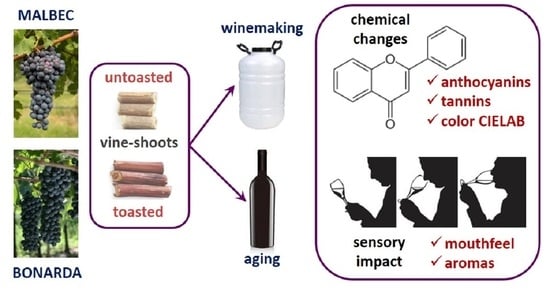
Application of Vine-Shoot Chips during Winemaking and Aging of Malbec and Bonarda Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vine-Shoot Chips
2.2. Experimental Design
2.2.1. Application of Vine-Shoots during Winemaking (Experiment A)
2.2.2. Application of Vine-Shoots during Wine Aging (Experiment B)
2.3. Wine General Analytical Parameters
2.4. Global Phenolic Composition and Color Parameters
2.5. Anthocyanins and Derived Pigments
2.6. Sensory Analysis
2.7. Data Analysis
3. Results and Discussion
3.1. Experiment A: Application of Vine-Shoots during Winemaking
3.1.1. General Chemical Composition of Wines
3.1.2. Global Phenolic Parameters and Wine Color
3.1.3. Wine Anthocyanin Profile
3.2. Experiment B: Application of Vine-Shoots during Wine Aging
3.2.1. General Chemical Composition of Wines
3.2.2. Global Phenolic Parameters and Wine Color
3.2.3. Wine Anthocyanin Profile
3.2.4. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velasco, M.C.V.; de Cerio, M.C.D.; Dietrich, T.; Rodríguez, E. Subproductos hortofrutícolas para una bioeconomía circular. Mediterráneo Econ. 2018, 31, 251–272. [Google Scholar]
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Vine-shoot waste aqueous extracts for re-use in agriculture obtained by different extraction techniques: Phenolic, volatile, and mineral compounds. J. Agric. Food Chem. 2014, 62, 10861–10872. [Google Scholar] [CrossRef] [PubMed]
- Peralbo-Molina, T.; Luque de Castro, M.D. Potential of residues from the Mediterranean agriculture and agrifood industry. Trends Food Sci. Technol. 2013, 32, 16–24. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Pardo, F.; Alonso, G.L.; Salinas, M.R. An innovative use of vine-shoots residues and their “feedback” effect on wine quality. Innov. Food Sci. Emerg. Technol. 2016, 37, 18–26. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Pardo, F.; Alonso, G.L.; Salinas, M.R. Moscatel vine-shoot extracts as a grapevine biostimulant to enhance wine quality. Food Res. Int. 2017, 98, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gómez, R.; Pérez-Álvarez, E.P.; Salinas, R.; Gonzalo-Diago, A.; Zalacain, A.; Garde-Cerdan, T. Effect of vine-shoot and oak extract foliar grapevine applications on oenological parameters, phenolic acids and glutathione content of white musts and wines. OENO One 2020, 54, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Gómez, R.; Sánchez-Vioque, R.; Santana-Méridas, O.; Martín-Bejerano, M.; Alonso, G.L.; Salinas, M.R.; Zalacain, A. A potential use of vine-shoot wastes: The antioxidant, antifeedant and phytotoxic activities of their aqueous extracts. Ind. Crops Prod. 2017, 97, 120–127. [Google Scholar] [CrossRef]
- Delgado de la Torre, M.P.; Priego-Capote, F.; Luque de Castro, M.D. Comparative profiling analysis of woody flavouring from vine-shoots and oak chips. J. Sci. Food Agric. 2014, 94, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, C.; Sánchez-Gómez, R.; Salinas, M.R.; Alonso, G.L.; Zalacain, A. Effect of post-pruning vine-shoots storage on the evolution of high-value compounds. Ind. Crops Prod. 2017, 109, 730–736. [Google Scholar] [CrossRef]
- Houillé, B.; Besseau, S.; Courdavault, V.; Oudin, A.; Glévarec, G.; Delanoue, G.; Lanoue, A. Biosynthetic origin of e -resveratrol accumulation in grape canes during postharvest storage. J. Agric. Food Chem. 2015, 63, 1631–1638. [Google Scholar] [CrossRef]
