Review of the Effects of Grapevine Smoke Exposure and Technologies to Assess Smoke Contamination and Taint in Grapes and Wine
Abstract
:1. Introduction
2. Composition of Smoke from Bushfires
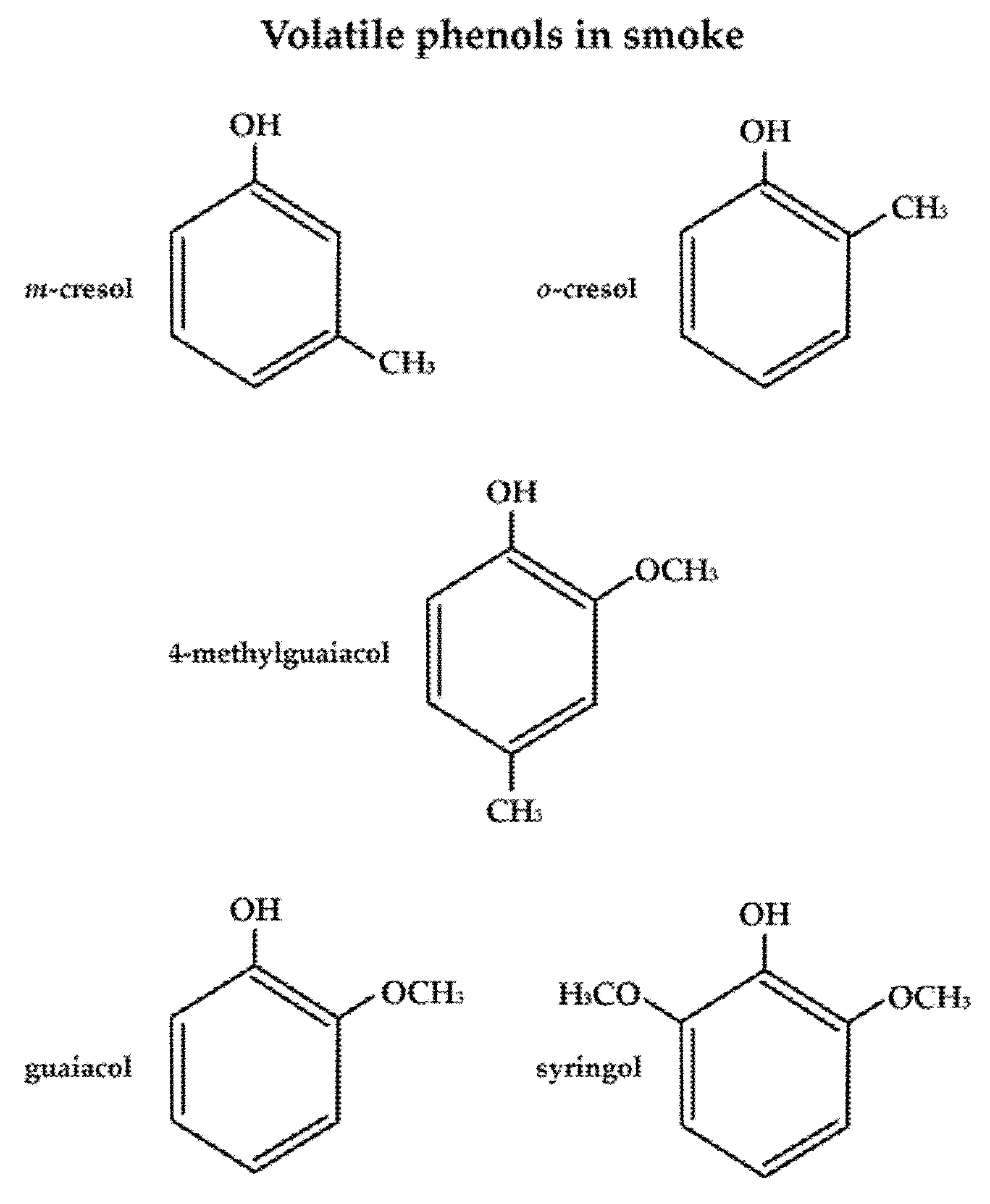
2.1. Volatile Organic Compounds Responsible for Smoke Taint Aromas and Detection Thresholds
2.2. Uptake and Accumulation of Smoke Volatiles into Grapevines and Grapes
2.3. Entry and Location of Smoke Volatiles in Grape Berries
2.4. Entry Through Leaves
2.5. Uptake Through Roots
3. Influence of Vine Phenology, Grapevine Cultivar, and Duration of Smoke Exposure on Smoke Taint Development
3.1. Influence of Vine Phenology and Duration of Smoke Exposure
3.2. Influence of Cultivar on Sensory Properties Following Smoke Exposure
4. Effect of Smoke Exposure on Vine Physiology and Fruit Production, and Carry-Over of Smoke Compounds to Following Years
5. Glycosylation and Hydrolysis of Volatiles into Wine
6. Pre- and Postharvest Methods for Reducing Smoke Contamination and Smoke Taint in Wine
7. Current and Emerging Methods of Assessing Grapevine Smoke Contamination and Smoke Taint in Wine
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Vries, C.; Mokwena, L.; Buica, A.; McKay, M. Determination of volatile phenol in Cabernet Sauvignon wines, made from smoke-affected grapes, by using HS-SPME GC-MS. S. Afr. J. Enol. Vitic. 2016, 37, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Hayasaka, Y.; Baldock, G.; Pardon, K.; Jeffery, D.; Herderich, M. Investigation into the formation of guaiacol conjugates in berries and leaves of grapevine Vitis vinifera L. cv. Cabernet Sauvignon using stable isotope tracers combined with HPLC-MS and MS/MS analysis. J. Agric. Food Chem. 2010, 58, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Kennison, K.; Wilkinson, K.; Williams, H.; Smith, J.; Gibberd, M. Smoke-derived taint in wine: Effect of postharvest smoke exposure of grapes on the chemical composition and sensory characteristics of wine. J. Agric. Food Chem. 2007, 55, 10897–10901. [Google Scholar] [CrossRef] [PubMed]
- Ristic, R.; van der Hulst, L.; Capone, D.; Wilkinson, K. Impact of Bottle Aging on Smoke-Tainted Wines from Different Grape Cultivars. J. Agric. Food Chem. 2017, 65, 4146–4152. [Google Scholar] [CrossRef]
- Summerson, V.; Gonzalez Viejo, C.; Szeto, C.; Wilkinson, K.L.; Torrico, D.D.; Pang, A.; Bei, R.D.; Fuentes, S. Classification of Smoke Contaminated Cabernet Sauvignon Berries and Leaves Based on Chemical Fingerprinting and Machine Learning Algorithms. Sensors 2020, 20, 5099. [Google Scholar] [CrossRef]
