Claw Characteristics of Culled Sows from Three Farrow-to-Finish Greek Farms. Part 1: Claw Length Measurements, Lesion Scores and Their Association
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participating Farms
2.2. Slaughterhouse Sampling Protocol
2.3. Macroscopical Examination of Sow Claw Lesions
2.4. Statistical Analysis
3. Results
3.1. Claw Length Measurements
3.2. Macroscopic Examination
3.3. Associations between Claw Length and Lesion Scores
3.4. Associations between Length of Weight Bearing Claws and Corresponding Dewclaws
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torrison, J.; Cameron, R. Integumentary system, skin, hoof, and claw. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 292–312. [Google Scholar]
- Johnson, A.; Garcia, A.; Karriker, L.A.; Stalder, K.J. Sow Lateral Toe Growth and Lesion Presence on Hooves When Housed in Gestation Stalls. J. Anim. Sci. Livest. Prod. 2020, 4, 7. [Google Scholar]
- Tubbs, R.C. Lameness in sows: Solving a preventable problem. Vet. Med. 1988, 83, 610–616. [Google Scholar]
- de Sevilla, X.F.; Fabrega, E.; Tibau, J.; Casellas, J. Effect of leg conformation on survivability of Duroc, Landrace and Large White sows. J. Anim. Sci. 2008, 86, 2392–2400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Sevilla, X.F.; Fàbrega, E.; Tibau, J.; Casellas, J. Competing risk analyses of longevity in Duroc sows with a special emphasis on leg conformation. Animal 2009, 3, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, R.F.; Stalder, K.J.; Karriker, L.A.; Sadler, L.J.; Hill, H.T.; Kaisand, J.; Johnson, A.K. The effect of hoof abnormalities on sow behavior and performance. Livest. Sci. 2012, 145, 230–238. [Google Scholar] [CrossRef]
- Pluym, L.M.; Van Nuffel, A.; Van Weyenberg, S.; Maes, D. Prevalence of lameness and claw lesions during different stages in the reproductive cycle of sows and the impact on reproduction results. Animal 2013, 7, 1174–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisgara, M.; Skampardonis, V.; Angelidou, E.; Kouroupides, S.; Leontides, L. Associations between claw lesions and reproductive performance of sows in three Greek herds. Vet. Czech. Med. 2015, 60, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Lisgara, M.; Skampardonis, V.; Kouroupides, S.; Leontides, L. Hoof lesions and lameness in sows of three Greek swine herds. J. Swine Health Prod. 2015, 23, 244–251. [Google Scholar]