- Cebrián-Tarancón, C.; Sánchez-Goómez, R.; Gómez-Alonso, S.; Hermosín-Gutierrez, I.; Mena-Morales, A.; García-Romero, E.; Salinas, M.R.; Zalacain, A. Vine-shoot tannins: Effect of post-pruning storage and toasting treatment. J. Agric. Food Chem. 2018, 66, 5556–5562. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Effect of toasting on non-volatile and volatile vine-shoots low molecular weight phenolic compounds. Food Chem. 2016, 204, 499–505. [Google Scholar] [CrossRef]
- Cebrián-Tarancón, C.; Sánchez-Gómez, R.; Salinas, M.R.; Alonso, G.L.; Oliva, J.; Zalacain, A. Toasted vine-shoot chips as enological additive. Food Chem. 2018, 263, 96–103. [Google Scholar] [CrossRef]
- Cebrián-Tarancón, C.; Sánchez-Gómez, R.; Carot, J.M.; Zalacain, A.; Alonso, G.; Salinas, M.R. Assessment of vine-shoots in a model wines as enological additives. Food Chem. 2019, 288, 86–95. [Google Scholar] [CrossRef]
- Cebrián-Tarancón, C.; Sánchez-Gómez, R.; Cabrita, M.J.; García, R.; Zalacain, A.; Alonso, G.; Salinas, M.R. Winemaking with vine-shoots. Modulating the composition of wines by using their own resources. Food Res. Int. 2019, 121, 117–126. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Lencina, A.G.; Cano-López, M.; Pardo-Mínguez, F.; López-Roca, J.M.; Gómez-Plaza, E. The use of oak chips during the ageing of a red wine in stainless steel tanks or used barrels: Effect of the contact time and size of the oak chips on aroma compounds. Aust. J. Grape Wine Res. 2008, 14, 63–70. [Google Scholar] [CrossRef]
- Kyraleou, M.; Teissedre, P.L.; Tzanakouli, E.; Kotseridis, Y.; Proxenia, N.; Chira, C.; Ligas, I.; Kallithraka, S. Addition of wood chips in red wine during and after alcoholic fermentation: Differences in color parameters, phenolic content and volatile composition. OENO One 2016, 50, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Laqui-Estaña, J.; López-Solís, R.; Peña-Neira, A.; Medel-Marabolía, M.; Obreque-Slier, E. Wines in contact with oak wood: The impact of the variety (Carménère and Cabernet Sauvignon), format (barrels, chips and staves), and aging time on the phenolic composition. J. Sci. Food Agric. 2019, 99, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Vitivinicultura (INV). Informe Anual de Superficie 2020. Available online: https://www.argentina.gob.ar/inv/vinos/estadisticas/superficie/anuarios (accessed on 19 May 2021).
- Organisation Internationale de la Vigne et du Vin (OIV). Compendium of International Methods of Analysis of Wines and Musts; OIV: Paris, France, 2012; Volume 1. [Google Scholar]
- Harbertson, J.F.; Picciotto, E.A.; Adams, D.O. Measurement of polymeric pigments in grape berry extracts and wines using a protein precipitation assay combined with bisulfite bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar]
- Heredia, T.M.; Adams, D.O.; Fields, K.C.; Held, P.G.; Harbertson, J.F. Evaluation of a comprehensive Red wine phenolics assay using a microplate reader. Am. J. Enol. Vitic. 2006, 57, 497–502. [Google Scholar]
- Gordillo, B.; Rodríguez-Pulido, F.J.; Escudero-Gilete, M.L.; González-Miret, M.L.; Heredia, F.J. Comprehensive colorimetric study of anthocyanic copigmentation in model solutions. Effects of pH and molar ratio. J. Agric. Food Chem. 2012, 60, 2896–2905. [Google Scholar] [CrossRef]
- Commission Internationale de l’Eclairage (CIE). Technical Report Colorimetry; Commission Internationale de l’Eclairage Central Bureau; CIE: Vienna, Austria, 2004. [Google Scholar]
- Gama, J. Colorscience: Color Science Methods and Data. R Package Version 1.0.8. Available online: http://CRAN.R-project.org/package=colorscience (accessed on 1 April 2021).