- Summerson, V.; Viejo, C.G.; Torrico, D.D.; Pang, A.; Fuentes, S. Detection of smoke-derived compounds from bushfires in Cabernet-Sauvignon grapes, must, and wine using Near-Infrared spectroscopy and machine learning algorithms. OENO One 2020, 54, 1105–1119. [Google Scholar] [CrossRef]
- Cain, N.; Hancock, F.; Rogers, P.; Downey, M. The effect of grape variety and smoking duration on the accumulation of smoke taint compounds in wine. Wine Vitic. J. 2013, 28, 48–49. [Google Scholar]
- Kennison, K.; Ward, G.; Fisher, D. Key Information on Smoke Effect in Grapes and Wine: What Can Be done to Identify and Reduce Smoke Effect in Grape and Wine Production? Department of Agriculture and Food Western Australia. Western Australia Agriculture Authority. 2011. Available online: https://www.djdaxx.com/wp-content/uploads/smoke_effect_in_grapes_and_wine.pdf (accessed on 10 October 2020).
- Dungey, K.A.; Hayasaka, Y.; Wilkinson, K.L. Quantitative analysis of glycoconjugate precursors of guaiacol in smoke-affected grapes using liquid chromatography–tandem mass spectrometry based stable isotope dilution analysis. Food Chem. 2011, 126, 801–806. [Google Scholar] [CrossRef]
- Noestheden, M.; Dennis, E.G.; Zandberg, W.F. Quantitating Volatile Phenols in Cabernet Franc Berries and Wine after On-Vine Exposure to Smoke from a Simulated Forest Fire. J. Agric. Food Chem. 2018, 66, 695–703. [Google Scholar] [CrossRef]
- Kennison, K.; Gibberd, M.; Pollnitz, A.; Wilkinson, K. Smoke-Derived Taint in Wine: The Release of Smoke-Derived Volatile Phenols during Fermentation of Merlot Juice following Grapevine Exposure to Smoke. J. Agric. Food Chem. 2008, 56, 7379–7383. [Google Scholar] [CrossRef]
- CSIRO; Australian Government Bureau of Meteorology. State of the Climate 2018. Available online: http://www.bom.gov.au/state-of-the-climate/2018/State-of-the-Climate-2018.pdf (accessed on 10 October 2020).
- Flannigan, M.D.; Krawchuk, M.A.; de Groot, W.J.; Wotton, B.M.; Gowman, L.M. Implications of changing climate for global wildland fire. Int. J. Wildland Fire 2009, 18, 483–507. [Google Scholar] [CrossRef]
- Climate Council. This Is not Normal: Climate Change and Escalating Bushfire Risk; Climate Council Briefing Paper 2019. Available online: https://www.climatecouncil.org.au/wp-content/uploads/2019/11/bushfire-briefing-paper_18-november.pdf (accessed on 15 September 2020).
