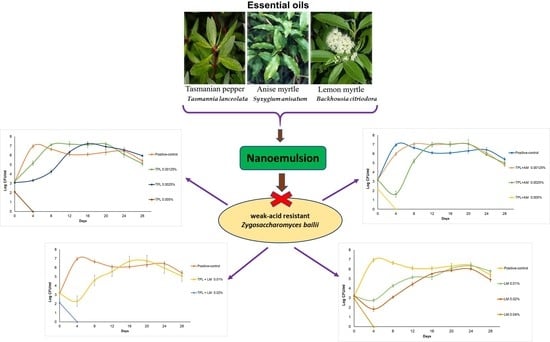
Antimicrobial Activity of Nanoencapsulated Essential Oils of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum against Weak-Acid Resistant Zygosaccharomyces bailii in Clear Apple Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oils, Reagents, and Apple Juice
2.2. Culture Preparation and Inoculation
2.3. Addition of Essential Oil Nanoemulsion and Sodium Benzoate in Apple Juice
2.4. Statistical Analysis
3. Results and Discussion
Effect of Sodium Benzoate and Essential Oils on Zygosaccharomyces bailii Cell Counts in Apple Juice during Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faleiro, M. The mode of antibacterial action of essential oils. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex Research Center: Norristown, PA, USA, 2011; Volume 2, pp. 1143–1156. [Google Scholar]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomised, double-blinded, placebo-controlled trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Gortzi, O.; Lalas, S.; Chinou, I.; Tsaknis, J. Reevaluation of antimicrobial and antioxidant activity of thymus spp. Extracts before and after encapsulation in liposomes. J. Food Prot. 2006, 69, 2998–3005. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Piper, P. Potential safety issues surrounding the use of benzoate preservatives. Beverages 2018, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Dehghan, P.; Mohammadi, A.; Mohammadzadeh-Aghdash, H.; Ezzati Nazhad Dolatabadi, J. Pharmacokinetic and toxicological aspects of potassium sorbate food additive and its constituents. Trends Food Sci. Technol. 2018, 80, 123–130. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N.K. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Siddique, S.; Perveen, Z.; Nawaz, S.; Shahzad, K.; Ali, Z. Chemical composition and antimicrobial activities of essential oils of six species from family Myrtaceae. J. Essent. Oil Bear. Plants 2015, 18, 950–956. [Google Scholar] [CrossRef]
- Nabet, N.; Boudries, H.; Loupassaki, S.; Souagui, S.; Madani, K.; Carbonell-Barrachina, Á.A. Chemical composition, antimicrobial and antioxidant activities of Thymus fontanesii Boiss. et Reut. and Origanum glandulosum Desf. essential oils. Int. Food Res. J. 2017, 24, 2518–2525. [Google Scholar]
- Almadiy, A.A.; Nenaah, G.E.; Al Assiuty, B.A.; Moussa, E.A.; Mira, N.M. Chemical composition and antibacterial activity of essential oils and major fractions of four Achillea species and their nanoemulsions against foodborne bacteria. LWT—Food Sci. Technol. 2016, 69, 529–537. [Google Scholar] [CrossRef]
- Loeffler, M.; Beiser, S.; Suriyarak, S.; Gibis, M.; Weiss, J. Antimicrobial efficacy of emulsified essential oil components against weak acid–adapted spoilage yeasts in clear and cloudy apple juice. J. Food Prot. 2014, 77, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Gottardi, D.; Malik, A.; Guerzoni, M.E. Chemical composition, in vitro anti-yeast activity and fruit juice preservation potential of lemon grass oil. LWT—Food Sci. Technol. 2014, 57, 731–737. [Google Scholar] [CrossRef]
- Azeredo, D.R.P.; Alvarenga, V.; Sant’Ana, A.S.; Sabaa Srur, A.U.O. An overview of microorganisms and factors contributing for the microbial stability of carbonated soft drinks. Food Res. Int. 2016, 82, 136–144. [Google Scholar] [CrossRef]
- Rawat, S. Food spoilage: Microorganisms and their prevention. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Fleet, G.H. Yeast spoilage of foods and beverages. In The Yeasts, 5th ed.; Boekhout, C.P.K.W.F., Ed.; Elsevier: London, UK, 2011; pp. 53–63. [Google Scholar]
- Roberts, T.; Cordier, J.-L.; Gram, L.; Tompkin, R.; Pitt, J.; Gorris, L.; Swanson, K. Soft drinks, fruit juices, concentrates, and fruit preserves. In Micro-Organisms in Foods 6; Springer: Boston, MA, USA, 2005; pp. 544–573. [Google Scholar]
- Piper, J.D.; Piper, P.W. Benzoate and sorbate salts: A systematic review of the potential hazards of these invaluable preservatives and the expanding spectrum of clinical uses for sodium benzoate. Compr. Rev. Food Sci. Food Saf. 2017, 16, 868–880. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.I.; Hocking, A.D. The ecology of fungal food spoilage. In Fungi and Food Spoilage; Springer: Boston, MA, USA, 2009; pp. 3–9. [Google Scholar]