- Blanco-Vega, D.; López-Bellido, F.J.; Alía-Robledo, J.M.; Hermosín-Gutiérrez, I. HPLC–DAD–ESI-MS/MS characterization of pyranoanthocyanins pigments formed in model wine. J. Agric. Food Chem. 2011, 59, 9523–9531. [Google Scholar] [CrossRef]
- Delarue, J. Flash Profile. In Novel Techniques in Sensory Characterization and Consumer Profiling, 1st ed.; Varela, P., Ares, A., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 175–205. [Google Scholar]
- Lê, S.; Worch, T. Analyzing Sensory Data with R; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; 372p. [Google Scholar]
- Husson, F.; Lê, S.; Cadoret, M. SensoMineR: Sensory Data Analysis. R Package Version 1.23. Available online: https://CRAN.R-project.org/package=SensoMineR (accessed on 20 April 2021).
- Vivas, N.; Augustin, A.; Lonvaud-Funel, A. Influence of oak wood and grape tannins on the lactic acid bacterium Oenococcus oeni (Leuconostoc oenos, 8413). J. Sci. Food Agric. 2000, 80, 1675–1678. [Google Scholar] [CrossRef]
- Dumitriu, G.D.; Peinado, R.A.; Coteac, V.V.; de Lerma, N.L. Volatilome fingerprint of red wines aged with chips or staves: Influence of the aging time and toasting degree. Food Chem. 2020, 310, 125801. [Google Scholar] [CrossRef]
- Kilmister, R.L.; Mazza, M.; Baker, N.K.; Faulkner, P.; Downey, M.O. A role for anthocyanin in determining wine tannin concentration in Shiraz. Food Chem. 2014, 152, 475–482. [Google Scholar] [CrossRef]
- Adams, D.O.; Harbertson, J.F.; Picciotto, E.A. Fractionation of Red Wine Polymeric Pigments by Protein Precipitation and Bisulfite Bleaching. In Red Wine Color; Waterhouse, A.L., Kennedy, J.A., Eds.; American Chemical Society: Washington, DC, USA, 2004; Volume 886, pp. 275–288. [Google Scholar] [CrossRef]
- Gordillo, B.; Cejudo-Bastante, M.J.; Rodríguez-Pulido, F.J.; Jara-Palacios, M.J.; Ramírez-Pérez, P.; González-Miret, M.L.; Heredia, F.J. Impact of adding white pomace to red grapes on the phenolic composition and color stability of Syrah wines from a warm climate. J. Agric. Food Chem. 2014, 62, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Fanzone, M.L.; Sari, S.E.; Mestre, M.V.; Catania, A.A.; Catelén, M.J.; Jofré, V.P.; González-Miret, M.L.; Combina, M.; Vazquez, F.; Maturano, Y.P. Combination of pre-fermentative and fermentative strategies to produce Malbec wines of lower alcohol and pH, with high chemical and sensory quality. OENO One 2020, 54, 1041–1058. [Google Scholar] [CrossRef]
- Martínez, J.A.; Melgosa, M.; Pérez, M.M.; Hita, E.; Negueruela, A.I. Note. Visual and instrumental color evaluation in red wines. Food Sci. Technol. Int. 2001, 7, 439–444. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-Sanjosé, M.L. Application of absorbance values used in wineries for estimating CIELAB parameters in red wines. Food Chem. 2003, 81, 301–306. [Google Scholar] [CrossRef]
- Fanzone, M.; Zamora, F.; Jofré, V.; Assof, M.; Gómez-Cordovés, C.; Peña-Neira, Á. Phenolic characterisation of red wines from different grape varieties cultivated in Mendoza province (Argentina). J. Sci. Food Agric. 2012, 92, 704–718. [Google Scholar] [CrossRef]