- Fuentes, S.; Summerson, V.; Viejo, C.G.; Tongson, E.; Lipovetzky, N.; Wilkinson, K.L.; Szeto, C.; Unnithan, R.R. Assessment of Smoke Contamination in Grapevine Berries and Taint in Wines Due to Bushfires Using a Low-Cost E-Nose and an Artificial Intelligence Approach. Sensors 2020, 20, 5108. [Google Scholar] [CrossRef] [PubMed]
- Noestheden, M.; Noyovitz, B.; Riordan-Short, S.; Dennis, E.G.; Zandberg, W.F. Smoke from simulated forest fire alters secondary metabolites in Vitis vinifera L. berries and wine. Planta 2018. [Google Scholar] [CrossRef]
- Collins, C.; Gao, H.; Wilkinson, K. An observational study into the recovery of grapevines (Vitis vinifera L.) following a bushfire. Am. J. Enol. Vitic. 2014, 65, 285–292. [Google Scholar] [CrossRef]
- Kelly, D.; Zerihun, A.; Singh, D.; von Eckstaedt, C.V.; Gibberd, M.; Grice, K.; Downey, M. Exposure of grapes to smoke of vegetation with varying lignin composition and accretion of lignin derived putative smoke taint compounds in wine. Food Chem. 2012, 135, 787–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krstic, M.; Johnson, D.; Herderich, M. Review of smoke taint in wine: Smoke-derived volatile phenols and their glycosidic metabolites in grapes and vines as biomarkers for smoke exposure and their role in the sensory perception of smoke taint. Aust. J. Grape Wine Res. 2015, 21, 537–553. [Google Scholar] [CrossRef]
- Sheppard, S.; Dhesi, M.; Eggers, N. Effect of Pre- and Postveraison Smoke Exposure on Guaiacol and 4-Methylguaiacol Concentration in Mature Grapes. Am. J. Enol. Vitic. 2009, 60, 98–103. [Google Scholar]
- Bell, T.; Stephens, S.; Moritz, M. Short-term physiological effects of smoke on grapevine leaves. Int. J. Wildland Fire 2013, 22, 933–946. [Google Scholar] [CrossRef] [Green Version]
- Noestheden, M.; Dennis, E.G.; Romero-Montalvo, E.; DiLabio, G.A.; Zandberg, W.F. Detailed characterization of glycosylated sensory-active volatile phenols in smoke-exposed grapes and wine. Food Chem. 2018, 259, 147–156. [Google Scholar] [CrossRef]
- Parker, M.; Osidacz, P.; Baldock, G.A.; Hayasaka, Y.; Black, C.A.; Pardon, K.H.; Jeffery, D.W.; Geue, J.P.; Herderich, M.J.; Francis, I.L. Contribution of several volatile phenols and their glycoconjugates to smoke-related sensory properties of red wine. J. Agric. Food Chem. 2012, 60, 2629–2637. [Google Scholar] [CrossRef]
- Härtl, K.; Huang, F.-C.; Giri, A.P.; Franz-Oberdorf, K.; Frotscher, J.; Shao, Y.; Hoffmann, T.; Schwab, W. Glucosylation of Smoke-Derived Volatiles in Grapevine (Vitis vinifera) is Catalyzed by a Promiscuous Resveratrol/Guaiacol Glucosyltransferase. J. Agric. Food Chem. 2017, 65, 5681–5689. [Google Scholar] [CrossRef]
- Ristic, R.; Boss, P.; Wilkinson, K. Influence of Fruit Maturity at Harvest on the Intensity of Smoke Taint in Wine. Molecules 2015, 20, 8913–8927. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Chong, H.; Pitt, K.; Cleary, M.; Dokoozlian, N.; Downey, M. Guaiacol and 4-methylguaiacol accumulate in wines made from smoke-affected fruit because of hydrolysis of their conjugates. Aust. J. Grape Wine Res. 2011, 17, S13–S21. [Google Scholar] [CrossRef]
- Singh, D.; Zerihun, A.; Kelly, D.; Cain, N.M.; Nankervis, P.; Downey, M.O. A GC-MS Based Analytical Method for Detection of Smoke Taint Associated Phenols in Smoke Affected Wines. Curr. Bioact. Compd. 2012, 8, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, S.; Tongson, E. Advances in smoke contamination detection systems for grapevine canopies and berries. Wine Vitic. J. 2017, 32, 36. [Google Scholar]
- Fudge, A.; Ristic, R.; Wollan, D.; Wilkinson, K. Amelioration of smoke taint in wine by reverse osmosis and solid phase adsorption. Aust. J. Grape Wine Res. 2011, 17, S41–S48. [Google Scholar] [CrossRef]
- Noestheden, M.; Thiessen, K.; Dennis, E.G.; Tiet, B.; Zandberg, W.F. Quantitating Organoleptic Volatile Phenols in Smoke-Exposed Vitis vinifera Berries. J. Agric. Food Chem. 2017, 65, 8418–8425. [Google Scholar] [CrossRef]
- Whiting, J.; Krstic, M. Understanding the Sensitivity to Timing and Management Options to Mitigate the Negative Impacts of Bush Fire Smoke on Grape and Wine Quality-Scoping Study; Department of Primary Industries: Knoxfield, VIC, Australia, 2007. [Google Scholar]
- Department of Primary Industries. Impacts of Smoke on Grapes and Wine in Victoria; Department of Primary Industries: Mildura, VIC, Australia, 2009; Available online: http://wine.wsu.edu/research-extension/files/2012/10/DPI-fact-sheet_Impacts-of-smoke-on-grapes-and-wine-in-Victoria_final.pdf (accessed on 10 October 2020).
- Claughton, D.; Jeffery, C.; Pritchard, M.; Hough, C.; Wheaton, C. Wine Industry’s ‘Black Summer’ as Cost of Smoke Taint, Burnt Vineyards, and Lost Sales Add Up. ABC News. 28 February 2020. Available online: https://www.abc.net.au/news/rural/2020-02-28/fire-and-smoke-costs-wine-industry-40-million-dollars/11972450 (accessed on 9 September 2020).
