Effect of 1-Methylcyclopropene (1-MCP) and Storage Atmosphere on the Volatile Aroma Composition of Cloudy and Clear Apple Juices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apple Harvesting, 1-MCP Treatment, and Storage
2.2. Juice Preparation
2.3. Volatile Analysis from the Juice
2.4. Volatile Analysis from Whole Apples
2.5. Experimental Design and Statistical Analysis
3. Results
3.1. Volatile Aroma Composition
3.2. Effect of Harvest Maturity, 1-MCP, and Storage Conditions on the Volatile Composition
3.2.1. McIntosh Juice
3.2.2. Honeycrisp Juice
4. Discussion
4.1. Volatile Aroma Composition
4.2. Effect of 1-MCP, Storage Atmosphere, and Juice Processing
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rupasinghe, H.P.V.; Thilakarathna, S. Apple juice. In Handbook of Functional Beverages and Human Health, 1st ed.; Shahidi, F., Alasalvar, C., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 93–106. [Google Scholar]
- Nikfardjam, M.P.; Maier, D. Development of a headspace trap HRGC/MS method for the assessment of the relevance of certain aroma compounds on the sensorial characteristics of commercial apple juice. Food Chem. 2011, 126, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Graell, J.; Lopez, M.; Fuentes, T.; Echeverría, G.; Lara, I. Quality and volatile emission changes of ‘Mondial Gala’ apples during on-tree maturation and postharvest storage in air or controlled atmosphere. Food Sci. Technol. Int. 2008, 14, 285–294. [Google Scholar] [CrossRef]
- López, M.; Villatoro, C.; Fuentes, T.; Graell, J.; Lara, I.; Echeverría, G. Volatile compounds, quality parameters and consumer acceptance of ‘Pink Lady®’ apples stored in different conditions. Postharvest Biol. Technol. 2007, 43, 55–66. [Google Scholar] [CrossRef]
- Kondo, S.; Setha, S.; Rudell, D.R.; Buchanan, D.A.; Mattheis, J.P. Aroma volatile biosynthesis in apples affected by 1-MCP and methyl jasmonate. Postharvest Biol. Technol. 2005, 36, 61–68. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Murr, D.; Paliyath, G.; Skog, L. Inhibitory effect of 1-MCP on ripening and superficial scald development in ‘McIntosh’ and ‘Delicious’ apples. J. Hortic. Sci. Biotechnol. 2000, 75, 271–276. [Google Scholar] [CrossRef]
- Yan, T.; Qin, H.; Zhang, P.; Tian, S.; Li, J.; Li, B. Effects of 1-methylcyclopropene combined with ξ-polylysine on quality and volatile components of Fuji apples during shelf life after cold storage. Shipin Kexue (Bejing) 2018, 39, 207–214. [Google Scholar]