- Lisgara, M.; Skampardonis, V.; Leontides, L. Effect of diet supplementation with chelated zinc, copper and manganese on hoof lesions of loose housed sows. Porc. Health Manag. 2016, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Skampardonis, V.; Lisgara, M.; Papatsiros, V.; Leontides, L. Effect of sow diets supplementation with chelated trace minerals on their reproductive performance. J. Hell. Vet. Med. Soc. 2016, 67, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Varagka, N.; Lisgara, M.; Skampardonis, V.; Psychas, V.; Leontides, L. Partial substitution, with their chelated complexes, of the inorganic zinc, copper and manganese in sow diets reduced the laminitic lesion in the claws and improved the morphometric characteristics of the hoof horn of sows from three Greek herds. Porc. Health Manag. 2016, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Varagka, N.; Lisgara, M.; Skampardonis, V.; Psychas, V.; Leontides, L. Pathological evaluation of claw lesions in culled sows from a Greek herd. J. Swine Health Prod. 2016, 24, 72–80. [Google Scholar]
- Hartnett, P.; Boyle, L.A.; O’Driscoll, K. The effect of group composition and mineral supplementation during rearing on the behavior and welfare of replacement gilts. Transl. Anim. Sci. 2020, 4, 1038–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Amstel, S.; Doherty, T. Claw horn growth and wear rates, toe length, and claw size in commercial pigs: A pilot study. J. Swine Health Prod. 2010, 18, 239–243. [Google Scholar]
- Grégoire, J.; Bergeron, R.; D’Allaire, S.; Meunier-Salaün, M.C.; Devillers, N. Assessment of lameness in sows using gait, footprints, postural behaviour and foot lesion analysis. Animal 2013, 7, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Calderón Díaz, J.A.; Stienezena, I.M.J.; Leonard, F.C.; Boyle, L.A. The effect of overgrown claws on behaviour and claw abnormalities of sows in farrowing crates. Appl. Anim. Behav. Sci. 2015, 166, 44–51. [Google Scholar] [CrossRef]
- Newman, S.J.; Rohrbach, B.W.; Wilson, M.E.; Torrison, J.; Van Amstel, S. Characterization of histopathologic lesions among pigs with overgrown claws. J. Swine Health Prod. 2015, 23, 91–96. [Google Scholar]
- Sasaki, Y.; Ushijima, R.; Sueyoshi, M. Field study of hind limb claw lesions and claw measures in sows. Anim. Sci. J. 2015, 86, 351–357. [Google Scholar] [CrossRef]
- van Riet, M.M.J.; Janssens, G.P.J.; Cornillie, P.; Van Den Broeck, W.; Nalon, E.; Ampe, B.; Tuyttens, F.A.M.; Maes, D.; Du Laing, G.; Millet, S. Marginal dietary zinc concentration affects claw conformation measurements but not histological claw characteristics in weaned pigs. Vet. J. 2016, 209, 98–107. [Google Scholar] [CrossRef]
