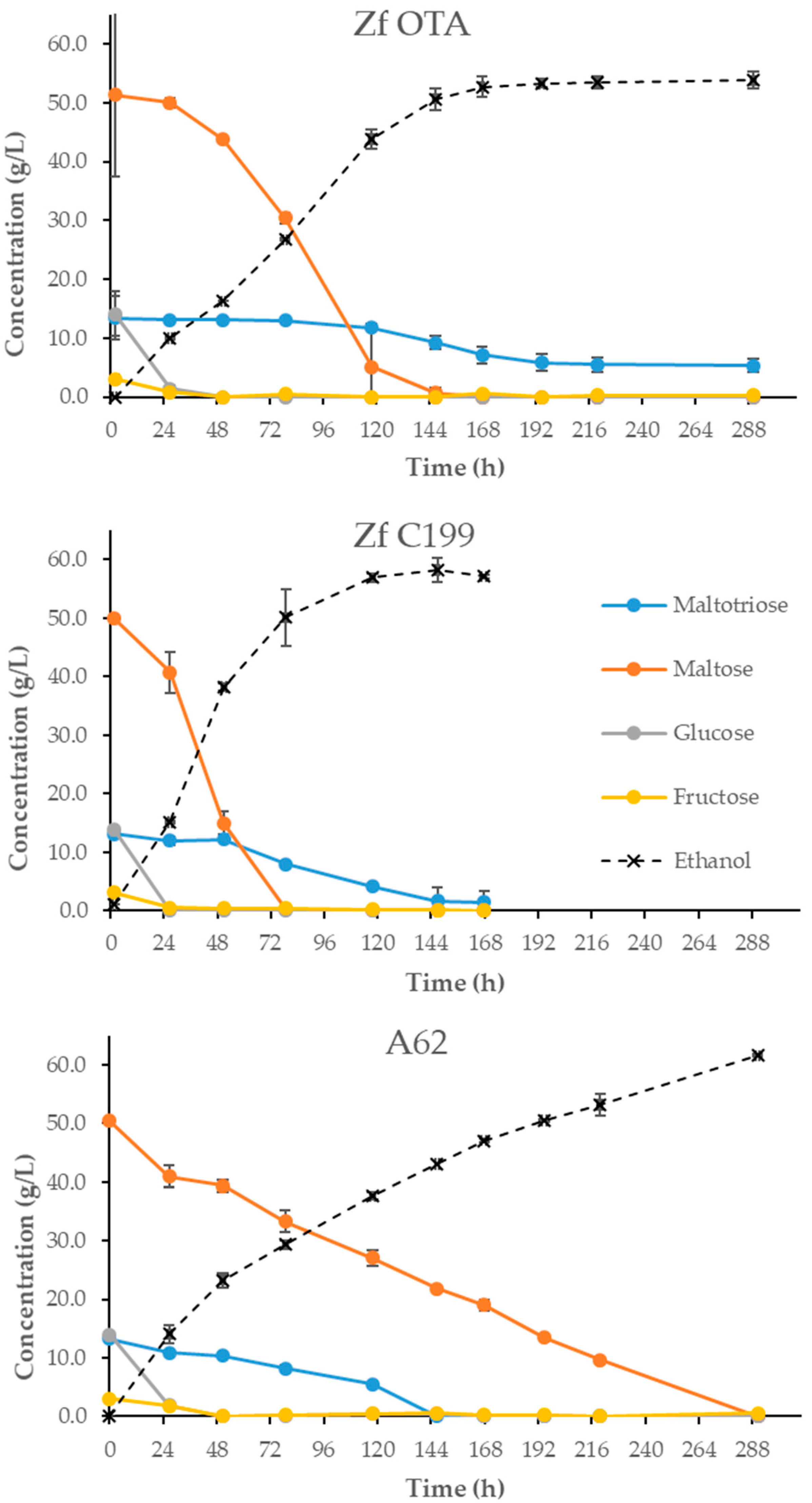
The most notable feature of
Z. florentina strains was their ability to utilize the main wort sugars and, as a consequence, to reach good apparent attenuation levels. In 12 °P wort, the alcohol by volume (ABV) levels produced were comparable to a commercial ale yeast due, in particular, to their ability to utilize maltotriose. Maltose was fully depleted in all fermentation conditions and, although the consumption of maltotriose was reduced in stronger wort, the apparent attenuation levels of 74% are still comparable to those of many commercial brewing yeast strains. This is a relatively rare trait among wild yeasts [
7], as many of the reported wild yeasts shown to have beer brewing potential still lack the ability to ferment maltotriose [
26,
27,
28]. This applies to wild
S. cerevisiae strains as well [
29]. Contrary to wild strains, most domesticated brewing strains of
S. cerevisiae have an AGT1 gene, which codes transporters for the uptake of common sugars in wort, including maltotriose [
29,
30]. The other important genes for maltotriose utilization can be found in the MAL gene family [
30]. This gene family is also found from wild
Saccharomyces, but without recombination, these genes have not been found to code transporters for maltotriose utilization [
31,
32], emphasizing the role of domestication in the evolution of maltotriose utilization. Less is known about the genes behind maltotriose consumption within the non-
Saccharomyces species. With the diastatic strains, the consumption of maltotriose can be explained by α-glucosidase secreted outside the cell to break down the longer-chain sugars. However, as the strain of
Z. florentina studied here was not able to grow on starch-agar, indicating no diastatic power, it is more likely that the sugar was taken up into the cell through transmembrane transporters. Considering that maltotriose-positive species in nature are rare, as is maltotriose itself, a possibility is that these transporters were originally designed for uptake of some other sugars. For the genetic studies of yeast metabolism, all maltotriose-positive wild yeasts offer valuable material.