- Oszmianski, J.; Wojdylo, A.; Kolniak, J. Effect of enzymatic mash treatment and storage on phenolic composition, antioxidant activity, and turbidity of cloudy apple juice. J. Agric. Food Chem. 2009, 57, 7078–7085. [Google Scholar] [CrossRef]
- Scaman, C.H.; Jim, V.J.W.; Hartnett, C. Free galactose concentrations in fresh and stored apples (Malus domestica) and processed apple products. J. Agric. Food Chem. 2004, 52, 511–517. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 752. [Google Scholar] [CrossRef]
- Flath, R.A.; Black, D.R.; Guadagni, D.G.; McFadden, W.H.; Schultz, T.H. Identification and organoleptic evaluation of compounds in Delicious apple essence. J. Agric. Food Chem. 1967, 15, 29–35. [Google Scholar] [CrossRef]
- Komthong, P.; Igura, N.; Shimoda, M. Effect of ascorbic acid on the odours of cloudy apple juice. Food Chem. 2007, 100, 1342–1349. [Google Scholar] [CrossRef]
- Jennings, W.; Tang, C. Volatile components of apricot. J. Agric. Food Chem. 1967, 15, 24–28. [Google Scholar] [CrossRef]
- Sampaio, K.L.; Garruti, D.S.; Franco, M.R.B.; Janzantti, N.S.; Da Silva, M.A. Aroma volatiles recovered in the water phase of cashew apple (Anacardium occidentale L.) juice during concentration. J. Sci. Food Agric. 2011, 91, 1801–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, C.C.; Suffet, I. Development of a standard method—Analysis of compounds causing tastes and odors in drinking water. Water Sci. Technol. 1999, 40, 279–285. [Google Scholar] [CrossRef]
- Sapers, G.M.; Abbott, J.; Massie, O.; Watada, A.; Finney, E.E. Volatile composition of McIntosh apple juice as a function of maturity and ripeness indices. J. Food Sci. 1977, 42, 44–47. [Google Scholar] [CrossRef]
- Schmutzer, G.R.; Magdas, A.D.; David, L.I.; Moldovan, Z. Determination of the volatile components of apple juice using solid phase microextraction and gas chromatography-mass spectrometry. Anal. Lett. 2014, 47, 1683–1696. [Google Scholar] [CrossRef]
- Martínez Vega, M.; Varming, C.; Skov, T.; Toldam-Andersen, T. Post-harvest ripening increase cultivar specific sensory and analytical aroma profile in apple juice: A study of four commercial cultivars in Denmark. Acta Agric. Scand. Sect. B 2014, 64, 244–251. [Google Scholar] [CrossRef]
- Poll, L. Influence of storage temperature on sensory evaluation and composition of volatiles of McIntosh apple juice. Lebensm. Wiss. Technol. 1983, 16, 220–223. [Google Scholar]
- Echeverrıa, G.; Fuentes, T.; Graell, J.; Lara, I.; López, M. Aroma volatile compounds of ‘Fuji’ apples in relation to harvest date and cold storage technology: A comparison of two seasons. Postharvest Biol. Technol. 2004, 32, 29–44. [Google Scholar] [CrossRef]