- Kennison, K.; Wilkinson, K.; Pollnitz, A.; Williams, H.; Gibberd, M. Effect of timing and duration of grapevine exposure to smoke on the composition and sensory properties of wine. Aust. J. Grape Wine Res. 2009, 15, 228–237. [Google Scholar] [CrossRef]
- Ristic, R.; Fudge, A.; Pinchbeck, K.; De Bei, R.; Fuentes, S.; Hayasaka, Y.; Tyerman, S.; Wilkinson, K. Impact of grapevine exposure to smoke on vine physiology and the composition and sensory properties of wine. Theor. Exp. Plant Physiol. 2016, 28, 67–83. [Google Scholar] [CrossRef]
- Wang, H.; Chambers, E., IV. Sensory characteristics of various concentrations of phenolic compounds potentially associated with smoked aroma in foods. Molecules 2018, 23, 780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostyra, E.; Baryłko-Pikielna, N. Volatiles composition and flavour profile identity of smoke flavourings. Food Qual. Prefer. 2006, 17, 85–95. [Google Scholar] [CrossRef]
- Nawawi, D.S.; Syafii, W.; Tomoda, I.; Uchida, Y.; Akiyama, T.; Yokoyama, T.; Matsumoto, Y. Characteristics and Reactivity of Lignin in Acacia and Eucalyptus Woods. J. Wood Chem. Technol. 2017, 37, 273–282. [Google Scholar] [CrossRef]
- De Vries, C.; Buica, A.; McKay, J.B.M. The impact of smoke from vegetation fires on sensory characteristics of cabernet sauvignon wines made from affected grapes. S. Afr. J. Enol. Vitic. 2016, 37, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Favell, J.W.; Noestheden, M.; Lyons, S.-M.; Zandberg, W.F. Development and evaluation of a vineyard-based strategy to mitigate smoke-taint in wine grapes. J. Agric. Food Chem. 2019, 67, 14137–14142. [Google Scholar] [CrossRef] [PubMed]
- Härtl, K.; Schwab, W. Smoke Taint in Wine-How smoke-derived volatiles accumulate in grapevines. Wines Vines 2018, 99/02, 62–64. [Google Scholar]
- Pollnitz, A.P.; Pardon, K.H.; Sykes, M.; Sefton, M.A. The effects of sample preparation and gas chromatograph injection techniques on the accuracy of measuring guaiacol, 4-methylguaiacol and other volatile oak compounds in oak extracts by stable isotope dilution analyses. J. Agric. Food Chem. 2004, 52, 3244–3252. [Google Scholar] [CrossRef]
- Allen, D.; Bui, A.D.; Cain, N.; Rose, G.; Downey, M. Analysis of free and bound phenolics in wine and grapes by GC-MS after automated SPE. Anal. Bioanal. Chem. 2013, 405, 9869–9877. [Google Scholar] [CrossRef] [PubMed]
- Simos, C. The implications of smoke taint and management practices. Aust. Vitic. Jan/Feb 2008, 12, 77–80. [Google Scholar]
- Hayasaka, Y.; Baldock, G.; Parker, M.; Pardon, K.; Black, C.; Herderich, M.; Jeffery, D. Glycosylation of smoke-derived volatile phenols in grapes as a consequence of grapevine exposure to bushfire smoke. J. Agric. Food Chem. 2010, 58, 10989–10998. [Google Scholar] [CrossRef]
- Kelly, D.; Zerihun, A. The Effect of Phenol Composition on the Sensory Profile of Smoke Affected Wines. Molecules 2015, 20, 9536–9549. [Google Scholar] [CrossRef] [Green Version]
- Kennison, K. Bushfire Generated Smoke Taint in Grapes and Wine. Final Report to Grape and Wine Research and Development Corporation; RD 05/02-3; Department of Agriculture and Food Western Australia: Perth, WA, Australia, 2009.
- Boidron, J.-N.; Chatonnet, P.; Pons, M. Influence du bois sur certaines substances odorantes des vins. OENO One 1988, 22, 275–294. [Google Scholar] [CrossRef]
- Simpson, R.; Amon, J.; Daw, A. Off-flavour in wine caused by guaiacol [1986]. Food Technol. Aust. 2013, 38, 31–33. [Google Scholar]
- Pardo-Garcia, A.; Wilkinson, K.; Culbert, J.; Lloyd, N.; Alonso, G.L.; Salinas, M.R. Accumulation of Glycoconjugates of 3-Methyl-4-hydroxyoctanoic Acid in Fruits, Leaves, and Shoots of Vitis vinifera cv. Monastrell following Foliar Applications of Oak Extract or Oak Lactone. J. Agric. Food Chem. 2015, 63, 4533–4538. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Garcia, A.; Wilkinson, K.; Culbert, J.; Lloyd, N.; Alonso, G.; Salinas, M.R. Accumulation of guaiacol glycoconjugates in fruit, leaves and shoots of Vitis vinifera cv. Monastrell following foliar applications of guaiacol or oak extract to grapevines. Food Chem. 2017, 217, 782–789. [Google Scholar] [CrossRef]
- The Australian Wine Research Institute. Smoke Taint-Entry into Grapes and Vineyard Risk Factors. 2015. Available online: https://www.awri.com.au/wp-content/uploads/2012/04/smoke-taint-entry-into-grapes-and-vineyard-risk-factors.pdf (accessed on 15 September 2020).