- Canals, R.; Llaudy, M.C.; Canals, J.M.; Zamora, F. Influence of the elimination and addition of seeds on the colour, phenolic composition and astringency of red wine. Eur. Food Res. Technol. 2008, 226, 1183–1190. [Google Scholar] [CrossRef]
- Casassa, L.F.; Dermutz, N.P.; Mawdsley, P.F.W.; Thompson, M.; Catania, A.A.; Collins, T.S.; Ashmore, P.L.; du Fresne, F.; Gasic, G.; Dodson Peterson, J.C. Whole cluster and dried stem additions effects on chemical and sensory properties of Pinot noir wines over two vintages. Am. J. Enol. Vitic. 2021, 72, 21–35. [Google Scholar] [CrossRef]
- Baiano, A.; De Gianni, A.; Mentana, A.; Quinto, M.; Centonze, D.; Del Nobile, M.A. Effects of the treatment with oak chips on color-related phenolics, volatile composition, and sensory profile of red wines: The case of Aglianico and Montepulciano. Eur. Food Res. Technol. 2016, 242, 745–767. [Google Scholar] [CrossRef]
- Gordillo, B.; Baca-Bocanegra, B.; Rodríguez-Pulido, F.J.; González-Miret, M.L.; Estévez, I.G.; Quijada-Morín, N.; Heredia, F.J.; Escribano-Bailón, M.T. Optimisation of an oak chips-grape mix maceration process. Influence of chip dose and maceration time. Food Chem. 2016, 206, 249–259. [Google Scholar] [CrossRef] [Green Version]
- de Esteban, M.L.G.; Ubeda, C.; Heredia, F.J.; Catania, A.A.; Assof, M.V.; Fanzone, M.L.; Jofre, V.P. Impact of closure type and storage temperature on chemical and sensory composition of Malbec wines (Mendoza, Argentina) during aging in bottle. Food Res. Int. 2019, 125, 108553. [Google Scholar] [CrossRef]
- Del Barrio-Galán, R.; Medel-Marabolí, M.; Peña-Neira, A. Effect of different aging techniques on the polysaccharide and phenolic composition and sensory characteristics of Syrah red wines fermented using different yeast strains. Food Chem. 2015, 179, 116–126. [Google Scholar] [CrossRef]
- Del Álamo, S.M.; Escudero, J.A.F.; De Castro, T.R. Changes in phenolic compounds and colour parameters of red wine aged with oak chips and in oak barrels. Food Sci. Technol. Int. 2004, 10, 233–241. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Evolution of flavanols, anthocyanins, and their derivatives during the aging of red wines elaborated from grapes harvested at different stages of ripening. J. Agric. Food Chem. 2004, 52, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Cadahía, E.; de Simón, B.F.; Sanz, M.; Poveda, P.; Colio, J. Chemical and chromatic characteristics of Tempranillo, Cabernet Sauvignon and Merlot wines from DO Navarra aged in Spanish and French oak barrels. Food Chem. 2009, 115, 639–649. [Google Scholar] [CrossRef]
- Avizcuri, J.M.; Sáenz-Navajas, M.P.; Echávarri, J.F.; Ferreira, V.; Fernández-Zurbano, P. Evaluation of the impact of initial red wine composition on changes in color and anthocyanin content during bottle storage. Food Chem. 2016, 213, 123–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baiano, A.; De Gianni, A. Timing of the treatment with oak chips: The case of Nero di Troia wine. Eur. Food Res. Technol. 2016, 242, 1343–1353. [Google Scholar] [CrossRef]
- Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O. Effect of oak constituents and oxygen on the evolution of malvidin-3-glucoside and (+)-catechin in model wine. Am. J. Enol. Vitic. 2006, 57, 377–381. [Google Scholar]
- Rentzsch, M.; Schwarz, M.; Winterhalter, P. Pyranoanthocyanins—An overview on structures, occurrence, and pathways of formation. Trends Food Sci. Technol. 2007, 18, 526–534. [Google Scholar] [CrossRef]
- Escribano-Bailón, T.; Álvarez-García, M.; Rivas-Gonzalo, J.C.; Heredia, F.J.; Santos-Buelga, C. Color and stability of pigments derived from the acetaldehyde-mediated condensation between malvidin 3-O-glucoside and (+)-catechin. J. Agric. Food Chem. 2001, 49, 1213–1217. [Google Scholar] [CrossRef]
- Cadoret, M.; Husson, F. Construction and evaluation of confidence ellipses applied at sensory data. Food Qual. Prefer. 2013, 28, 106–115. [Google Scholar] [CrossRef]










| Treatments | Ethanol (% v/v) | pH | Titratable Acidity (g/L) | Volatile Acidity (g/L) | Malic Acid (g/L) | Lactic Acid (g/L) |
|---|---|---|---|---|---|---|
| Malbec | ||||||
| C | 14.24 * ± 0.04 a | 3.74 ± 0.04 a | 6.19 ± 0.09 a | 0.65 ± 0.04 b | 0.90 ± 0.15 a | 0.87 ± 0.09 b |
| CHWT | 14.45 ± 0.03 b | 3.72 ± 0.05 a | 6.07 ± 0.03 a | 0.46 ± 0.02 a | 1.77 ± 0.20 b | ND |
| CHT | 14.40 ± 0.01 b | 3.69 ± 0.01 a | 6.11 ± 0.07 a | 0.52 ± 0.03 a | 1.73 ± 0.08 b | ND |
| p-value | 0.0003 | 0.2754 | 0.1616 | 0.0005 | 0.0007 | <0.0001 |
| Bonarda | ||||||
| C | 14.90 ± 0.51 a | 3.53 ± 0.04 a | 6.63 ± 0.08 a | 0.40 ± 0.03 a | 1.49 ± 0.01 b | ND |
| CHWT | 15.01 ± 0.57 a | 3.57 ± 0.03 a | 6.56 ± 0.07 a | 0.41 ± 0.03 a | 1.41 ± 0.03 a | ND |
| CHT | 14.92 ± 0.42 a | 3.58 ± 0.03 a | 6.50 ± 0.11 a | 0.41 ± 0.03 a | 1.43 ± 0.05 ab | ND |
| p-value | 0.9601 | 0.2584 | 0.2835 | 0.6122 | 0.0499 | NA |
| Aging Time | Vine-Shoot Treatment | Ethanol (% v/v) | pH | Titratable Acidity (g/L) | Volatile Acidity (g/L) |
|---|---|---|---|---|---|
| 1M | C | 14.10 * ± 0.04 a | 3.78 ± 0.04 a | 6.07 ± 0.08 a | 0.66 ± 0.04 b |
| CHWT | 14.31 ± 0.03 b | 3.76 ± 0.05 a | 5.95 ± 0.03 a | 0.46 ± 0.02 a | |
| CHT | 14.26 ± 0.01 b | 3.73 ± 0.01 a | 5.99 ± 0.07 a | 0.53 ± 0.03 a | |
| p-value | 0.0003 | 0.2735 | 0.1602 | 0.0004 | |
| 2M | C | 14.09 ± 0.01 b | 3.81 ± 0.01 a | 5.72 ± 0.01 b | 0.72 ± 0.01 a |
| CHWT | 14.06 ± 0.00 a | 3.84 ± 0.01 b | 5.68 ± 0.01 a | 0.72 ± 0.01 a | |
| CHT | 14.07 ± 0.01 ab | 3.84 ± 0.01 b | 5.73 ± 0.02 b | 0.74 ± 0.01 a | |
| p-value | 0.0110 | 0.0151 | 0.0041 | 0.0787 | |
| 4M | C | 14.11 ± 0.03 a | 3.79 ± 0.01 a | 5.66 ± 0.02 a | 0.70 ± 0.00 a |
| CHWT | 14.10 ± 0.02 a | 3.82 ± 0.00 b | 5.66 ± 0.01 a | 0.70 ± 0.01 a | |
| CHT | 14.14 ± 0.04 a | 3.82 ± 0.01 b | 5.74 ± 0.01 b | 0.72 ± 0.01 b | |
| p-value | 0.4315 | 0.0039 | 0.0003 | 0.0104 | |
| Two-way ANOVA | |||||
| Aging time (F1) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Vine-shoot treatment (F2) | 0.0002 | 0.5475 | 0.0081 | <0.0001 | |