- Aaby, K.; Haffner, K.; Skrede, G. Aroma quality of Gravenstein apples influenced by regular and controlled atmosphere storage. LWT Food Sci. Technol. 2002, 35, 254–259. [Google Scholar] [CrossRef]
- Komthong, P.; Katoh, T.; Igura, N.; Shimoda, M. Changes in the odours of apple juice during enzymatic browning. Food Qual. Preference 2006, 17, 497–504. [Google Scholar] [CrossRef]
- Plotto, A.; McDaniel, M.R.; Mattheis, J.P. Characterization of changes in ‘Gala’ apple aroma during storage using Osme analysis, a gas chromatography-olfactometry technique. J. Am. Soc. Hortic. Sci. 2000, 125, 714–722. [Google Scholar] [CrossRef] [Green Version]
- Dimick, P.S.; Hoskin, J.C.; Acree, T.E. Review of apple flavor-State of the art. Crit. Rev. Food Sci. Nutr. 1983, 18, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Schreier, P.; Drawert, F.; Steiger, G.; Mick, W. Effect of enzyme treatment of apple pulp with a commercial pectinase and cellulase on the volatiles of the juice. J. Food Sci. 1978, 43, 1797–1800. [Google Scholar] [CrossRef]
- Paillard, N.M.M.; Rouri, O. Hexanal and 2-hexenal production by mashed apple tissues. Lebensm. Wiss. Technol. 1984, 17, 345–350. [Google Scholar]
- Su, S.; Wiley, R. Changes in apple juice flavor compounds during processing. J. Food Sci. 1998, 63, 688–691. [Google Scholar] [CrossRef]
- Lara, I.; Echeverría, G.; Graell, J.; López, M.L. Volatile emission after controlled atmosphere storage of Mondial Gala apples (Malus domestica): Relationship to some involved enzyme activities. J. Agric. Food Chem. 2007, 55, 6087–6095. [Google Scholar] [CrossRef]
- Mattheis, J.P.; Fan, X.; Argenta, L.C. Interactive responses of Gala apple fruit volatile production to controlled atmosphere storage and chemical inhibition of ethylene action. J. Agric. Food Chem. 2005, 53, 4510–4516. [Google Scholar] [CrossRef]
- Kader, A.A. Mode of action of oxygen and carbon dioxide on postharvest physiology of ‘Bartlett’ pears. ISHS Acta Hortic. 1988, 258, 161–168. [Google Scholar] [CrossRef]
- Yang, S.F.; Hoffman, N.E. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Fellman, J.K.; Rudell, D.R.; Mattinson, D.S.; Mattheis, J. Relationship of harvest maturity to flavor regeneration after CA storage of ‘Delicious’ apples. Postharvest Biol. Technol. 2003, 27, 39–51. [Google Scholar] [CrossRef]