- van Riet, M.M.J.; Bos, E.J.; Ampe, B.; Bikker, P.; Vanhauteghem, D.; Van Bockstaele, F.; Cornillie, P.; Van Den Broeck, W.; Du Laing, G.; Maes, D.; et al. Long-term impact of zinc supplementation in sows: Impact on claw quality. J. Swine Health Prod. 2018, 26, 10–24. [Google Scholar]
- Jørgensen, B.; Andersen, T. Genetics of Leg Weakness in Boars at the Danish Pig Breeding Stations. Acta Agric. Scand. 1990, 40, 59–69. [Google Scholar] [CrossRef]
- Quintanilla, R.L.; Varona, L.; Noguera, J.L. Testing genetic determinism in rate of hoof growth in pigs using Bayes Factors. Livest. Sci. 2006, 105, 50–56. [Google Scholar] [CrossRef]
- Fernàndez de Sevilla, X.; Fàbrega, E.; Tibau, J.; Casellas, J. Genetic background and phenotypic characterization over two farrowings of leg conformation defects in Landrace and Large White sows. J. Anim. Sci. 2009, 87, 1606–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, B.; Onteru, S.K.; Nikkilä, M.T.; Stalder, K.J.; Rothschild, M.F. Identification of genetic markers associated with fatness and leg weakness traits in the pig. Anim. Genet. 2009, 40, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Dohoo, I.R.; Ducrot, C.; Fourichon, C.; Donald, A.; Hurnik, D. An overview of techniques for dealing with large numbers of independent variables in epidemiologic studies. Prev. Vet. Med. 1997, 29, 221–239. [Google Scholar] [CrossRef]
- Berghaus, R.D.; Lombard, J.E.; Gardner, I.A.; Farver, T.B. Factor analysis of a Johne’s disease risk assessment questionnaire with evaluation of factor scores and a subset of original questions as predictors of observed clinical paratuberculosis. Prev. Vet. Med. 2005, 72, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Costello, A.B.; & Osborne, J.W. Best Practices in Exploratory Factor Analysis: Four Recommendations for Getting the Most from Your Analysis. Pract. Assess. Res. Evaluation. 2005, 10, 1–9. [Google Scholar]
- Stevens, J. Confirmatory and exploratory factor analysis. In Multivariate Statistics for the Social Sciences, 3rd ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1996; p. 366. [Google Scholar]
- Balogh, E.P.; Miller, B.T.; Ball, J.R. Improving Diagnosis in Health Care; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Wang, C.; Wu, Y.; Shu, D.; Wei, H.; Zhou, Y.; Peng, J. An Analysis of Culling Patterns during the Breeding Cycle and Lifetime Production from the Aspect of Culling Reasons for Gilts and Sows in Southwest China. Animals 2019, 9, 160. [Google Scholar] [CrossRef] [Green Version]
- Tinkle, A.K.; Duberstein, K.J.; Wilson, M.E.; Parsley, M.A.; Beckman, M.K.; Torrison, J.; Azain, M.J.; Dove, C.R. Functional claw trimming improves the gait and locomotion of sows. Livest. Sci. 2017, 195, 53–57. [Google Scholar] [CrossRef]