- Hoj, P.; Pretorius, I.; Blair, R. Investigations Conducted into the Nature and Amelioration of Taints in Grapes and Wine, Caused by Smoke Resulting from Bushfires. In The Australian Wine Research Institute Annual Report 2003; The Australian Wine Research Institute: Adelaide, SA, Australia, 2003; pp. 37–39. [Google Scholar]
- Beattie, G.A.; Seibel, J.R. Uptake and localization of gaseous phenol and p-cresol in plant leaves. Chemosphere 2007, 68, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gil, A.; Garde-Cerdán, T.; Martínez, L.; Alonso, G.; Salinas, M.R. Effect of Oak Extract Application to Verdejo Grapevines on Grape and Wine Aroma. J. Agric. Food Chem. 2011, 59, 3253–3263. [Google Scholar] [CrossRef]
- Martínez-Gil, A.; Garde-Cerdán, T.; Zalacain, A.; Pardo-García, A.; Salinas, M.R. Applications of an oak extract on Petit Verdot grapevines. Influence on grape and wine volatile compounds. Food Chem. 2012, 132, 1836–1845. [Google Scholar] [CrossRef]
- Pardo-García, A.I.; de la Hoz, K.S.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Effect of vine foliar treatments on the varietal aroma of Monastrell wines. Food Chem. 2014, 163, 258–266. [Google Scholar] [CrossRef]
- Australian Wine Rearch Institute. Annual Report 2003; Australian Wine Rearch Institute: Adelaide, SA, Australia, 2003. [Google Scholar]
- Kennison, K.; Wilkinson, K.; Pollnitz, A.; Williams, H.; Gibberd, M. Effect of smoke application to field-grown Merlot grapevines at key phenological growth stages on wine sensory and chemical properties. Aust. J. Grape Wine Res. 2011, 17, 5–12. [Google Scholar] [CrossRef]
- Brodison, K. Bulletin 4847: Effect of Smoke in Grape and Wine Production; Department of Agriculture and Food Western Australia: Perth, WA, Australia, 2013. Available online: http://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=7DCE37912829907FD8A627BFB05F4822?doi=10.1.1.392.6887&rep=rep1&type=pdf (accessed on 10 October 2020).
- Calder, J.; Lifferth, G.; Moritz, M.; St Clair, S. Physiological Effects of Smoke Exposure on Deciduous and Conifer Tree Species. Int. J. For. Res. 2010, 2010, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.; Ripley, B.; van Staden, J. The effect of smoke on the photosynthetic gas exchange of Chrysanthemoides monilifera. S. Afr. J. Bot. 2002, 68, 525–531. [Google Scholar] [CrossRef] [Green Version]
- During, H. Photochemical and non-photochemical responses of glasshouse-grown grape to combined light and water stress. Vitis 1998, 37, 1–4. [Google Scholar]
- Sepúlveda, G.; Kliewer, W.M.; Ryugo, K. Effect of high temperature on grapevines (Vitis vinifera L.). I. Translocation of 14C-photosynthates. Am. J. Enol. Vitic. 1986, 37, 13–19. [Google Scholar]
- Hayasaka, Y.; Dungey, K.; Baldock, G.; Kennison, K.; Wilkinson, K. Identification of a β-d-glucopyranoside precursor to guaiacol in grape juice following grapevine exposure to smoke. Anal. Chim. Acta 2010, 660, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gil, A.; Angenieux, M.; Pardo-García, A.; Alonso, G.; Ojeda, H.; Salinas, R. Glycosidic aroma precursors of Syrah and Chardonnay grapes after an oak extract application to the grapevines. Food Chem. 2013, 138, 956–965. [Google Scholar] [CrossRef]
- Mayr, C.M.; Parker, M.; Baldock, G.A.; Black, C.A.; Pardon, K.H.; Williamson, P.O.; Herderich, M.J.; Francis, I.L. Determination of the Importance of In-Mouth Release of Volatile Phenol Glycoconjugates to the Flavor of Smoke-Tainted Wines. J. Agric. Food Chem. 2014, 62, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Ristic, R.; Osidacz, P.; Pinchbeck, K.; Hayasaka, Y.; Fudge, A.; Wilkinson, K. The effect of winemaking techniques on the intensity of smoke taint in wine. Aust. J. Grape Wine Res. 2011, 17, S29–S40. [Google Scholar] [CrossRef]
- Otero, R.R.C.; Iranzo, J.F.U.; Briones-Perez, A.I.; Potgieter, N.; Villena, M.A.; Pretorius, I.S.; Rensburg, P.V. Characterization of the β-Glucosidase Activity Produced by Enological Strains of Non-Saccharomyces Yeasts. J. Food Sci. 2003, 68, 2564–2569. [Google Scholar] [CrossRef]
- Government of Western Australia: Department of Primary Industries and Regional Development. Management of Grapes and Wine after Smoke Events. 2018. Available online: https://www.agric.wa.gov.au/fire/management-grapes-and-wine-after-smoke-events (accessed on 25 August 2020).
- van der Hulst, L.; Munguia, P.; Culbert, J.A.; Ford, C.M.; Burton, R.A.; Wilkinson, K.L. Accumulation of volatile phenol glycoconjugates in grapes following grapevine exposure to smoke and potential mitigation of smoke taint by foliar application of kaolin. Planta 2019, 249, 941–952. [Google Scholar] [CrossRef]
- Fudge, A.; Schiettecatte, M.; Ristic, R.; Hayasaka, Y.; Wilkinson, K. Amelioration of smoke taint in wine by treatment with commercial fining agents. Aust. J. Grape Wine Res. 2012, 18, 302–307. [Google Scholar] [CrossRef]
- Ulrich, T. When the Smoke Cleared: California Winemakers Face Tough Pre-Bottling Decisions for 2008 Wines. Available online: https://winesvinesanalytics.com/sections/printout_article.cfm?article=feature&content=65507 (accessed on 25 October 2020).