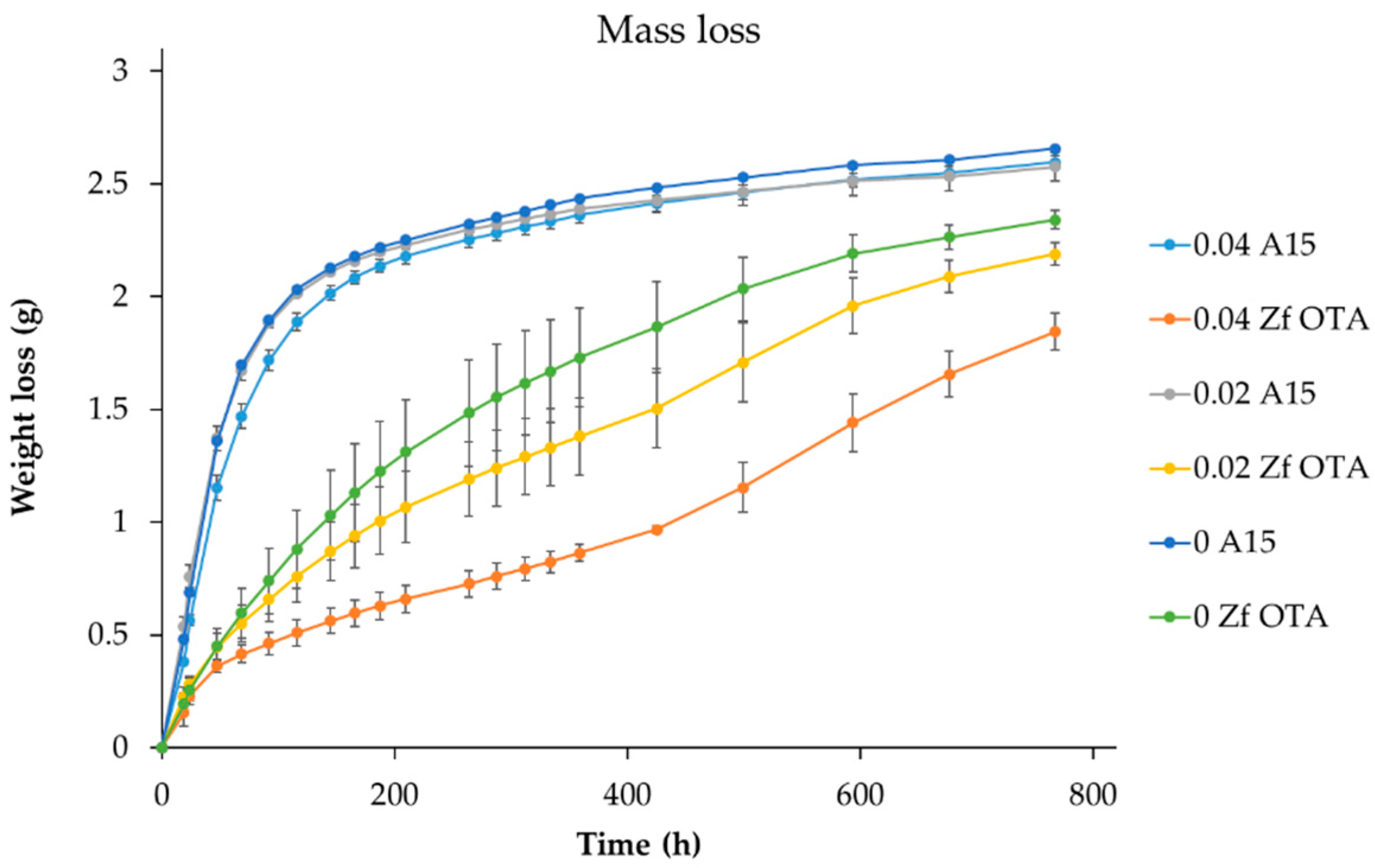
A further requirement for a brewing strain is the ability to tolerate the levels of ethanol found in standard beers. Results suggest that the Finnish
Z. florentina strain is most effective when the wort’s starting gravity is below 10 °P. At higher gravities, the relative ABV yields decreased and this can be, at least partly, attributed to ethanol levels reaching 5% ABV. Although growth was observed eventually during microplate screening experiments at ethanol levels of 5%, the lag-phase was clearly extended. Fermentation trials with worts supplemented with different levels of ethanol confirmed the inhibitory effect. Interestingly, however, once fermentation was initiated, the fermentation rates were relatively similar, with or without ethanol exposure, suggesting that ethanol tolerance may be achieved through physiological adaptation. In the study of Methner et al. [
8], only one of the further-selected yeast strains (belonging to species
S. pombe) reached ABV-values over 5% within 14 days. Maltotriose-negative, but maltose-positive wild
Saccharomyces spp. have been reported to reach ABV values of around 6% of ethanol [
9,
26]. High ethanol levels require high sugar levels, limiting the occurrence of such environments in nature, and the ability to take advantage of such occasions is often attributed to the
Saccharomyces spp., especially to
S. cerevisiae [
33]. The sensitivity of
Z. florentina to ethanol may limit its potential application in brewing. Ethanol tolerance is, however, a malleable trait and evolutionary engineering has been applied successfully to brewing strains to improve their ability to withstand the conditions associated with high gravity wort [
34]. Similar strategies could feasibly be applied to
Z. florentina.
The fermentation efficiency of
Z. florentina was reduced also at greater fermentation volumes. Increasing from the 2 L-scale to the 10 L-scale cut down the attenuation rates. In higher volumes, especially in cylindroconical vessels, the hydrostatic pressure and carbon dioxide pressure in suspension increase, affecting the conditions of cells in suspension [
35]. Thus, a slightly weakened performance with the
Z. florentina strain could be expected. Scaling-up often necessitates the optimization of conditions—like pitching rate, wort aeration and fermentation temperature—and this may be the case here. However, as the strain of
Z. florentina used in this study has had no history of domestication, and had only one fermentation step prior to the main fermentation, the preferred option would be adaptive steps to increase tolerance against higher volumes and ethanol levels. The simplest option would be repeated repitching. While investigating the suitability of the
Lachancea thremotolerans strain for repitching, Domizio et al. [
