Transcatheter Closure of PFO and ASD: Multimodality Imaging for Patient Selection and Perioperative Guidance
Abstract
:1. Introduction
2. Focused Anatomy of Atrial Septum and ASD
3. PFO
3.1. Indication: Patient Screening and Standardized Approach to PFO-Related Event
3.2. Perioperative Monitoring
4. ASD
4.1. Indication
4.2. Perioperative Monitoring
5. Training and Quality Measures
6. Final Consideration
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AO | AOrta |
| AoV | Aortic Valve |
| ASD | Atrial Septal Defect |
| AV | Artero-Venous |
| AVV | Atrio-Ventricular Valves |
| CMR | Cardiac Magnetic Resonance |
| DWI | Diffusion-Weighted magnetic resonance Imaging |
| CS | Coronary Sinus |
| EDV | End-Diastolic Volume |
| ESUS | Embolic Stroke of Unknown Source |
| ESV | End-systolic Volume |
| FO | Fossa Ovalis |
| HITS | High Intensity Transient Signals |
| ICE | Intracardiac Echocardiography |
| IVC | Inferior Vena Cava |
| LA | Left Atrium |
| PFO | Patent Foramen Ovale |
| RA | Right Atrium |
| PV | Pulmonary Valve |
| RV | Right Ventricle |
| SVC | Superior Vena Cava |
| TCD | Trans-Cranial Doppler |
| TEE | Trans-Esophageal Echocardiography |
| TTE | Trans-Thoracic Echocardiography |
| TV | Tricuspid Valve |
References
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e81–e192. [Google Scholar] [CrossRef] [PubMed]
- Pristipino, C.; Sievert, H.; D’Ascenzo, F.; Mas, J.L.; Meier, B.; Scacciatella, P.; Hildick-Smith, D.; Gaita, F.; Toni, D.; Kyrle, P.; et al. European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. Eur. Heart J. 2019, 40, 3182–3195. [Google Scholar] [CrossRef]
- Thaler, D.E.; Ruthazer, R.; Di Angelantonio, E.; Di Tullio, M.R.; Donovan, J.S.; Elkind, M.S.; Griffith, J.; Homma, S.; Jaigobin, C.; Mas, J.-L.; et al. Neuroimaging Findings in Cryptogenic Stroke Patients With and Without Patent Foramen Ovale. Stroke 2013, 44, 675–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donti, A.; Assenza, G.E.; Mariucci, E. Transcatheter closure of patent foramen ovale in patients with cryptogenic stroke. G. Ital. Cardiol. 2019, 20, 73–84. [Google Scholar]
- Silvestry, F.E.; Cohen, M.S.; Armsby, L.B.; Burkule, N.J.; Fleishman, C.E.; Hijazi, Z.M.; Lang, R.M.; Rome, J.J.; Wang, Y. Guidelines for the Echocardiographic Assessment of Atrial Septal Defect and Patent Foramen Ovale: From the American Society of Echocardiography and Society for Cardiac Angiography and Interventions. J. Am. Soc. Echocardiogr. 2015, 28, 910–958. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K.; Abdelgadir, I.; Boudjemline, Y.; Hijazi, Z.M. Patent foramen ovale (PFO) closure versus medical therapy for prevention of recurrent stroke in patients with prior cryptogenic stroke: A systematic review and meta-analysis of randomized controlled trials. Catheter. Cardiovasc. Interv. 2018, 92, 165–173. [Google Scholar] [CrossRef]
- Allen, L.M.; Hasso, A.N.; Handwerker, J.; Farid, H. Sequence-specific MR Imaging Findings That Are Useful in Dating Ischemic Stroke. Radiographics 2012, 32, 1285–1297. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.A.; Vangala, H.; Tong, D.C.; Campbell, D.M.; Balgude, A.; Eyngorn, I.; Beraud, A.S.; Olivot, J.M.; Hsia, A.W.; Bernstein, R.A.; et al. MRI guides diagnostic approach for ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1201–1205. [Google Scholar] [CrossRef]
- Chung, J.; Park, S.H.; Kim, N.; Kim, W.; Park, J.H.; Ko, Y.; Yang, M.H.; Jang, M.S.; Han, M.; Jung, C.; et al. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Classification and Vascular Territory of Ischemic Stroke Lesions Diagnosed by Diffusion-Weighted Imaging. J. Am. Heart Assoc. 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Kamel, H.; Healey, J.S. Cardioembolic Stroke. Circ. Res. 2017, 120, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Jickling, G.C.; Stamova, B.; Ander, B.P.; Zhan, X.; Tian, Y.; Liu, D.; Xu, H.; Johnston, S.C.; Verro, P.; Sharp, F.R. Profiles of lacunar and nonlacunar stroke. Ann. Neurol. 2011, 70, 477–485. [Google Scholar] [CrossRef]
- Shi, Y.; Wardlaw, J.M. Update on cerebral small vessel disease: A dynamic whole-brain disease. Stroke Vasc. Neurol. 2016, 1, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Sohn, H.; Sun, B.J.; Song, J.-K.; Kang, D.-W.; Kim, J.S.; Kwon, S.U. Imaging Characteristics of Ischemic Strokes Related to Patent Foramen Ovale. Stroke 2013, 44, 3350–3356. [Google Scholar] [CrossRef] [Green Version]
- Katsanos, A.H.; Psaltopoulou, T.; Sergentanis, T.N.; Frogoudaki, A.; Vrettou, A.-R.; Ikonomidis, I.; Paraskevaidis, I.; Parissis, J.; Bogiatzi, C.; Zompola, C.; et al. Transcranial Doppler versus transthoracic echocardiography for the detection of patent foramen ovale in patients with cryptogenic cerebral ischemia: A systematic review and diagnostic test accuracy meta-analysis. Ann. Neurol. 2016, 79, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.H.; Osten, M.; Leventhal, A.; Bach, Y.; Yoo, D.; Mansour, D.; Benson, L.; Wilson, W.M.; Horlicket, E. Percutaneous Intervention to Treat Platypnea-Orthodeoxia Syndrome: The Toronto Experience. JACC Cardiovasc. Interv. 