- Karaman, K.; Sagdic, O.; Yilmaz, M.T. Multiple response surface optimization for effects of processing parameters on physicochemical and bioactive properties of apple juice inoculated with Zygosaccharomyces rouxii and Zygosaccharomyces bailii. LWT—Food Sci. Technol. 2016, 69, 258–272. [Google Scholar] [CrossRef]
- Piper, P.W. Resistance of yeasts to weak organic acid food preservatives. In Advances in Applied Microbiology; Laskin, A.I., Sariaslani, S., Gadd, G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 77, pp. 97–113. [Google Scholar]
- Stratford, M.; Steels, H.; Nebe-von-Caron, G.; Novodvorska, M.; Hayer, K.; Archer, D.B. Extreme resistance to weak-acid preservatives in the spoilage yeast Zygosaccharomyces bailii. Int. J. Food Microbiol. 2013, 166, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Stratford, M.; James, S.A. Non-alcoholic beverages and yeasts. In Yeasts in Food: Beneficial and Detrimental Aspects; Boekhout, T., Robert, V., Eds.; Woodhead Publishing: Sawston, UK, 2003; pp. 309–345. [Google Scholar]
- Dos Santos, S.; Paula, V.; Medeiros Salgado, A.; Guedes Torres, A.; Signori Pereira, K. Benzene as a chemical hazard in processed foods. Int. J. Food Sci. 2015, 2015, 545640. [Google Scholar] [CrossRef]
- Mollapour, M.; Shepherd, A.; Piper, P.W. Novel stress responses facilitate Saccharomyces cerevisiae growth in the presence of the monocarboxylate preservatives. Yeast 2008, 25, 169. [Google Scholar] [CrossRef]
- Piper, P.W. Yeast superoxide dismutase mutants reveal a pro-oxidant action of weak organic acid food preservatives. Free Radic. Biol. Med. 1999, 27, 1219–1227. [Google Scholar] [CrossRef]
- Ullah, A.; Orij, R.; Brul, S.; Smits, G.J. Quantitative analysis of the modes of growth inhibition by weak organic acids in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2012, 78, 8377–8387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piper, P.; Mahe, Y.; Thompson, S.; Pandjaitan, R.; Holyoak, C.; Egner, R.; Mühlbauer, M.; Coote, P.; Kuchler, K. The Pdr12 abc transporter is required for the development of weak organic acid resistance in yeast. EMBO J. 1998, 17, 4257–4265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuehlke, J.; Petrova, B.; Edwards, C. Advances in the control of wine spoilage by Ygosaccharomyces and Dekkera/Brettanomyces. Annu. Rev. Food Sci. Technol. 2013, 4, 57–78. [Google Scholar] [CrossRef]
- Kalathenos, P.; Sutherland, J.; Roberts, T. Resistance of some wine spoilage yeasts to combinations of ethanol and acids present in wine. J. Appl. Bacteriol. 1995, 78, 245–250. [Google Scholar] [CrossRef]
- Tapia de Daza, M.; Argaiz, A.; López-Malo, A.; Díaz, R. Microbial stability assessment in high and intermediate moisture foods: Special emphasis on fruit products. In Food Preservation By Moisture Control–Fundamental and Applications; Barbosa-Canovas, G.V., Welti-Chanes, J., Eds.; Techtonic: Lanchester, PA, USA, 1995; pp. 575–601. [Google Scholar]
- López-Malo, A.; Palou, E. Modeling the growth/no-growth interface of Zygosaccharomyces bailii in mango puree. J. Food Sci. 2000, 65, 516–520. [Google Scholar] [CrossRef]
- Stratford, M.; Capell, C. Soft drinks: Microbiology. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Finglas, P., Toldra, F., Eds.; Academic Press: Oxford, UK, 2003; pp. 5358–5366. [Google Scholar]
- Reyes-Jurado, F.; Franco-Vega, A.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Essential oils: Antimicrobial activities, extraction methods, and their modeling. Food Eng. Rev. 2015, 7, 275–297. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [Green Version]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Donsì, F.; Annunziata, M.; Vincensi, M.; Ferrari, G. Design of nanoemulsion-based delivery systems of natural antimicrobials: Effect of the emulsifier. J. Biotechnol. 2012, 159, 342–350. [Google Scholar] [CrossRef]