| Interaction (F1 × F2) | <0.0001 | 0.0310 | 0.0367 | <0.0001 | |
| Aging Time | Vine-Shoot Treatment | Ethanol (% v/v) | pH | Titratable Acidity (g/L) | Volatile Acidity (g/L) |
|---|---|---|---|---|---|
| 1M | C | 14.75 * ± 0.50 a | 3.57 ± 0.05 a | 6.50 ± 0.08 a | 0.40 ± 0.03 a |
| CHWT | 14.85 ± 0.56 a | 3.61 ± 0.03 a | 6.43 ± 0.07 a | 0.42 ± 0.03 a | |
| CHT | 14.77 ± 0.42 a | 3.62 ± 0.03 a | 6.37 ± 0.11 a | 0.42 ± 0.03 a | |
| p-value | 0.9645 | 0.2506 | 0.2835 | 0.5637 | |
| 2M | C | 14.64 ± 0.02 a | 3.67 ± 0.01 a | 6.06 ± 0.04 a | 0.57 ± 0.01 a |
| CHWT | 14.68 ± 0.10 ab | 3.69 ± 0.02 ab | 6.10 ± 0.06 a | 0.56 ± 0.02 a | |
| CHT | 14.81 ± 0.01 b | 3.71 ± 0.01 b | 6.27 ± 0.06 b | 0.59 ± 0.01 a | |
| p-value | 0.0341 | 0.0370 | 0.0063 | 0.0983 | |
| 4M | C | 14.71 ± 0.00 a | 3.65 ± 0.01 a | 5.99 ± 0.01 c | 0.57 ± 0.01 a |
| CHWT | 14.89 ± 0.00 c | 3.71 ± 0.01 b | 5.93 ± 0.01 a | 0.62 ± 0.01 b | |
| CHT | 14.87 ± 0.01 b | 3.71 ± 0.01 b | 5.96 ± 0.01 b | 0.62 ± 0.00 b | |
| p-value | <0.0001 | <0.0001 | 0.0001 | <0.0001 | |
| Two-way ANOVA | |||||
| Aging time (F1) | 0.6943 | <0.0001 | <0.0001 | <0.0001 | |
| Vine-shoot treatment (F2) | 0.6481 | 0.0007 | 0.2861 | 0.0067 | |
| Interaction (F1 × F2) | 0.9650 | 0.7684 | 0.0016 | 0.1173 | |
| Parameter | p-value a for Factor | ||
|---|---|---|---|
| F1 | F2 | F1 × F2 b | |
| Malbec | |||
| Total phenols | <0.0001 | 0.3637 | 0.0225 |
| Total tannins | <0.0001 | 0.0049 | 0.4915 |
| Total anthocyanins | 0.0001 | <0.0001 | 0.0027 |
| LPP | 0.0001 | 0.0285 | 0.7361 |
| SPP | 0.0001 | 0.3571 | 0.9841 |
| TPP | 0.1784 | 0.0001 | 0.0790 |
| L* | <0.0001 | <0.0001 | 0.0047 |
| C*ab | <0.0001 | <0.0001 | 0.0175 |
| hab | <0.0001 | 0.0121 | 0.0612 |
| Bonarda | |||
| Total phenols | <0.0001 | 0.0001 | 0.1165 |
| Total tannins | <0.0001 | 0.0019 | 0.9012 |
| Total anthocyanins | <0.0001 | 0.0018 | 0.3873 |
| LPP | 0.5928 | 0.0011 | 0.9884 |
| SPP | <0.0001 | 0.0047 | 0.2178 |
| TPP | <0.0001 | <0.0001 | 0.8834 |
| L* | 0.0001 | 0.0002 | 0.3416 |
| C*ab | 0.0001 | <0.0001 | 0.2540 |
| hab | <0.0001 | <0.0001 | 0.0389 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanzone, M.; Catania, A.; Assof, M.; Jofré, V.; Prieto, J.; Gil Quiroga, D.; Lacognata Sottano, J.; Sari, S. Application of Vine-Shoot Chips during Winemaking and Aging of Malbec and Bonarda Wines. Beverages 2021, 7, 51. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7030051
Fanzone M, Catania A, Assof M, Jofré V, Prieto J, Gil Quiroga D, Lacognata Sottano J, Sari S. Application of Vine-Shoot Chips during Winemaking and Aging of Malbec and Bonarda Wines. Beverages. 2021; 7(3):51. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7030051
Chicago/Turabian StyleFanzone, Martín, Anibal Catania, Mariela Assof, Viviana Jofré, Jorge Prieto, Daniela Gil Quiroga, Juan Lacognata Sottano, and Santiago Sari. 2021. "Application of Vine-Shoot Chips during Winemaking and Aging of Malbec and Bonarda Wines" Beverages 7, no. 3: 51. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7030051