- van Riet, M.M.J.; Janssens, G.P.J.; Ampe, B.; Nalon, E.; Bos, E.J.; Pluym, L.; Vangeyte, J.; Tuyttens, F.A.M.; Maes, D.; Millet, S. Factors influencing claw lesion scoring in sows. Prev. Vet. Med. 2020, 175, 104859. [Google Scholar] [CrossRef] [Green Version]
- Calderón Díaz, J.C.; Fahey, A.G.; KilBride, A.L.; Green, L.E.; Boyle, L.A. Longitudinal study of the effect of rubber slat mats on locomotory ability, body, limb and claw lesions, and dirtiness of group housed sows. J. Anim. Sci. 2013, 91, 3940–3954. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Li, J.L.; Wei, H.K.; Zhou, Y.F.; Tan, J.J.; Sun, H.Q.; Jiang, S.W.; Peng, J. Analysis of influencing factors of boar claw lesion and lameness. Anim. Sci. J. 2018, 89, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.J.; Muelling, C.M.; Fakler, T.M. Formation of keratins in the bovine claw: Roles of hormones, minerals, and vitamins in functional claw integrity. J. Dairy Sci. 2004, 87, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Van Riet, M.M.J.; Millet, S.; Aluwé, M.; Janssens, G.P.J. Impact of nutrition on lameness and claw health in sows. Livest. Sci. 2013, 156, 24–35. [Google Scholar] [CrossRef]
- Muelling, C.K.W. Nutritional influences on horn quality and hoof health. WCDS Adv. Diary Technol. 2009, 21, 283–291. [Google Scholar]
- Pollitt, C.C. Anatomy and physiology of the inner hoof wall. Clin. Tech. Equine Pract. 2004, 3, 3–21. [Google Scholar] [CrossRef]
- Karriker, L.A.; Abell, C.E.; Pairis-Garcia, M.D.; Holt, W.A.; Sun, G.; Coetzee, J.F.; Johnson, A.K.; Hoff, S.J.; Stalder, K.J. Validation of a lameness model in sows using physiological and mechanical measurements. J. Anim. Sci. 2013, 91, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Anil, S.S.; Anil, L.; Deen, J.; Baidoo, S.K.; Walker, R.D. Factors associated with claw lesions in gestating sows. J. Swine Health Prod. 2007, 15, 78–83. [Google Scholar]
- Pluym, L.; Van Nuffel, A.; Dewulf, J.; Cools, A.; Vangroenweghe, F.; Van Hoorebeke, S.; Maes, D. Prevalence and risk factors of claw lesions and lameness in pregnant sows in two types of group housing. Vet. Med. (Praha) 2011, 56, 101–109. [Google Scholar] [CrossRef]
- Carvalho, V.; Naas, I.; Bucklin, R.; Shearer, J.; Shearer, K.; Massafera, V.; Souza, S. Effects of trimming on dairy cattle hoof weight bearing surfaces and pressure distributions. Braz. J. Vet. Res. Anim. Sci. 2006, 43, 518–525. [Google Scholar] [CrossRef] [Green Version]
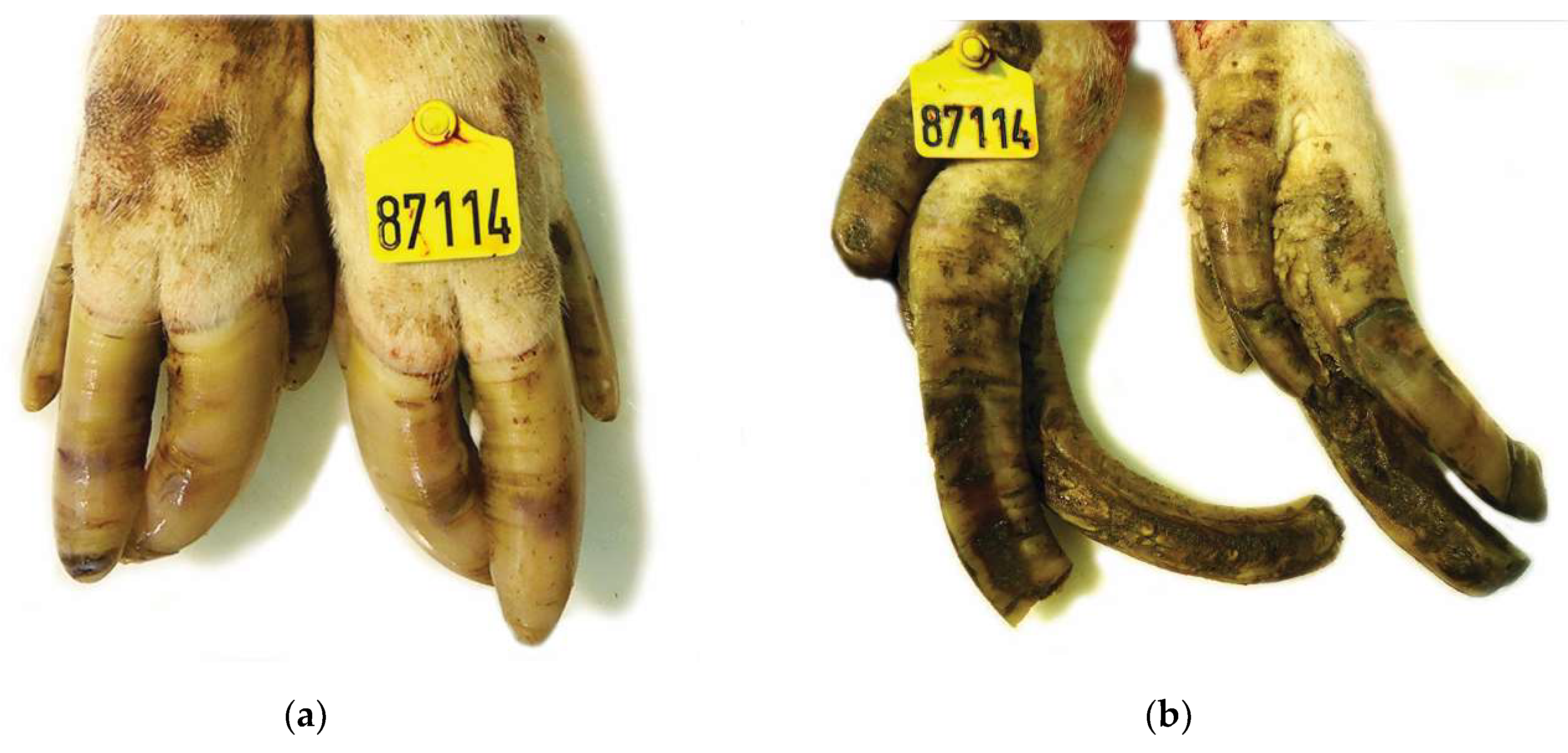