- Clarke, M. Australian Native Food Industry Stocktake; RIRDC Publication No. 12/066; Union Offset Printing: Canberra, Australia, 2012. [Google Scholar]
- Scheman, A.; Scheman, N.; Rakowski, E.-M. European directive fragrances in natural products. Dermatitis 2014, 25, 51–55. [Google Scholar] [CrossRef]
- Jones, G.L. Asia and Australia–essentially a well oiled connection. In Aromatic Plants from Asia, Their Chemistry and Application in Food and Therapy; Jirovetz, L., Dung, N.X., Varshney, V.K., Eds.; Har Krishnan Bhalla and Sons: Uttarakhand, India, 2007; p. 247. [Google Scholar]
- Ress, N.; Hailey, J.; Maronpot, R.; Bucher, J.; Travlos, G.; Haseman, J.; Orzech, D.; Johnson, J.; Hejtmancik, M. Toxicology and carcinogenesis studies of microencapsulated citral in rats and mice. Toxicol. Sci. 2003, 71, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Blewitt, M.; Southwell, I.A. Backhousia anisata Vickery, an alternative source of (e)-anethole. J. Essent. Oil Res. 2000, 12, 445–454. [Google Scholar] [CrossRef]
- Sultanbawa, Y. Tasmanian pepper leaf (Tasmannia lanceolata) oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 819–823. [Google Scholar]
- Smyth, H.E.; Sanderson, J.E.; Sultanbawa, Y. Lexicon for the sensory description of Australian native plant foods and ingredients. J. Sens. Stud. 2012, 27, 471–481. [Google Scholar] [CrossRef]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Sources of antioxidant activity in Australian native fruits. Identification and quantification of anthocyanins. J. Agric. Food Chem. 2006, 54, 9820–9826. [Google Scholar] [CrossRef]
- Pengelly, A. Indigenous and naturalised herbs: Tasmannia lanceolata: Mountain pepper. Aust. J. Med Herbal. 2002, 14, 71–74. [Google Scholar]
- Menary, R.C.; Dragar, V.A.; Thomas, S.; Read, C.D. Mountain Pepper Extract, Tasmannia Lanceolata: Quality Stabilisation and Registration: A Report for the Rural Industries Research and Development Corporation; RIRDC: Barton, Australia, 2003. [Google Scholar]
- Fujita, K.-I.; Kubo, I. Naturally occurring antifungal agents against Zygosaccharomyces bailii and their synergism. J. Agric. Food Chem. 2005, 53, 5187–5191. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.i.; Lee, S.H.; Ha, T.J. Antibacterial activity of polygodial. Phytother. Res. 2005, 19, 1013–1017. [Google Scholar] [CrossRef]
- Forbes-Smith, M.; Paton, J. Innovative products from Australian native foods. In Rural Industries Research and Development Corporation; RIRDC: Barton, Australia, 2002; pp. 1–78. [Google Scholar]
- Pengelly, A. Antimicrobial activity of lemon myrtle and tea tree oils. Aust. J. Med. Herbal. 2003, 15, 9. [Google Scholar]
- Southwell, I.A.; Russell, M.; Smith, R.L.; Archer, D.W. Backhousia citriodora f. Muell. (myrtaceae), a superior source of citral. J. Essent. Oil Res. 2000, 12, 735–741. [Google Scholar] [CrossRef]
- Southwell, I.; Russell, M.; Smith, R. Chemical composition of some novel aromatic oils from the Australian flora. In Proceedings of the International Conference on Medicinal and Aromatic Plants (Part II), Budabest, Hungary, 8–11 July 2001; pp. 79–89. [Google Scholar]
- Brophy, J.J.; Boland, D.J. The leaf essential oil of two chemotypes of Backhousia anisata Vickery. Flavour Fragr. J. 1991, 6, 187–188. [Google Scholar] [CrossRef]
- Souza, E.L.; Stamford, T.L.M.; Lima, E.O.; Trajano, V.N. Effectiveness of Origanum vulgare l. Essential oil to inhibit the growth of food spoiling yeasts. Food Control 2007, 18, 409–413. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E. Antibacterial activities of plant essential oils and their components against Escherichia coli o157: H7 and salmonella enterica in apple juice. J. Agric. Food Chem. 2004, 52, 6042–6048. [Google Scholar]
- McCallion, R.F.; Cole, A.; Walker, J.; Blunt, J.; Munro, M. Antibiotic substances from New Zealand plants. Planta Med. 1982, 44, 134–138. [Google Scholar] [CrossRef]