- Zhang, L.P.; Shen, Y.X.; Bu, Q.Z.; Ji, S.J. Effects of 1-methylcyclopropene on the metabolic pathways of aroma-related compounds in Nanguo pear. J. Food Process. Preserv. 2013, 38, 1749–1758. [Google Scholar] [CrossRef]
- Poll, L. The effect of pulp holding time on the volatile components in apple juice (with and without pectolytic enzyme treatment). Lebensm. Wiss. Technol. 1988, 21, 87–91. [Google Scholar]
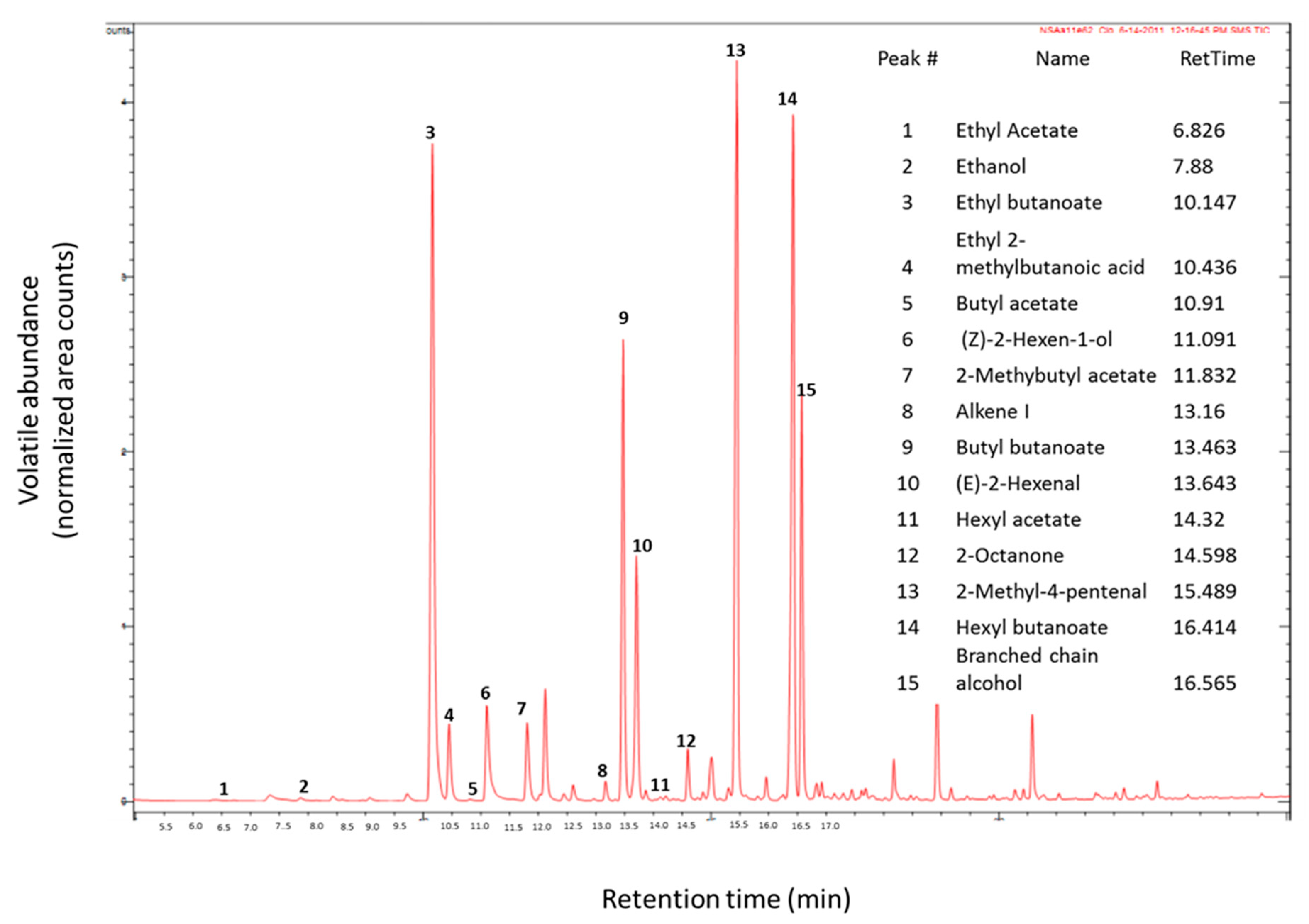
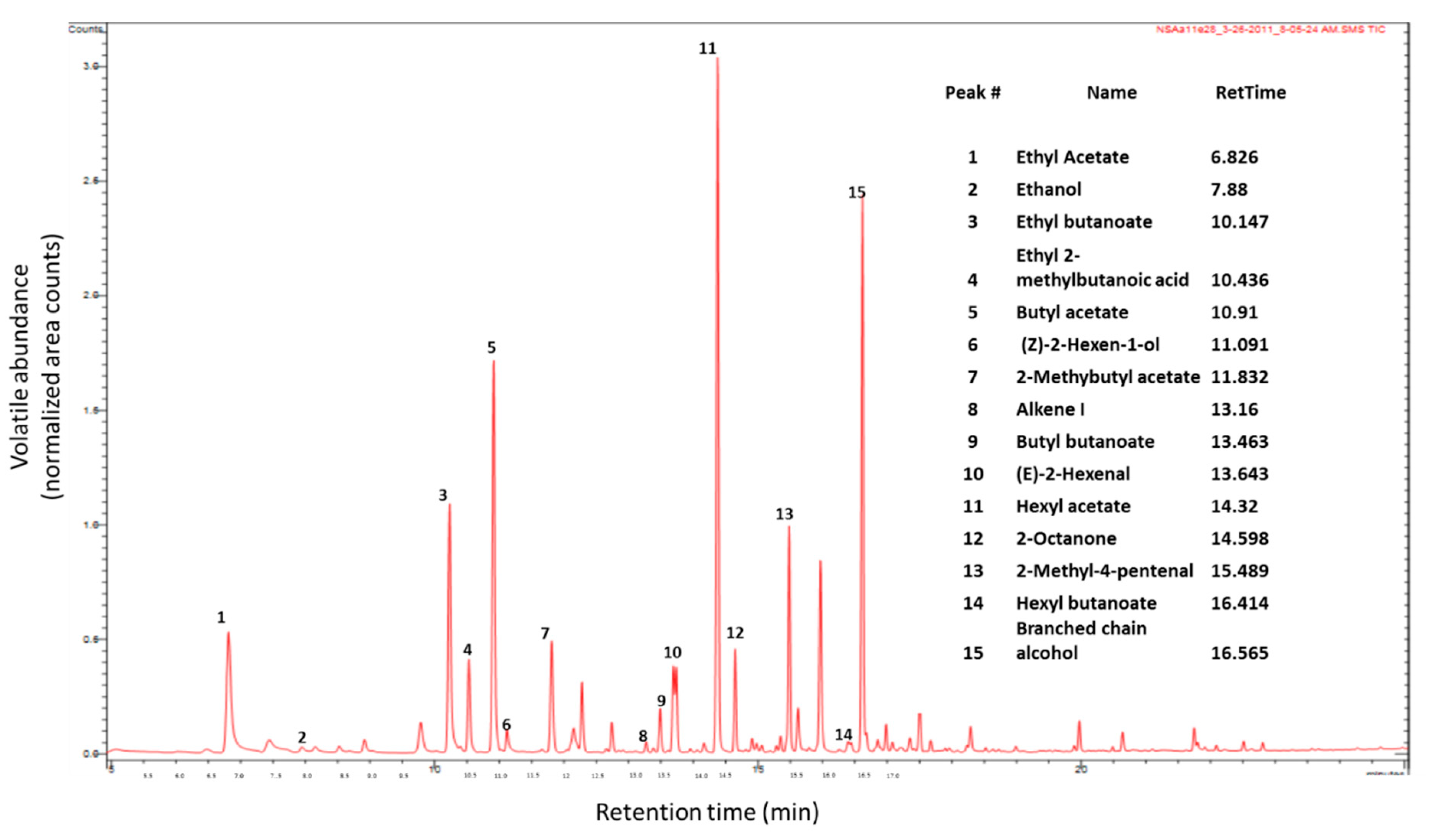
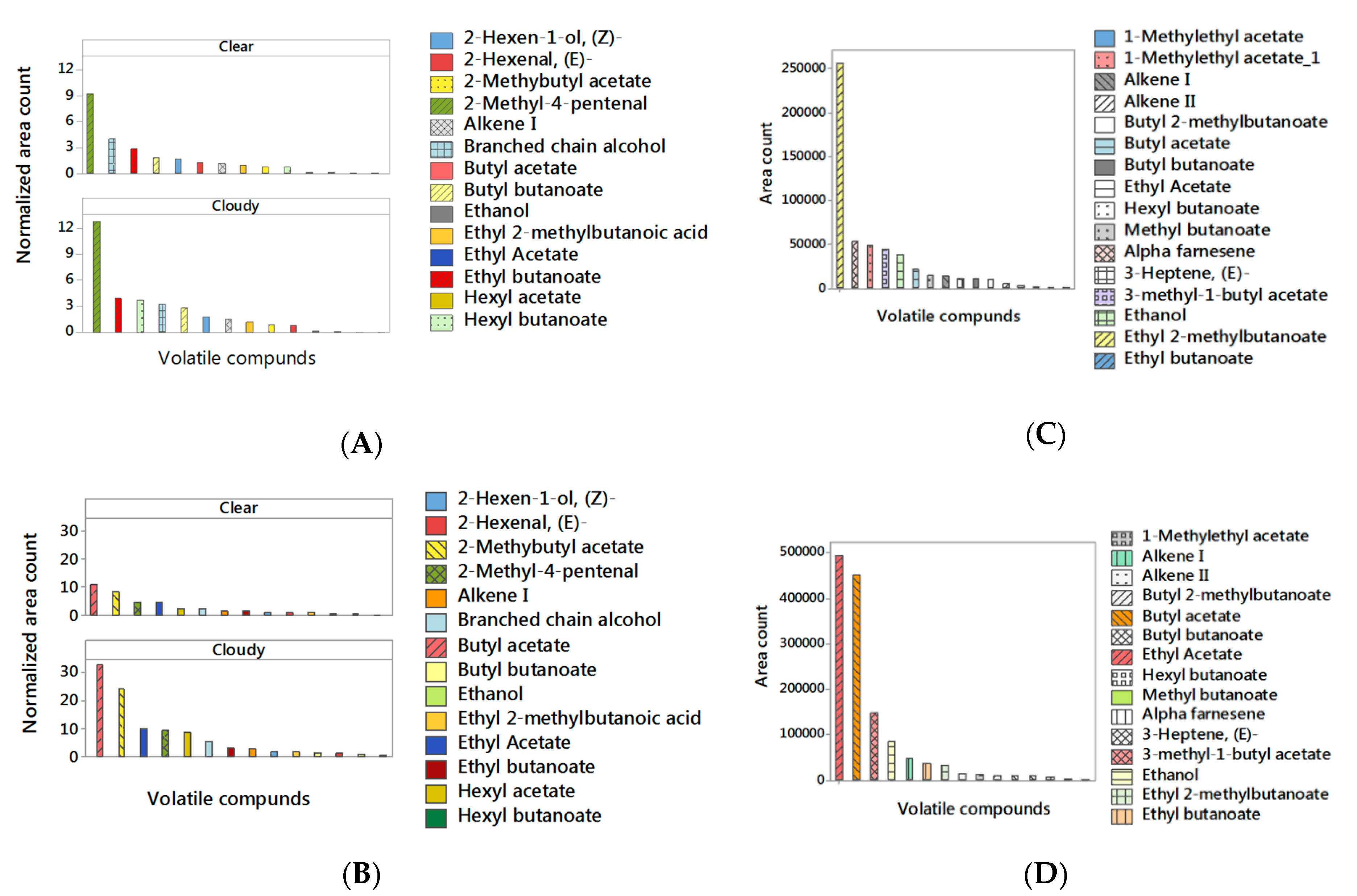
| Volatile Compounds | Retention Time (min) | Experimental Retention Index (RI) a | Odor Property | Odor Threshold (mg/L) |
|---|---|---|---|---|
| Esters | ||||
| Ethyl acetate | 6.83 | 898 | Ether like [2] | 7.50 [12] |
| Ethyl butanoate | 10.15 | 1049 | Sweet fruity [13] | 0.001 [14] |
| Butyl acetate | 10.91 | 1087 | Sweet fruity [13] | 0.066 [12,14] |
| 2-Methyl butyl acetate | 11.83 | 1137 | Fresh [13] | 0.011 [14] |
| Butyl butanoate | 13.46 | 1233 | Fresh [13] | 0.10 [14] |
| Hexyl acetate | 14.32 | 1287 | Sweet fruity [13] | 0.002 [14] |
| Hexyl butanoate | 16.41 | 1433 | ||
| Aldehydes | ||||
| 2-(E)-hexenal | 13.64 | 1244 | Green apple like [2] | 0.011 [12] |
| 2-Methyl-4-pentenal | 15.49 | 1367 | Desirable, green grass, fruity [15] | |
| Alcohols | ||||
| Ethanol | 7.88 | 944 | Sweet [2] | 716 [12] |
| (Z)-2-hexen-1-ol | 11.09 | 1096 | Fresh leaf green [13] | 0.07 [16] |
| Unidentified branched-chain alcohol | 16.56 | 1444 | ||
| Acids | ||||
| Ethyl 2-methylbutanoic acid | 10.43 | 1063 | ||
| Hydrocarbon | ||||
| Alkene I | 13.16 | 1214 |
| Treatment Combinations | Normalized Peak Area Counts a | ||||||
|---|---|---|---|---|---|---|---|
| Ethyl Butanoate | Butyl Butanoate | Hexyl Butanoate | 2-Methyl Butyl Acetate | Total Volatile h | |||
| Harvest | Comm. b | 0.59 ± 0.06 B | 0.31 ± 0.05 B | 0.20 ± 0.29 B | 0.43 ± 0.13 B | 20.31 ± 3.28 B | |
| (H) | Late | 2.47 ± 0.06 A | 2.01 ± 0.05 A | 0.89 ± 0.29 A | 1.16 ± 0.13 A | 37.14 ± 3.28 A | |
| 1-MCP | 1-MCP | 1.48 ± 0.06 A | 0.94 ± 0.05 A | 0.41 ± 0.29 A | 0.79 ± 0.13 A | 27.87 ± 3.28 A | |
| (M) | Control | 1.09 ± 0.06 A | 0.79 ± 0.05 A | 0.43 ± 0.29 A | 0.80 ± 0.13 A | 29.58 ± 3.28 A | |
| Atmos. c | CA d | 0.99 ± 0.06 A | 0.64 ± 0.05 A | 0.31 ± 0.29 A | 0.42 ± 0.13 B | 21.07 ± 3.28 B | |
| (A) | RA e | 1.62 ± 0.06 A | 1.14 ± 0.05 A | 0.57 ± 0.29 A | 0.16 ± 0.13 A | 36.38 ± 3.28 A | |
| Juice | Clear | 0.94 ± 0.06 A | 0.61 ± 0.05 A | 0.16 ± 0.29 B | 0.72 ± 0.13 A | 24.37 ± 3.28 A | |
| (J) | Cloudy | 1.69 ± 0.06 A | 1.18 ± 0.05 A | 1.10 ± 0.29 A | 0.87 ± 0.13 A | 33.08 ± 3.28 A | |