- The Australian Wine Research Institute. Smoke Taint Analysis. Available online: https://www.awri.com.au/wp-content/uploads/awri_smoke_analysis_faq.pdf (accessed on 25 October 2020).
- Fudge, A.; Wilkinson, K.; Ristic, R.; Cozzolino, D. Classification of smoke tainted wines using mid-infrared spectroscopy and chemometrics. J. Agric. Food Chem. 2012, 60, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Fudge, A.; Wilkinson, K.; Ristic, R.; Cozzolino, D. Synchronous two-dimensional MIR correlation spectroscopy (2D-COS) as a novel method for screening smoke tainted wine. Food Chem. 2013, 139, 115–119. [Google Scholar] [CrossRef]
- Barbin, D.F.; De Souza Madureira Felicio, A.L.; Sun, D.-W.; Nixdorf, S.L.; Hirooka, E.Y. Application of infrared spectral techniques on quality and compositional attributes of coffee: An overview. Food Res. Int. 2014, 61, 23–32. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, C.T.; Lopo, M.; Ricardo, N.; Lopes, J. A Review on the Applications of Portable Near-Infrared Spectrometers in the Agro-Food Industry. Appl. Spectrosc. 2013, 67, 1215–1233. [Google Scholar] [CrossRef]
- Urraca, R.; Sanz-Garcia, A.; Tardaguila, J.; Diago, M.P. Estimation of total soluble solids in grape berries using a handheld NIR spectrometer under field conditions. J. Sci. Food Agric. 2016, 96, 3007–3016. [Google Scholar] [CrossRef]
- Hall, A. Remote Sensing Applications for Viticultural Terroir Analysis. Elements 2018, 14, 185–190. [Google Scholar] [CrossRef]
- Fuentes, S.; De Bei, R.; Pech, J.; Tyerman, S. Computational water stress indices obtained from thermal image analysis of grapevine canopies. Irrig. Sci. 2012, 30, 523–536. [Google Scholar] [CrossRef]
- Poblete-Echeverría, C.; Sepulveda-Reyes, D.; Ortega-Farias, S.; Zuñiga, M.; Fuentes, S. Plant water stress detection based on aerial and terrestrial infrared thermography: A study case from vineyard and olive orchard. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014), Brisbane, QLD, Australia, 17–22 August 2014; 1112, pp. 141–146. [Google Scholar]
- Poblete-Echeverría, C.; Ortega-Farías, S.; Zuñiga, M.; Lobos, G.; Romero, S.; Estrada, F.; Fuentes, S. Use of infrared thermography on canopies as indicator of water stress in ‘Arbequina’olive orchards. In Proceedings of the VII International Symposium on Olive Growing, San Juan, Argentina, 25–29 September 2012; 1057, pp. 399–403. [Google Scholar]
- Pou, A.; Diago, M.P.; Medrano, H.; Baluja, J.; Tardaguila, J. Validation of thermal indices for water status identification in grapevine. Agric. Water Manag. 2014, 134, 60–72. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.; Reginato, R.; Pinter, P., Jr. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Gontia, N.; Tiwari, K. Development of crop water stress index of wheat crop for scheduling irrigation using infrared thermometry. Agric. Water Manag. 2008, 95, 1144–1152. [Google Scholar] [CrossRef]
- Fuentes, S.; Tongson, E.; Summerson, V.; Viejo, C.G. Advances in Artificial Intelligence to Assess Smoke Contamination in Grapevines and Taint in Wines Due to Increased Bushfire Events. Wine Vitic. J. 2020, 35, 26–29. [Google Scholar]
- Fuentes, S.; Tongson, E.J.; De Bei, R.; Gonzalez Viejo, C.; Ristic, R.; Tyerman, S.; Wilkinson, K. Non-Invasive Tools to Detect Smoke Contamination in Grapevine Canopies, Berries and Wine: A Remote Sensing and Machine Learning Modeling Approach. Sensors 2019, 19, 3335. [Google Scholar] [CrossRef] [Green Version]
- Dolatabadi, Z.; Rad, A.H.E.; Farzaneh, V.; Feizabad, S.H.A.; Estiri, S.H.; Bakhshabadi, H. Modeling of the lycopene extraction from tomato pulps. Food Chem. 2016, 190, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Viejo, C.G.; Torrico, D.D.; Dunshea, F.R.; Fuentes, S. Emerging technologies based on artificial intelligence to assess the quality and consumer preference of beverages. Beverages 2019, 5, 62. [Google Scholar] [CrossRef] [Green Version]
- Gumus, Z.P.; Ertas, H.; Yasar, E.; Gumus, O. Classification of olive oils using chromatography, principal component analysis and artificial neural network modelling. J. Food Meas. Charact. 2018, 12, 1325–1333. [Google Scholar] [CrossRef]
- Pero, M.; Askari, G.; Skåra, T.; Skipnes, D.; Kiani, H. Change in the color of heat-treated, vacuum-packed broccoli stems and florets during storage: Effects of process conditions and modeling by an artificial neural network. J. Sci. Food Agric. 2018, 98, 4151–4159. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Viejo, C.; Torrico, D.D.; Dunshea, F.R.; Fuentes, S. Development of artificial neural network models to assess beer acceptability based on sensory properties using a robotic pourer: A comparative model approach to achieve an artificial intelligence system. Beverages 2019, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, S.; Tardaguila, J.; Fernandez-Novales, J.; Diago, M.P. Support vector machine and artificial neural network models for the classification of grapevine varieties using a portable NIR spectrophotometer. PLoS ONE 2015, 10, e0143197. [Google Scholar] [CrossRef] [PubMed]