- Himejima, M.; Kubo, I. Fungicidal activity of polygodial in combination with anethole and indole against candida albicans. J. Agric. Food Chem. 1993, 41, 1776–1779. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Kubo, I. Multifunctional action of antifungal polygodial against Saccharomyces cerevisiae: Involvement of pyrrole formation on cell surface in antifungal action. Bioorganic Med. Chem. 2005, 13, 6742–6747. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Taniguchi, M. Polygodial, an antifungal potentiator. J. Nat. Prod. 1988, 51, 22–29. [Google Scholar] [CrossRef]
- Lim, T.K. Tasmannia lanceolata. In Edible Medicinal and Non-Medicinal Plants: Volume 6, Fruits; Springer: Dordrecht, The Netherlands, 2013; pp. 493–499. [Google Scholar]
- Yano, Y.; Taniguchi, M.; Tanaka, T.; Oi, S.; Kubo, I. Protective effects of Ca2+ on cell membrane damage by polygodial in Saccharomyces cerevisiae. Agric. Biol. Chem. 1991, 55, 603–604. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.i.; Lee, S.H. Antifungal mechanism of polygodial. J. Agric. Food Chem. 2001, 49, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Machida, K.; Tanaka, T.; Taniguchi, M. Depletion of glutathione as a cause of the promotive effects of polygodial, a sesquiterpene on the production of reactive oxygen species in Saccharomyces cerevisiae. J. Biosci. Bioeng. 1999, 88, 526–530. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.i. Naturally occurring anti-salmonella agents. J. Agric. Food Chem. 2001, 49, 5750–5754. [Google Scholar] [CrossRef]
- Marques, A.M.; Lima, C.H.; Alviano, D.S.; Esteves, R.L.; Kaplan, M.A.C. Traditional use, chemical composition and antimicrobial activity of Pectis brevipedunculata essential oil: A correlated lemongrass species in brazil. Emir. J. Food Agric. 2013, 25, 798–808. [Google Scholar] [CrossRef] [Green Version]
- Aoudou, Y.; Léopold, T.N.; Michel, J.D.P.; Franccedil, E.; Moses, M.C. Antifungal properties of essential oils and some constituents to reduce foodborne pathogen. J. Yeast Fungal Res. 2010, 1, 001–008. [Google Scholar]
- Alderees, F.; Mereddy, R.; Webber, D.; Nirmal, N.; Sultanbawa, Y. Mechanism of action against food spoilage yeasts and bioactivity of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum plant solvent extracts. Foods 2018, 7, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghaddam, M.; Alymanesh, M.R.; Mehdizadeh, L.; Mirzaei, H.; Ghasemi Pirbalouti, A. Chemical composition and antibacterial activity of essential oil of Ocimum ciliatum, as a new source of methyl chavicol, against ten phytopathogens. Ind. Crop. Prod. 2014, 59, 144–148. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Cavanagh, H. Antibacterial activity of essential oils from Australian native plants. Phytother. Res. 2005, 19, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.R.; Wilkinson, J.M.; Cavanagh, H.M. Evaluation of common antibacterial screening methods utilized in essential oil research. J. Essent. Oil Res. 2003, 15, 428–433. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Mereddy, R.; Li, L.; Sultanbawa, Y. Formulation, characterization and antibacterial activity of lemon myrtle and anise myrtle essential oil in water nanoemulsion. Food Chem. 2018, 254, 1–7. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alderees, F.; Akter, S.; Mereddy, R.; Sultanbawa, Y. Antimicrobial Activity of Nanoencapsulated Essential Oils of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum against Weak-Acid Resistant Zygosaccharomyces bailii in Clear Apple Juice. Beverages 2021, 7, 67. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7030067
Alderees F, Akter S, Mereddy R, Sultanbawa Y. Antimicrobial Activity of Nanoencapsulated Essential Oils of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum against Weak-Acid Resistant Zygosaccharomyces bailii in Clear Apple Juice. Beverages. 2021; 7(3):67. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7030067
Chicago/Turabian StyleAlderees, Fahad, Saleha Akter, Ram Mereddy, and Yasmina Sultanbawa. 2021. "Antimicrobial Activity of Nanoencapsulated Essential Oils of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum against Weak-Acid Resistant Zygosaccharomyces bailii in Clear Apple Juice" Beverages 7, no. 3: 67. https://0-doi-org.brum.beds.ac.uk/10.3390/beverages7030067