| H × M | Comm. | 1-MCP | 1.22 ± 0.08 A,B | 0.62 ± 0.07 B,C | 0.31± 0.42 A,B | 0.61 ± 0.18 B,C | 25.80 ± 4.64 B |
| Comm. | Control | 0.25 ± 0.08 B | 0.14 ± 0.07 C | 0.13 ± 0.42 B | 0.26 ± 0.18 C | 14.82 ± 4.64 B | |
| Late | 1-MCP | 1.77 ± 0.08 A | 1.37 ± 0.07 A,B | 0.55 ± 0.42 A,B | 0.97 ± 0.18 A,B | 29.95 ± 4.64 A,B | |
| Late | Control | 3.40 ± 0.08 A | 2.87 ± 0.07 A | 1.46 ± 0.42 A | 1.34 ± 0.18 A | 44.33 ± 4.64 A | |
| H × A | Comm. | CA | 1.64 ± 0.08 B | 0.72 ± 0.07 B | 0.41 ± 0.42 A | 0.58 ± 0.18 B | 25.54 ± 4.64 B |
| Comm. | RA | 0.16 ± 0.08 C | 0.12 ± 0.07 B | 0.09 ± 0.42 B | 0.28 ± 0.18 B | 15.08 ± 4.64 B | |
| Late | CA | 0.56 ± 0.08 B,C | 0.57 ± 0.07 B | 0.24 ± 0.42 B | 0.26 ± 0.18 B | 16.60 ± 4.64 B | |
| Late | RA | 7.86 ± 0.08 A | 5.50 ± 0.07 A | 3.40 ± 0.42 A | 2.05 ± 0.18 A | 57.86 ± 4.64 A | |
| H × J | Comm. | Clear | 0.48 ± 0.08 B | 0.27 ± 0.07 B | 0.09 ± 0.42 B | 0.46 ± 0.18 B | 20.55± 4.64 B |
| Comm. | Cloudy | 0.72 ± 0.08 A | 0.36 ± 0.07 B | 0.42 ± 0.42 A | 0.41 ± 0.18 B | 20.08 ± 4.64 B | |
| Late | Clear | 1.71 ± 0.08 A | 1.24 ± 0.07 A | 0.27 ± 0.42 A | 0.98 ± 0.18 A | 28.19 ± 4.64 A | |
| Late | Cloudy | 3.50 ± 0.08 A | 3.31 ± 0.07 A | 2.92 ± 0.42 A | 1.33 ± 0.18 A | 46.09 ± 4.64 A | |
| Statistical Significance f | H *** | H *** | H *** | H *** | H *** | ||
| A *** | A *** | ||||||
| J *** | |||||||
| H × M * | H × M * | H × M * | H × M * | H × M ** | |||
| H × A *** | H × A *** | H × A *** | H × A *** | H × A *** | |||
| H × J ** | |||||||
| Lambda g | 0.2 | 0.2 | 0 | 1 | 1 | ||
| Treatment Combinations | Normalized Peak Area Counts a | |||||
|---|---|---|---|---|---|---|
| 2-Methyl 4-Pentenal | (E)-2- Hexenal | Branched- Chain Alcohol | (Z)-2-Hexen-1-ol | |||
| Harvest | Comm. b | 6.37 ± 0.19 B | 1.15 ± 0.05 A | 0.32 ± 0.37 B | 1.06 ± 0.07 B | |
| (H) | Late | 12.69 ± 0.19 A | 0.49 ± 0.05 B | 0.98 ± 0.37 A | 2.07 ± 0.07 A | |
| 1-MCP | 1-MCP | 9.73 ± 0.19 A | 0.84 ± 0.05 A | 0.55 ± 0.37 A | 1.40 ± 0.07 A | |
| (M) | Control | 8.81 ± 0.19 A | 0.62 ± 0.05 B | 0.58 ± 0.37 A | 1.57 ± 0.07 A | |
| Atmos. c | CA d | 8.26 ± 0.19 A | 0.78 ± 0.05 A | 0.37 ± 0.37 A | 1.65 ± 0.07 A | |
| (A) | RA e | 10.33 ± 0.19 A | 0.66 ± 0.05 A | 0.85 ± 0.37 A | 1.33 ± 0.07 B | |
| Juice | Clear | 7.67 ± 0.19 A | 0.97 ± 0.05 A | 0.51 ± 0.37 A | 1.50 ± 0.07 A | |
| (J) | Cloudy | 11.01 ± 0.19 A | 0.55± 0.05 B | 0.66 ± 0.37 A | 1.46 ± 0.07 A | |
| H × M | Comm. | 1-MCP | 9.30 ± 0.27 A | 1.12 ± 0.07 A | 0.63 ± 0.53 B | 1.20 ± 0.09 B,C |
| Comm. | Control | 4.00 ± 0.27 B | 1.19 ± 0.07 A | 0.16 ± 0.53 C | 0.94 ± 0.09 C | |