- Pralle, R.; Weigel, K.; White, H. Predicting blood β-hydroxybutyrate using milk Fourier transform infrared spectrum, milk composition, and producer-reported variables with multiple linear regression, partial least squares regression, and artificial neural network. J. Dairy Sci. 2018, 101, 4378–4387. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Kerr, W.L. Determining degree of roasting in cocoa beans by artificial neural network (ANN)-based electronic nose system and gas chromatography/mass spectrometry (GC/MS). J. Sci. Food Agric. 2018, 98, 3851–3859. [Google Scholar] [CrossRef]
- Shanmuganathan, S.; Samarasinghe, S. Artificial Neural Network Modelling; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Chandraratne, M.; Kulasiri, D.; Samarasinghe, S. Classification of lamb carcass using machine vision: Comparison of statistical and neural network analyses. J. Food Eng. 2007, 82, 26–34. [Google Scholar] [CrossRef]
- Carmel, L.; Levy, S.; Lancet, D.; Harel, D. A feature extraction method for chemical sensors in electronic noses. Sens. Actuators B Chem. 2003, 93, 67–76. [Google Scholar] [CrossRef]
- Cipriano, D.; Capelli, L. Evolution of electronic noses from research objects to engineered environmental odour monitoring systems: A review of standardization approaches. Biosensors 2019, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Hines, E.; Llobet, E.; Gardner, J. Electronic noses: A review of signal processing techniques. IEE Proc. Circuits Devices Syst. 1999, 146, 297–310. [Google Scholar] [CrossRef]
- Li, W.; Leung, H.; Kwan, C.; Linnell, B.R. E-nose vapor identification based on Dempster–Shafer fusion of multiple classifiers. IEEE Trans. Instrum. Meas. 2008, 57, 2273–2282. [Google Scholar] [CrossRef]
- Antolini, A.; Forniti, R.; Modesti, M.; Bellincontro, A.; Catelli, C.; Mencarelli, F. First Application of Ozone Postharvest Fumigation to Remove Smoke Taint from Grapes. Ozone Sci. Eng. 2020, 1–9. [Google Scholar] [CrossRef]
- Chapman, J.; Gangadoo, S.; Truong, V.K.; Cozzolino, D. Spectroscopic approaches for rapid beer and wine analysis. Curr. Opin. Food Sci. 2019, 28, 67–73. [Google Scholar] [CrossRef]
- Sorak, D.; Herberholz, L.; Iwascek, S.; Altinpinar, S.; Pfeifer, F.; Siesler, H.W. New developments and applications of handheld Raman, mid-infrared, and near-infrared spectrometers. Appl. Spectrosc. Rev. 2012, 47, 83–115. [Google Scholar] [CrossRef]
- O’Brien, N.A.; Hulse, C.A.; Friedrich, D.M.; Van Milligen, F.J.; von Gunten, M.K.; Pfeifer, F.; Siesler, H.W. Miniature near-infrared (NIR) spectrometer engine for handheld applications. In Proceedings of the Next-Generation Spectroscopic Technologies V, Baltimore, MD, USA, 23–24 April 2012; p. 837404. [Google Scholar]
- Gardner, J.W.; Bartlett, P.N. A brief history of electronic noses. Sens. Actuators B Chem. 1994, 18, 210–211. [Google Scholar] [CrossRef]
- Fuentes, S.; Tongson, E.; Torrico, D.D.; Gonzalez Viejo, C. Modeling Pinot Noir Aroma Profiles Based on Weather and Water Management Information Using Machine Learning Algorithms: A Vertical Vintage Analysis Using Artificial Intelligence. Foods 2020, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, S.; Torrico, D.D.; Tongson, E.; Gonzalez Viejo, C. Machine learning modeling of wine sensory profiles and color of vertical vintages of Pinot Noir based on chemical fingerprinting, weather and management data. Sensors 2020, 20, 3618. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Tongson, E.; Chen, J.; Gonzalez Viejo, C. A Digital Approach to Evaluate the Effect of Berry Cell Death on Pinot Noir Wines’ Quality Traits and Sensory Profiles Using Non-Destructive Near-Infrared Spectroscopy. Beverages 2020, 6, 39. [Google Scholar] [CrossRef]
| Compound | Aromas |
|---|---|
| Guaiacol | Smoky, phenolic, woody, musty/dusty, petroleum-like, sweet, sharp |
| 4-methylguaiacol | smoky, toasted, ash, vanilla-like, sweet, phenolic, sharp |
| m-cresol | smoky, petroleum-like, woody, musty/dusty |
| o-cresol | smoky, woody, musty/dusty, acrid, pungent, petroleum-like |
| Eugenol | Smoky, musty/dusty, woody, earthy, clove, vanilla-like, phenolic |
| Thymol | Smoky, woody, musty/dusty, cedar, petroleum-like, pungent |
| 4-ethylguaiacol | Smoky, woody, ashy, burnt, spicy, clove-like, sweet, cedar, musty/dusty, acrid, pungent |
| 4-ethylphenol | Clove, vanilla-like, phenolic |
| Compound | Aroma Detection Threshold (µg L−1) | |||
|---|---|---|---|---|
| Water | Model Wine | White Wine | Red Wine | |