28] observed an increase in ethanol yields after several generations, which the authors suspected to be related to improved tolerance against ethanol. Similarly, one could expect the improved tolerance also against higher hydrostatic pressure.
The advantageous traits possessed by
Z. florentina can only be considered relevant if they are accompanied by the production of metabolites that contribute positively to beer quality. In this respect, the generation of volatile aroma compounds (higher alcohols and esters) is particularly important. Relative to flavor threshold values, the highest aroma compound productivities were observed with 3-methylbutanol, ethyl acetate and 2-phenylethyl acetate. These compounds are frequently mentioned in wild yeast studies and produce banana, fruity and honey-like flavor notes [
8,
36]. As the levels of these compounds, however, remained under the flavor threshold values, none of the individual aromas dominated. Overall, the production of aroma compounds was decreased as the fermentation volume was increased. In regards to aroma volatile productivity, the most optimal conditions in this study were at the 2 L-scale at 20 °C. To what extent this outcome was due to suitable circumstances (volume and temperature) and how much to other conditions (pitching rate, wort strength, etc.) remains a subject for further studies.
The beer produced with
Z. florentina was of an acceptable quality and, importantly, had distinct flavor notes, distinguishing it from the reference beer produced by the commercial ale strain WLP 380. In particular, there was a specific fruity (main attribute red currant), tropical fruity (main attribute pineapple) and wine-like aroma profile according to the tasting scheme of Meier-Dörnberg et al. [
20]. Distinctive beer aroma profiles have similarly been described for other non-
Saccharoymces species used for beer production [
14,
27,
37,
38,
39,
40,
41]. Both yeast strains included in the sensory trials are POF
+ and produced 4-vinylgauaicol and 4-vinylphenol in concentrations above the aroma threshold. Interestingly though, in
Z. florentina beer, no phenolic/spicy/clove-like flavor notes were recognized by the tasting panel. Therefore, the authors hypothesize that the fruity and vinous aroma covers, or integrates, the phenolic flavor, so that the beers did not have the expected wheat-beer flavor profile. This phenomenon has not been reported previously, but comparable observations have been made for other flavor compounds. The corn-like flavor of dimethylsulfide, for example, may be masked by the presence of phenylethanol [
42], and the worty flavor notes of various aldehydes can be covered by esters [
43] or ethanol [
44]. A positive outcome of the sensory analysis was the absence of any notable off-flavor, further strengthening the case for