| Late | 1-MCP | 10.17 ± 0.27 A | 0.65 ± 0.07 B | 0.48 ± 0.53 B | 1.63 ± 0.09 B | |
| Late | Control | 15.50 ± 0.27 A | 0.38 ± 0.07 C | 2.01 ± 0.53 A | 2.63 ± 0.09 A | |
| H × A | Comm. | CA | 9.54 ± 0.27 B | 1.01 ± 0.07 A | 0.82 ± 0.53 B | 1.16 ± 0.09 A |
| Comm. | RA | 3.84 ± 0.27 C | 1.33 ± 0.07 A | 0.13 ± 0.53 C | 0.97 ± 0.09 B | |
| Late | CA | 7.07 ± 0.27 B,C | 0.63 ± 0.07 B | 0.17 ± 0.53 C | 2.35 ± 0.09 A | |
| Late | RA | 19.96 ± 0.27 A | 0.39 ± 0.07 C | 5.76 ± 0.53 A | 1.83 ± 0.09 A | |
| H × J | Comm. | Clear | 6.13 ± 0.27 B | 1.41 ± 0.07 A | 0.31 ± 0.53 B | 1.34 ± 0.09 A |
| Comm. | Cloudy | 6.62 ± 0.27 B | 0.96 ± 0.07 A,B | 0.33 ± 0.53 B | 0.85 ± 0.09 C | |
| Late | Clear | 9.38 ± 0.27 A | 0.70 ± 0.07 B | 0.85 ± 0.53 A | 1.69 ± 0.09 A | |
| Late | Cloudy | 16.51 ± 0.27 A | 0.36 ± 0.07 C | 1.12 ± 0.53 A | 2.53 ± 0.09 A | |
| Statistical Significance f | H *** | H *** | H * | H *** | ||
| A * | ||||||
| M ** | ||||||
| J *** | ||||||
| H × M *** | H × M ** | H × M *** | H × M *** | |||
| H × A *** | H × A *** | H × A *** | ||||
| H × J * | H × J *** | |||||
| Lambda g | 0.5 | 0.5 | 0 | 0 | ||
| Treatment Combinations | Normalized Peak Area Counts a | ||||||
|---|---|---|---|---|---|---|---|
| Butyl Acetate | 2-Methyl Butyl Acetate | Ethyl Acetate | Hexyl Acetate | Total Volatile | |||
| Harvest (H) | Comm. b | 16.17 ± 0.09 A | 14.26 ± 0.20 A | 3.57 ± 0.03 A | 4.77 ± 0.06 A | 58.44 ± 8.17 A | |
| Late | 16.46 ± 0.10 A | 12.44 ± 0.21 A | 4.24 ± 0.03 A | 3.36 ± 0.06 A | 48.31 ± 8.43 A | ||
| 1-MCP (M) | 1-MCP | 15.64 ± 0.10 A | 15.22 ± 0.20 A | 1.41 ± 0.03 B | 4.08 ± 0.06 A | 50.09 ± 8.17 A | |
| Control | 17.02 ± 0.10 A | 11.58 ± 0.20 A | 9.33 ± 0.03 A | 3.96 ± 0.06 A | 56.37± 8.43 A | ||
| Atmos. c (A) | CA d | 13.95 ± 0.10 B | 19.78 ± 0.20 A | 2.69 ± 0.03 B | 2.82 ± 0.06 B | 57.91± 8.17 A | |
| RA e | 19.09 ± 0.10 A | 8.15 ± 0.20 B | 5.51 ± 0.03 A | 5.54 ± 0.06 A | 48.75± 8.43 A | ||
| Juice (J) | Clear | 9.08 ± 0.10 B | 6.48 ± 0.20 B | 2.53 ± 0.03 B | 1.79 ± 0.06 B | 30.90 ± 8.17 B | |
| Cloudy | 29.33 ± 0.10 A | 22.63 ± 0.20 A | 5.81 ± 0.03 A | 7.72 ± 0.06 A | 91.36 ± 8.43 A | ||
| H × M | Comm. | 1-MCP | 14.70 ± 0.14 A | 16.70 ± 0.28 A | 0.90 ± 0.04 B | 4.23 ± 0.08 A | 53.62 ± 11.6 A |
| Comm. | Control | 17.79 ± 0.14 A | 12.02 ± 0.28 A | 11.01 ± 0.04 A | 5.36 ± 0.08 A | 63.70 ± 11.6 A | |
| Late | 1-MCP | 16.64 ± 0.15 A | 13.81± 0.30 A | 2.13 ± 0.04 B | 3.95 ± 0.09 A | 46.79 ± 11.6 A | |
| Late | Control | 16.29 ± 0.15 A | 11.14± 0.30 A | 7.86 ± 0.04 A | 2.84 ± 0.099 A | 49.88 ± 12.3 A | |