| Guaiacol | 0.48–5.5 | 20 | 20–95 | 75 |
| 4-methylguaiacol | 10 | 30 | 65 | 65 |
| 4-ethylguaiacol | 25 | 47 | 70 | 110–150 |
| 4-ethylphenol | 130 | 440 | 1100 | 605–1200 |
| Period | Growth Stage | Smoke Uptake Risk |
|---|---|---|
| P1 | 10-cm long shoots | Low |
| Flowering | Low | |
| P2 | Pea-sized grape berries | Variable (low to medium) |
| Onset of bunch closure | Variable (low to medium) | |
| Start of veraison to 3 days after onset | Variable (low to medium) | |
| P3 | 7 days post-veraison to harvest | High |
| Technique | Details | References |
|---|---|---|
| Hand harvest berries and maintain their integrity | Minimize skin rupturing as long as possible as contact of juice with skins can lead to higher concentrations of smoke-derived volatile compounds in the final wine | [31,44] |
| Avoid the inclusion of leaves in fruit harvest | Contaminated leaf material may increase levels of volatile phenols, leading to an increased risk of smoke taint | [31,44] |
| Plucking leaves and washing grapevines | Leaf plucking and high-pressure cold-water wash in the vineyard may remove ash; however, washing the entire canopy may increase smoke compounds in grapes | [47,53] |
| Cooling fruit | Processing berries at 10 °C causes less extraction of smoke-derived compounds compared to processing berries at 25 °C | [31,44] |
| Whole bunch press | Whole bunch processing may minimize the release of volatile phenols from smoke | [44,73] |
| Separate press fractions | Fruit cooling may reduce smoky aromas in the first 400 L/t. Free-run juice may contain less smoky aromas | [31,44,73] |
| Conduct trials with fining agents before fermentation | Variable results have been demonstrated with PVPP and isinglass in reducing smoke taint characters. Fining with activated carbon has been effective | [31,44,72,73] |
| Yeast selection | Yeast selection may affect the levels of smoke compounds in the final wine and alter smoke-related aromas and flavors | [31,44,68,73] |
| Keep fermentation time on skins to a minimum | Minimizing fermentation time on skins has been shown to reduce smoky aromas and flavors | [11,44,68] |
| The use of oak chips and tannins | Increases the complexity of wine and may reduce the intensity of smoky aromas and flavors | [68] |
| Reverse osmosis treatment of wine | Effective in reducing smoky characteristics; however, overtime smoky aromas and flavors were found to reappear | [29] |
| Foliar application of kaolin or biofilm | Foliar applications of kaolin or a biofilm consisting of phospholipids have been shown to provide some form of protection from volatile phenol uptake | [40,71] |
| Market wine for quick sale and immediate consumption | Smoky characteristics have been found to increase over time as the wine ages in the bottle | [4,29,44,73] |
| Phenological Stage | E-L Stage | Description | Smoke Compounds Measurements | Amelioration Strategy | Citation |
|---|---|---|---|---|---|
| Veraison | 35 | Berries color and enlarge | High-performance liquid chromatography (HPLC) | Refer to Table 4 | [2,5,6,9,15,59,88,90,99,100,101,102,103,104,105,106] |
| Post-veraison | 36 | Berries have moderate sugar content | |||
| 37 | Berries not fully ripe | Near-infrared spectroscopy (NIR), electronic nose (e-nose), and artificial intelligence (AI) | |||
| Harvest | 38 | Berries ripe for harvest | |||
| Postharvest processing | Description | Smoke Compounds measurements | Amelioration strategy | Citation | |
| Winemaking | Involves micro- and industrial vinification at all stages | High-performance liquid chromatography (HPLC) | Refer to Table 4 | [6,15,59,76,88,90,99,100,101,102,103,104,105,106] | |
| Final product | Involves the wine before and after bottling | Near-infrared spectroscopy (NIR), electronic nose (e-nose), and artificial intelligence (AI) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Summerson, V.; Gonzalez Viejo, C.; Pang, A.; Torrico, D.D.; Fuentes, S. Review of the Effects of Grapevine Smoke Exposure and Technologies to Assess Smoke Contamination and Taint in Grapes and Wine. Beverages 2021, 7, 7. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7010007
Summerson V, Gonzalez Viejo C, Pang A, Torrico DD, Fuentes S. Review of the Effects of Grapevine Smoke Exposure and Technologies to Assess Smoke Contamination and Taint in Grapes and Wine. Beverages. 2021; 7(1):7. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7010007
Chicago/Turabian StyleSummerson, Vasiliki, Claudia Gonzalez Viejo, Alexis Pang, Damir D. Torrico, and Sigfredo Fuentes. 2021. "Review of the Effects of Grapevine Smoke Exposure and Technologies to Assess Smoke Contamination and Taint in Grapes and Wine" Beverages 7, no. 1: 7. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7010007