Z. florentina as a potentially valuable brewing strain.
Irrespective of functional properties, any species considered for application in the food industry must be completely safe to handle, produce a safe product and not pose a contamination risk in the production facility. In this regard,
Z. florentina can be found on the inventory list of microorganisms with technological beneficial use [
45] with a recorded history in food production. In addition, there are no reported pathogenicity cases related to the species, which is line with the strains’ reported inability to grow at 37 °C (culture collection data, [
11]). To assess the safety of the product, biogenic amine levels in the beers were measured also, but only trace levels were found and these were comparable to levels found in beers produced by commercial yeasts [
25]. Although there are no known health concerns related to the species, in order for the strain to be considered suitable for commercial production, its genome should be sequenced and potential for pathogenicity determined at the gene level to prevent possible health implications if the strain acquired a higher temperature tolerance. Additionally, the ability of the species to tolerate antifungal compounds could be assessed to ensure that any emergent pathogenicity was treatable. With regard to the risk of cross-contamination within brewing facilities, there are several strains of the species available from culture collections that were originally isolated from soft drinks [
11], suggesting that the species may have contamination capability. Indeed, our strain was able to tolerate a range of common preservatives including benzoate, ethanol and sulfite. Sorbate was the only preservative effective against the strain. Thus, in order to deploy the strain, a brewer should pay attention to hygiene and be careful not to introduce the yeast into other products produced in or near the same bottling lines.
A trait relevant to the potential application is temperature tolerance, as this has an impact on permissible process conditions, microbiological control and potential pathogenicity. Although the studied strain was not extremely cold tolerant, it clearly preferred cooler temperatures. This trait appears to be consistent amongst culture collection strains of the species [
11], as none of them were reported to be able to grow at human body temperature. Thus, optimizing fermentation conditions could involve lowering the fermentation temperatures, and ethanol tolerance could be further assessed at these lower temperatures. Temperature preference has also been shown to be crucial for successful isolation from the nature of different species [
46]. Multiple species often coexist and those that prefer ambient temperatures can have a competitive advantage relative to those with lower temperature optima for growth. Although,
Z. florentina is not cryophilic, preference for cooler temperatures was evident based on isolation time and the enrichment temperature of 12 °C. Lower enrichment temperature may also have helped the strain to tolerate the relatively high concentrations of ethanol (7.8% ABV) used in the isolation protocol. As the strain did not prefer the higher ethanol levels, streaking the enrichment media, even those without any signs of growth (haze, pressure), was a prerequisite for successful isolation.
While temperature preference may have played a role in the isolation of the species, the environmental niche (oak bark) is likely to have been just as important. A strong connection between oaks and
Saccharomyces spp. is well-established [
17,
46,
47], but oak trees host a diverse range of yeasts, many of which have been found to have traits suitable for brewing. While isolating
Saccharomyces spp. from oak in a previous study [
9], among the few identified non-
Saccharomyces isolates were species such as
T. delbrueckii and
L. thermotolerans, which have also shown promise as alternative brewing strains [
28,
48,
49]. Though less well characterized with respect to brewing performance,
Z. florentina also appears to show considerable potential in this regard. Why
Saccharomyces spp. and other yeast species appear to prefer oak is not yet understood. It may be that oaks are simply a safe harbor for the otherwise nomadic yeasts, as suggested by Goddard and Greig [
33]. Sampaio et al. [
46] demonstrated a positive correlation between the sugar content of bark (tree-species specific) and incidence of certain species, suggesting that species composition could be, at least partly, related to the availability of fermentable sugar. However, to validate this assumption, more data about the sugary exudates of the bark of different tree-species should be determined, and comprehensive sampling of tree species should be carried out. Current knowledge may be biased towards oaks as these are often the preferred choice to sample, as in the current study.
Overall, the isolated strain illustrated many interesting characteristics in regards to potential brewing application. The ability to utilize maltose and maltotriose in particular is an interesting feature from a brewing perspective. Additionally, the distinctive flavor profile of the beer suggests that the species may be suitable for product differentiation. Promising results obtained also with the type strain demonstrated that there is a significant potential in screening further strains of the species for their brewing potential. Future research could focus on adaptive evolution of the strain/species to tolerate higher ethanol levels and larger fermentation volumes. Additionally, tracking the sugar transporters responsible for maltose and maltotriose utilization could help to better understand the occurrence of these traits in nature.