| H × A | Comm. | CA | 12.93 ± 0.14 A | 20.43 ± 0.28 A | 2.55 ± 0.04 B | 3.39 ± 0.08 A | 65.88 ± 11.6 A |
| Comm. | RA | 20.23 ± 0.14 A | 9.20 ± 0.28 B | 4.91± 0.04 A | 6.50 ± 0.08 A | 51.84 ± 11.6 A | |
| Late | CA | 15.04 ± 0.15 A | 19.15 ± 0.30 A | 2.84 ± 0.04 B | 2.33 ± 0.09 B | 50.91 ± 12.3 A | |
| Late | RA | 18.01± 0.15 A | 7.17 ± 0.30 B | 6.16 ± 0.04 A | 4.68 ± 0.09 A | 45.85 ± 11.6 A | |
| H × J | Comm. | Clear | 11.95 ± 0.14 c | 9.61 ± 0.28 B | 3.19 ± 0.04 B | 2.56 ± 0.08 B | 45.07 ± 11.6 B |
| Comm. | Cloudy | 21.88 ± 0.14 A | 19.84 ± 0.28 A | 4.00 ± 0.04 A | 8.06 ± 0.08 A | 75.77 ± 11.6 A | |
| Late | Clear | 6.89 ± 0.15 C | 3.97 ± 0.30 B | 2.00 ± 0.04 B | 1.19 ± 0.09 B | 21.19 ± 12.3 C | |
| Late | Cloudy | 39.30 ± 0.15 A | 25.60 ± 0.30 A | 8.28 ± 0.04 A | 7.39 ± 0.09 A | 110.17 ± 11.6 A | |
| Statistical Significance f | M *** | M * | |||||
| A * | A *** | A *** | A *** | ||||
| J *** | J *** | J *** | J *** | J *** | |||
| H × M ** | |||||||
| H × J *** | H × J ** | H × J ** | H × J *** | ||||
| Lambda g | 0 | 0.5 | 0.2 | 0.3 | 0 | ||
| Treatment Combinations | Normalized Peak Area Counts a | ||||||
|---|---|---|---|---|---|---|---|
| 2-Methyl-4- Pentenal | (E)-2- Hexenal | Branched Chain Alcohol | (Z)-2- Hexen-1-ol | Ethanol | |||
| Harvest (H) | Comm. b | 6.62 ± 0.103 A | 1.02 ± 0.133 A | 1.07 ± 0.017 A | 1.33 ± 0.184 A | 0.56 ± 0.113 A | |
| Late | 4.57 ± 0.103 B | 0.51 ± 0.141 B | 1.11 ± 0.018 A | 1.61 ± 0.195 A | 0.71 ± 0.119 A | ||
| 1-MCP (M) | 1-MCP | 5.22 ± 0.107 A | 0.70 ± 0.137 A | 0.39 ± 0.018 B | 1.52 ± 0.189 A | 0.17 ± 0.116 B | |
| Control | 5.81 ± 0.107 A | 0.74 ± 0.137 A | 3.69 ± 0.018 A | 1.41 ± 0.189 A | 1.09 ± 0.116 A | ||
| Atmos. c (A) | CA d | 4.64 ± 0.107 B | 0.93 ± 0.137 A | 0.58 ± 0.018 B | 1.97 ± 0.189 A | 0.50 ± 0.116 A | |
| RA e | 6.52 ± 0.107 A | 0.56 ± 0.137 B | 2.23 ± 0.018 A | 0.97 ± 0.189 B | 0.77 ± 0.116 A | ||
| Juice (J) | Clear | 3.37 ± 0.107 B | 0.54 ± 0.137 B | 0.66 ± 0.018 B | 0.92 ± 0.189 B | 0.41 ± 0.116 B | |
| Cloudy | 8.98 ± 0.107 A | 0.96 ± 0.137 A | 1.88 ± 0.018 A | 2.02 ± 0.189 A | 0.86 ± 0.116 A | ||
| H × M | Comm. | 1-MCP | 5.59 ± 0.146 A | 0.85 ± 0.188 A | 0.31 ± 0.024 B | 1.38 ± 0.260 A | 0.10 ± 0.159 A |
| Comm. | Control | 7.85 ± 0.146 A | 1.22 ± 0.188 A | 5.10 ± 0.024 A | 1.29 ± 0.260 A | 1.02 ± 0.159 A | |
| Late | 1-MCP | 4.87 ± 0.155 A | 0.58 ± 0.199 A | 0.51 ± 0.024 B | 1.67 ± 0.275 A | 0.24 ± 0.169 A | |
| Late | Control | 4.29 ± 0.155 A | 0.45 ± 0.199 A | 2.72 ± 0.024 A | 1.54 ± 0.275 A | 1.18 ± 0.169 A | |