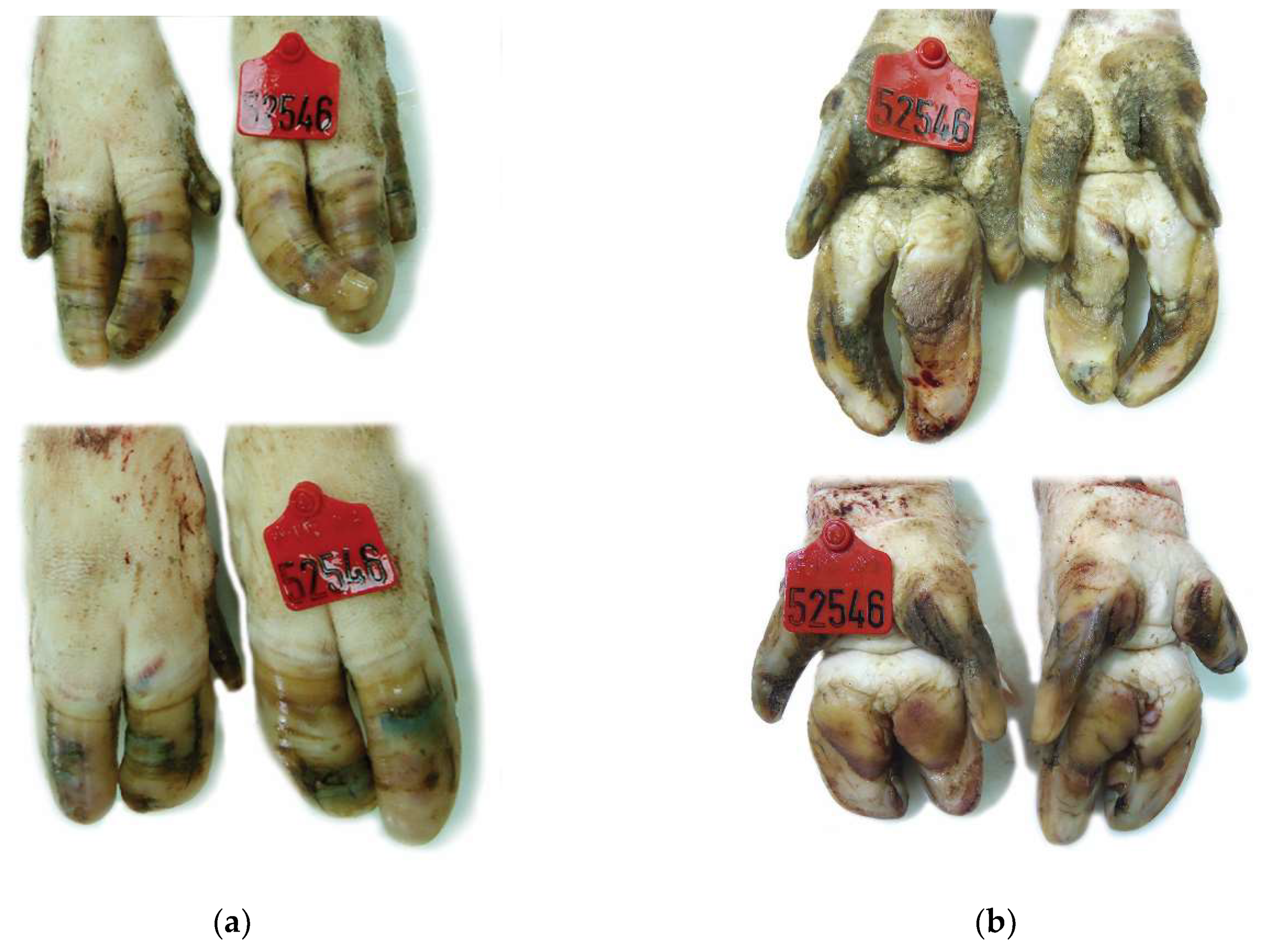
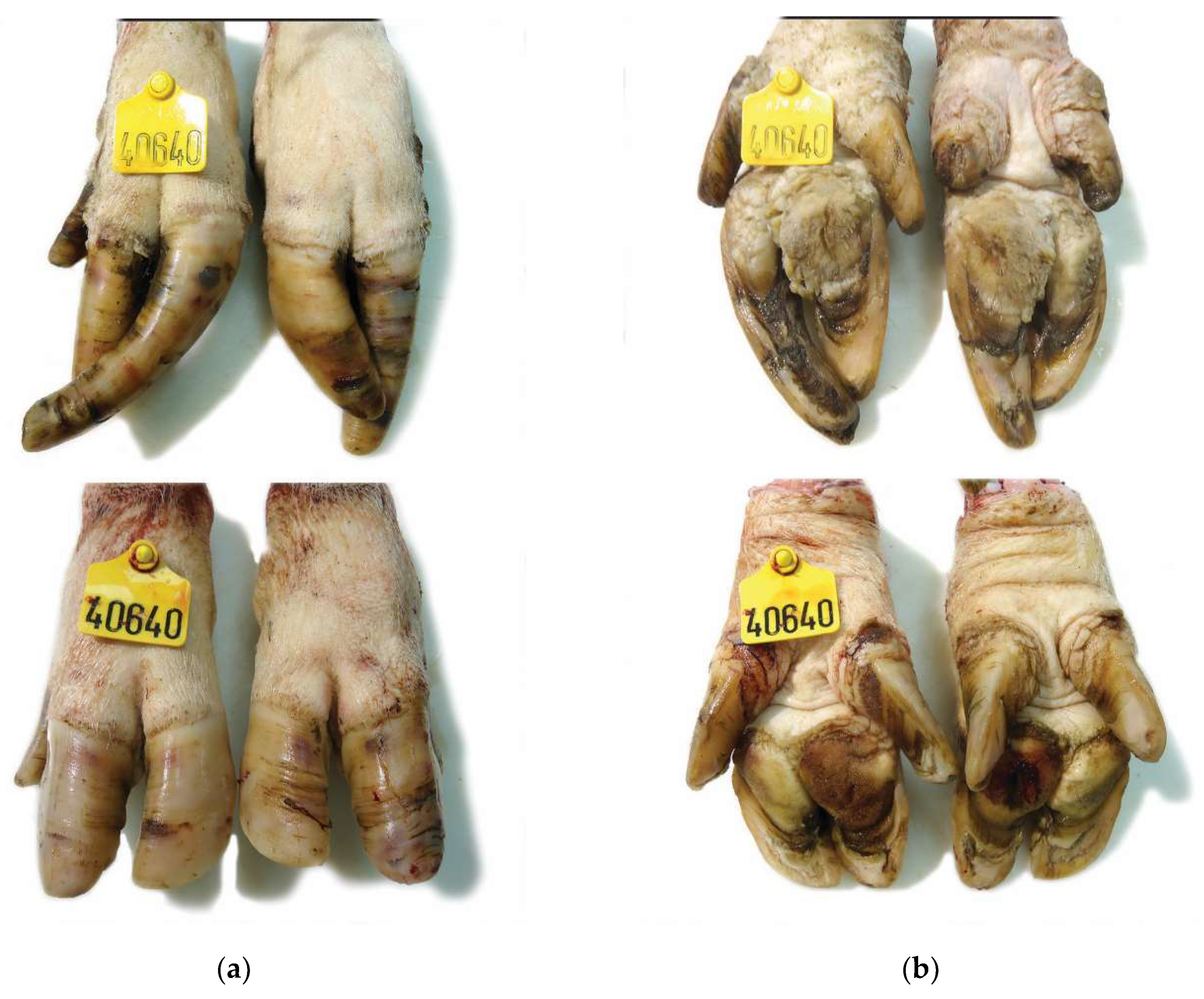
| Anatomical Site | Score 0 | Score 1 | Score 2 |
|---|---|---|---|
| Sole | No lesions or very small superficial cracks in the epidermis | Serious lesions in the epidermis not extending into the corium, heel–sole separation, or both | One or more deep cracks extending into the corium, severe heel–sole separation, or both |
| Heel | No lesions or very small superficial cracks in the epidermis | Hyperkeratinization and erosions in the epidermis not extending into the corium | Hyperkeratinization, deep cracks extending into the corium and often necrosis |
| White line | No lesions or very small superficial cracks in the epidermis | Wall-sole separation not extending into the corium | Wall-sole separation extending into the corium |
| Wall | No lesions or very small superficial cracks in the epidermis | Cracks not extending into the corium, often accompanied by bruising | Cracks extending into the corium, separation of the keratin, or both |
| Coronary band | No lesions or very small superficial cracks in the epidermis | Edema with purulent exudate, hemorrhage and necrosis, or both | NA |
| Genetic Lines-Farms | ||||
|---|---|---|---|---|
| A | B | C | p-Value | |
| Front right foot | ||||
| Medial claw lengths, cm | ||||
| Dorsal | 6.16 ± 1.34 a | 5.87 ± 1.10 a | 4.61 ± 0.47 b | <0.001 |
| Diagonal | 8.00 ± 1.48 a | 7.56 ± 1.06 a | 6.43 ± 0.57 b | <0.001 |
| Heel-Sole | 9.07 ± 1.44 a | 8.55 ± 1.14 a | 7.48 ± 0.60 b | <0.001 |
| Dewclaw | 4.63 ± 1.29 a | 3.85 ± 0.86 b | 3.19 ± 0.39 c | <0.001 |
| Lateral claw lengths, cm | ||||
| Dorsal | 6.19 ± 1.40 a | 5.71 ± 1.15 b | 4.73 ± 0.55 c | <0.001 |
| Diagonal | 7.98 ± 1.51 a | 7.34 ± 1.19 b | 6.39 ± 0.53 c | <0.001 |
| Heel-Sole | 9.32 ± 1.61 a | 8.55 ± 1.26 b | 7.66 ± 0.60 c | <0.001 |
| Dewclaw | 4.71 ± 1.01 a | 4.29 ±1.16 b | 3.53 ± 0.48 c | <0.001 |
| Dorsal length difference between lateral and medial hooves, cm | 0.51 ± 0.53 a | 0.46 ± 0.54 a | 0.26 ± 0.19 b | 0.009 |
| Front left foot | ||||
| Medial claw lengths, cm | ||||
| Dorsal | 6.04 ± 1.23 a | 5.94 ± 1.64 a | 4.51 ± 0.48 b | <0.001 |
| Diagonal | 7.91 ± 1.33 a | 7.69 ± 1.53 a | 6.51 ± 0.53 b | <0.001 |
| Heel-Sole | 8.68 ± 1.28 a | 8.55 ± 1.66 a | 7.27 ± 0.59 b | <0.001 |
| Dewclaw | 4.38 ± 1.10 a | 3.84 ± 1.10 b | 3.14 ±0.45 c | <0.001 |
| Lateral claw lengths, cm | ||||
| Dorsal | 6.24 ± 1.25 a | 5.91 ± 1.35 a | 4.78 ±0.56 b | <0.001 |
| Diagonal | 7.81 ± 1.25 a | 7.39 ± 1.46 b | 6.30 ± 0.48 c | <0.001 |
| Heel-Sole | 9.40 ± 1.42 a | 8.76 ± 1.46 b | 7.75 ± 0.67 c | <0.001 |
| Dewclaw | 5.10 ± 1.11 a | 4.19 ± 0.97 b | 3.58 ± 0.56 c | <0.001 |
| Dorsal length difference between lateral and medial hooves, cm | 0.51 ± 0.52 | 0.52 ± 0.83 | 0.31 ± 0.22 | 0.133 |
| Genetic Lines-Farms | ||||
|---|---|---|---|---|
| A | B | C | p-Value | |
| Rear right foot | ||||
| Medial claw lengths, cm | ||||
| Dorsal | 7.22 ± 2.21 a | 6.04 ± 1.32 b | 4.84 ± 0.56 c | <0.001 |
| Diagonal | 8.95 ± 2.49 a | 7.33 ± 1.23 b | 6.22 ± 0.53 c | <0.001 |
| Heel-Sole | 10.35 ± 2.83 a | 8.47 ± 1.31 b | 7.24 ± 0.65 c | <0.001 |
| Dewclaw | 5.95 ± 1.62 a | 4.69 ± 1.05 b | 3.89 ± 0.68 c | <0.001 |
| Lateral claw lengths, cm | ||||
| Dorsal | 8.17 ± 2.59 a | 6.40 ± 1.49 b | 5.35 ± 0.71 c | <0.001 |
| Diagonal | 10.03 ± 2.87 a | 7.88 ± 1.52 b | 6.76 ± 0.69 c | <0.001 |
| Heel-Sole | 11.46 ± 2.93 a | 9.15 ± 1.53 b | 7.95 ± 0.90 c | <0.001 |
| Dewclaw | 5.38 ± 1.37 a | 4.86 ± 1.08 a | 3.76 ± 0.55 b | <0.001 |
| Dorsal length difference between lateral and medial hooves, cm | 2.02 ± 2.21 a | 0.75 ± 0.76 b | 0.53 ± 0.44 b | <0.001 |
| Rear left foot | ||||
| Medial claw lengths, cm | ||||
| Dorsal | 7.33 ± 1.84 a | 5.89 ± 1.10 b | 4.83 ± 0.57 c | <0.001 |
| Diagonal | 8.98 ± 2.06 a | 7.28 ± 1.11 b | 6.38 ± 0.53 c | <0.001 |
| Heel-Sole | 10.09 ± 2.08 a | 8.19 ± 1.23 b | 7.06 ± 0.64 c | <0.001 |
| Dewclaw | 5.28 ± 1.46 a | 4.56 ± 0.98 b | 4.00 ± 0.80 c | <0.001 |
| Lateral claw lengths, cm | ||||
| Dorsal | 7.66 ± 2.00 a | 6.18 ± 1.17 b | 5.36 ± 0.92 c | <0.001 |
| Diagonal | 9.23 ± 2.40 a | 7.62 ± 1.17 b | 6.66 ± 0.99 c | <0.001 |
| Heel-Sole | 10.84 ± 2.43 a | 8.91 ± 1.16 b | 8.09 ± 1.20 c | <0.001 |
| Dewclaw | 5.48 ± 1.78 a | 4.93 ± 1.17 a | 3.96 ± 0.83 b | <0.001 |
| Dorsal length difference between lateral and medial hooves, cm | 1.48 ± 1.75 a | 0.64 ± 0.70 b | 0.58 ± 0.56 b | <0.001 |
| Sole | Heel | White Line | Wall | Coronary Band | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lesion Score | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | ||
| Right | Medial | A | 24.6 | 47.4 | 28.1 | 70.2 | 24.6 | 5.3 | 17.5 | 68.4 | 14.0 | 5.3 | 70.2 | 24.6 | 45.6 | 54.4 |
| B | 31.3 | 51.6 | 17.2 | 54.7 | 40.6 | 4.7 | 48.4 | 43.8 | 7.8 | 65.6 | 34.4 | 0.0 | 84.4 | 15.6 | ||
| C | 85.9 | 14.1 | 0.0 | 79.7 | 20.3 | 0.0 | 76.6 | 23.4 | 0.0 | 40.6 | 59.4 | 0.0 | 67.2 | 32.8 | ||
| Lateral | A | 14.0 | 57.9 | 28.1 | 57.9 | 36.8 | 5.3 | 19.3 | 66.7 | 14.0 | 3.5 | 63.2 | 33.3 | 43.9 | 56.1 | |
| B | 37.5 | 46.9 | 15.6 | 62.5 | 34.4 | 3.1 | 50,0 | 42.2 | 7.8 | 64.1 | 32.8 | 3.1 | 87.5 | 12.5 | ||
| C | 78.1 | 20.3 | 1.6 | 64.1 | 35.9 | 0.0 | 68.8 | 31.3 | 0.0 | 39.1 | 57.8 | 3.1 | 75.0 | 25.0 | ||