| H × A | Comm. | CA | 5.74 ± 0.146 A | 1.48± 0.188 A | 0.62 ± 0.024 A | 1.82 ± 0.260 A | 0.45 ± 0.159 A |
| Comm. | RA | 7.64 ± 0.146 A | 0.47 ± 0.188 A | 1.97 ± 0.024 A | 0.84 ± 0.260 A | 0.67 ± 0.159 A | |
| Late | CA | 3.75 ± 0.155A | 0.58 ± 0.199 A | 0.54 ± 0.024 A | 2.11 ± 0.275 A | 0.55 ± 0.169 A | |
| Late | RA | 5.57 ± 0.155 A | 0.45 ± 0.199 A | 2.53± 0.024 A | 1.11 ± 0.275 A | 0.87 ± 0.169 A | |
| H × J | Comm. | Clear | 5.68 ± 0.146 B | 0.71 ± 0.188 A | 0.87± 0.024 B,C | 1.07 ± 0.260 B | 0.55 ±0.159 A,B |
| Comm. | Cloudy | 7.73 ± 0.146 A,B | 1.48 ± 0.188 A | 1.34 ± 0.024 B | 1.60 ± 0.260 A,B | 0.57± 0.159 A,B | |
| Late | Clear | 2.00 ± 0.155 C | 0.41 ± 0.199 A | 0.51 ± 0.024 C | 0.76 ± 0.275 B | 0.26 ± 0.169 B | |
| Late | Cloudy | 10.44 ±0.155 A | 0.62 ± 0.199 A | 2.69 ± 0.024 A | 2.45 ± 0.275 A | 1.16 ± 0.169 A | |
| M × A | 1-MCP | CA | 4.03 ± 0.146 A | 1.04 ± 0.188 A | 0.20 ± 0.024 D | 2.18 ± 0.260 A | 0.08 ± 0.159 A |
| 1-MCP | RA | 6.75 ± 0.155 A | 0.47± 0.188 A | 0.91 ± 0.024 C | 0.87 ± 0.260 A | 0.26 ± 0.159 A | |
| Control | CA | 5.34 ± 0.155 A | 0.83 ± 0.199 A | 2.25± 0.024 B | 1.75 ± 0.275 A | 0.92 ± 0.169 A | |
| Control | RA | 6.31 ± 0.146 A | 0.67± 0.199 A | 6.35 ± 0.024 A | 1.08 ± 0.275 A | 1.27 ± 0.169 A | |
| Statistical significance f | H * | H *** | |||||
| M *** | M *** | ||||||
| A * | A *** | A *** | A *** | ||||
| J *** | J *** | J *** | J ** | J *** | |||
| H × M *** | |||||||
| M × A ** | |||||||
| H × J * | H × J ** | H × J ** | H × J * | ||||
| Lambda g | 0 | 0 | 0 | 1 | 1 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muche, B.M.; Jordan, M.; Forney, C.F.; Speers, R.A.; Rupasinghe, H.P.V. Effect of 1-Methylcyclopropene (1-MCP) and Storage Atmosphere on the Volatile Aroma Composition of Cloudy and Clear Apple Juices. Beverages 2020, 6, 59. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages6040059
Muche BM, Jordan M, Forney CF, Speers RA, Rupasinghe HPV. Effect of 1-Methylcyclopropene (1-MCP) and Storage Atmosphere on the Volatile Aroma Composition of Cloudy and Clear Apple Juices. Beverages. 2020; 6(4):59. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages6040059
Chicago/Turabian StyleMuche, Bizuayehu M., Michael Jordan, Charles F. Forney, R. Alex Speers, and H. P. Vasantha Rupasinghe. 2020. "Effect of 1-Methylcyclopropene (1-MCP) and Storage Atmosphere on the Volatile Aroma Composition of Cloudy and Clear Apple Juices" Beverages 6, no. 4: 59. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages6040059