| Left | Medial | A | 12.3 | 63.2 | 24.6 | 59.6 | 38.6 | 1.8 | 29.8 | 59.6 | 10.5 | 3.5 | 73.7 | 22.8 | 52.6 | 47.4 |
| B | 35.9 | 43.8 | 20.3 | 54.7 | 40.6 | 4.7 | 39.1 | 48.4 | 12.5 | 64.1 | 34.4 | 1.6 | 71.9 | 26.6 | ||
| C | 81.3 | 18.8 | 0.0 | 81.3 | 18.8 | 0.0 | 73.4 | 25.0 | 1.6 | 37.5 | 62.5 | 0.0 | 53.1 | 46.9 | ||
| Lateral | A | 19.3 | 54.4 | 26.3 | 47.4 | 43.9 | 8.8 | 17.5 | 71.9 | 10.5 | 5.3 | 66.7 | 28.1 | 45.6 | 54.4 | |
| B | 34.4 | 48.4 | 17.2 | 60.9 | 35.9 | 3.1 | 34.4 | 53.1 | 12.5 | 68.8 | 31.3 | 0.0 | 84.4 | 15.6 | ||
| C | 79.7 | 18.8 | 1.6 | 65.6 | 34.4 | 0.0 | 71.9 | 23.4 | 4.7 | 40.6 | 59.4 | 0.0 | 60.9 | 39.1 | ||
| Sole | Heel | White Line | Wall | Coronary Band | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lesion Score | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | ||
| Right | Medial | A | 71.9 | 23.4 | 4.7 | 82.8 | 15.6 | 1.6 | 56.3 | 40.6 | 3.1 | 35.9 | 57.8 | 6.3 | 62.5 | 37.5 |
| B | 45.3 | 45.3 | 9.4 | 82.8 | 15.6 | 1.6 | 45.3 | 51.6 | 3.1 | 60.9 | 34.4 | 4.7 | 75.0 | 25.0 | ||
| C | 10.7 | 44.6 | 44.6 | 58.9 | 32.1 | 8.9 | 14.3 | 51.8 | 33.9 | 8.9 | 30.4 | 60.7 | 26.8 | 73.2 | ||
| Lateral | A | 39.1 | 51.6 | 9.4 | 42.2 | 48.4 | 9.4 | 31.3 | 64.1 | 4.7 | 28.1 | 54.7 | 17.2 | 54.7 | 45.3 | |
| B | 25.0 | 56.3 | 18.8 | 32.8 | 59.4 | 7.8 | 28.1 | 60.9 | 10.9 | 40.6 | 56.3 | 3.1 | 62.5 | 37.5 | ||
| C | 3.5 | 19.3 | 77.2 | 17.5 | 50.9 | 31.6 | 1.8 | 42.1 | 56.1 | 0.0 | 31.6 | 68.4 | 15.8 | 84.2 | ||
| Left | Medial | A | 79.7 | 17.2 | 3.1 | 84.4 | 12.5 | 3.1 | 56.3 | 40.6 | 3.1 | 23.4 | 73.4 | 3.1 | 65.6 | 34.4 |
| B | 46.9 | 43.8 | 9.4 | 68.8 | 28.1 | 3.1 | 56.3 | 40.6 | 3.1 | 46.9 | 48.4 | 4.7 | 53.1 | 46.9 | ||
| C | 14.0 | 54.4 | 31.6 | 71.9 | 19.3 | 8.8 | 14.0 | 63.2 | 22.8 | 12.3 | 56.1 | 31.6 | 45.6 | 54.4 | ||
| Lateral | A | 48.4 | 39.1 | 12.5 | 39.1 | 53.1 | 7.8 | 40.6 | 50.0 | 9.4 | 25.0 | 65.6 | 9.4 | 60.9 | 39.1 | |
| B | 20.3 | 60.9 | 18.8 | 28.1 | 65.6 | 6.3 | 29.7 | 62.5 | 7.8 | 43.8 | 46.9 | 9.4 | 59.4 | 40.6 | ||
| C | 3.6 | 29.1 | 67.3 | 16.4 | 58.2 | 25.5 | 1.8 | 52.7 | 45.5 | 1.8 | 51.8 | 46.4 | 35.7 | 64.3 | ||
| Factor Score | Claw Lesion Scores | |
|---|---|---|
| Factor analysis of front feet claw lesions | Factor score 1 | For sole and white line lesions on the front right and on the front left foot |
| Factor score 2 | For wall lesions on front right and the front left foot | |
| Factor score 3 | For coronary band lesions on front right and the front left foot | |
| Factor score 4 | For heel lesions on front right and the front left foot | |
| Factor analysis of rear feet claw lesions | Factor score 1 | For sole and white line lesions on the rear right and on the rear left foot |
| Factor score 2 | For wall lesions on rear right and the rear left foot and coronary band lesions on the rear right foot | |
| Factor score 3 | For heel lesions on rear left medial and on rear right medial claws | |
| Factor score 4 | For coronary band lesions on rear left medial and on rear left lateral claws | |
| Factor score 5 | For heel and white line lesions on the rear left lateral claw |
| SLF | SLR | |||||
|---|---|---|---|---|---|---|
| Coef. | p-Value | 95% C.I. | Coef. | p-Value | 95% C.I. | |
| constant | 87.00 | <0.001 | 82.17; 91.83 | 98.60 | <0.001 | 89.53; 107.68 |
| herd | −3.73 | 0.001 | −5.84; −1.62 | −6.43 | 0.002 | −10.37; −2.50 |
| parity | 1.18 | <0.001 | 0.62; 1.75 | 1.40 | 0.001 | 0.56; 2.24 |
| Factor score 1 f | 8.30 | <0.001 | 6.54; 10.05 | NA | ||
| Factor score 2 f | - | - | - | NA | ||
| Factor score 3 f | - | - | - | NA | ||
| Factor score 4 f | 2.70 | 0.002 | 82.17; 91.83 | NA | ||
| Factor score 1 r | NA | 9.64 | <0.001 | 6.46; 12.82 | ||
| Factor score 2 r | NA | 5.31 | <0.001 | 2.65; 7.97 | ||
| Factor score 3 r | NA | - | - | - | ||
| Factor score 4 r | NA | 2.66 | 0.049 | 0.01; 5.31 | ||
| Factor score 5 r | NA | 4.88 | 0.001 | 2.15; 7.60 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, G.A.; Chalvatzi, S.; Kroustallas, F.; Skampardonis, V.; Cernat, M.; Marouda, C.; Psychas, V.; Poutahidis, T.; Leontides, L.; Fortomaris, P. Claw Characteristics of Culled Sows from Three Farrow-to-Finish Greek Farms. Part 1: Claw Length Measurements, Lesion Scores and Their Association. Vet. Sci. 2021, 8, 126. https://0-doi-org.brum.beds.ac.uk/10.3390/vetsci8070126
Papadopoulos GA, Chalvatzi S, Kroustallas F, Skampardonis V, Cernat M, Marouda C, Psychas V, Poutahidis T, Leontides L, Fortomaris P. Claw Characteristics of Culled Sows from Three Farrow-to-Finish Greek Farms. Part 1: Claw Length Measurements, Lesion Scores and Their Association. Veterinary Sciences. 2021; 8(7):126. https://0-doi-org.brum.beds.ac.uk/10.3390/vetsci8070126
Chicago/Turabian StylePapadopoulos, Georgios A., Sofia Chalvatzi, Fotios Kroustallas, Vassilis Skampardonis, Mihaela Cernat, Christina Marouda, Vassilios Psychas, Theofilos Poutahidis, Leonidas Leontides, and Paschalis Fortomaris. 2021. "Claw Characteristics of Culled Sows from Three Farrow-to-Finish Greek Farms. Part 1: Claw Length Measurements, Lesion Scores and Their Association" Veterinary Sciences 8, no. 7: 126. https://0-doi-org.brum.beds.ac.uk/10.3390/vetsci8070126