2016, 9, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Palacios, I.F.; Bach, R.G.; Bitar, S.R.; Sideris, E.B. Platypnea-orthodeoxia: Management by transcatheter buttoned device implantation. Catheter. Cardiovasc. Interv. 2001, 54, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Hijazi, Z.M.; Holmes, D.R.; Rihal, C.S.; Wiegers, S.E. Intracardiac Echocardiography in Structural Heart Disease Interventions. JACC Cardiovasc. Interv. 2018, 11, 2133–2147. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Kawel-Boehm, N.; Maceira, A.; Valsangiacomo-Buechel, E.R.; Vogel-Claussen, J.; Turkbey, E.B.; Williams, R.; Plein, S.; Tee, M.; Eng, J.; Bluemke, D.A. Normal values for cardiovascular magnetic resonance in adults and children. J. Cardiovasc. Magn. Reson. 2015, 17, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Zwijnenburg, R.D.; Baggen, V.J.; Witsenburg, M.; Boersma, E.; Roos-Hesselink, J.W.; Bosch, A.E.V.D. Risk Factors for Pulmonary Hypertension in Adults After Atrial Septal Defect Closure. Am. J. Cardiol. 2019, 123, 1336–1342. [Google Scholar] [CrossRef]
- Ooi, Y.K.; Kelleman, M.; Ehrlich, A.; Glanville, M.; Porter, A.; Kim, D.; Kogon, B.; Osteret, M.E. Transcatheter Versus Surgical Closure of Atrial Septal Defects in Children: A Value Comparison. JACC Cardiovasc. Interv. 2016, 9, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Roberson, D.A.; Cui, W.; Patel, D.; Tsang, W.; Sugeng, L.; Weinert, L.; Bharati, S.; Lang, R.M. Three-Dimensional Transesophageal Echocardiography of Atrial Septal Defect: A Qualitative and Quantitative Anatomic Study. J. Am. Soc. Echocardiogr. 2011, 24, 600–610. [Google Scholar] [CrossRef] [PubMed]
- McElhinney, D.B.; Quartermain, M.D.; Kenny, D.; Alboliras, E.; Amin, Z. Relative Risk Factors for Cardiac Erosion Following Transcatheter Closure of Atrial Septal Defects: A Case-Control Study. Circulation 2016, 133, 1738–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, B.A.; Andrade, M.J.; Badano, L.; Fox, K.F.; Flachskampf, F.A.; Lancellotti, P.; Varga, A.; Sicari, R.; Evangelista, A.; Nihoyannopoulos, P.; et al. European Association of Echocardiography recommendations for training, competence, and quality improvement in echocardiography. Eur. J. Echocardiogr. 2009, 10, 893–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
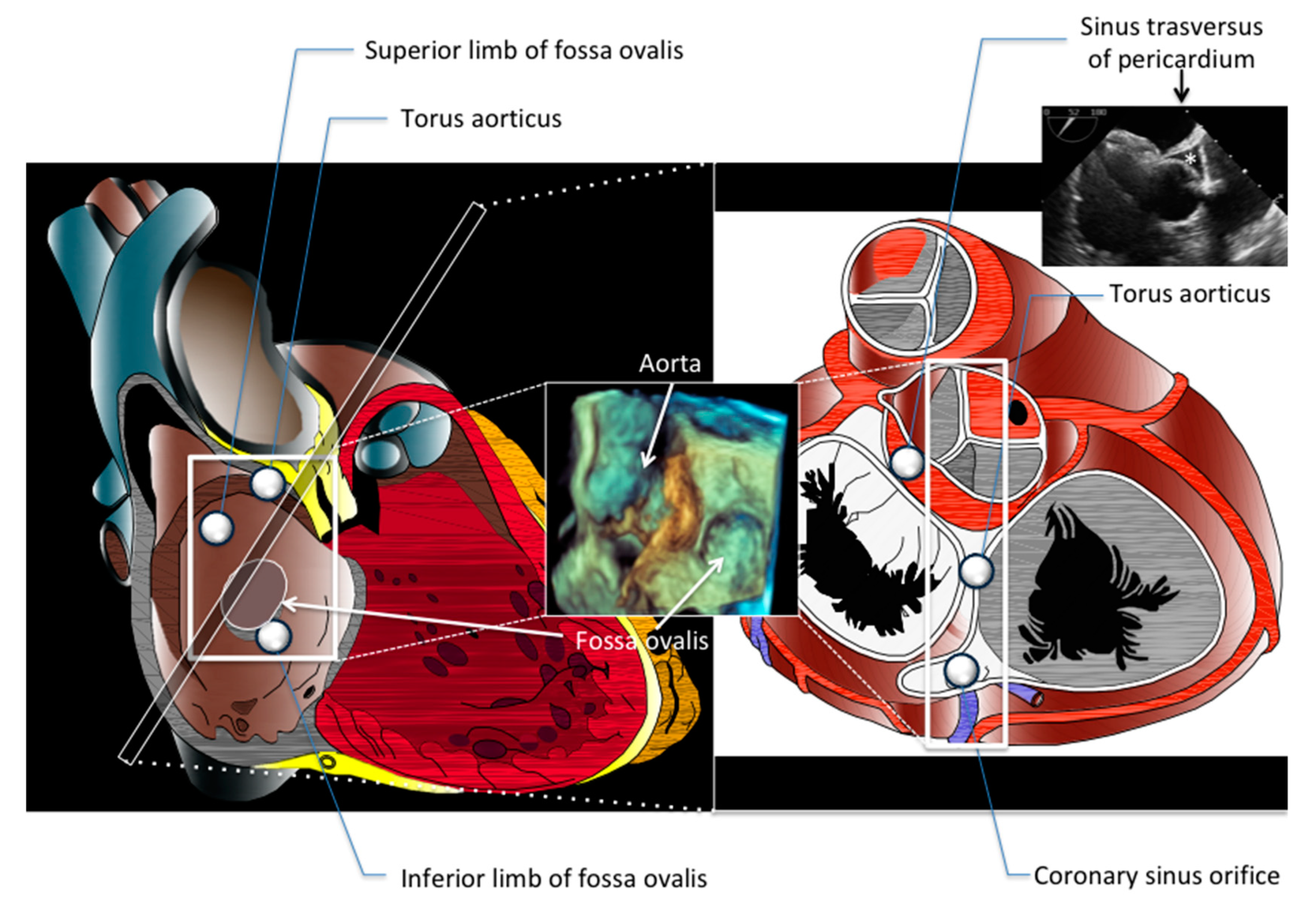





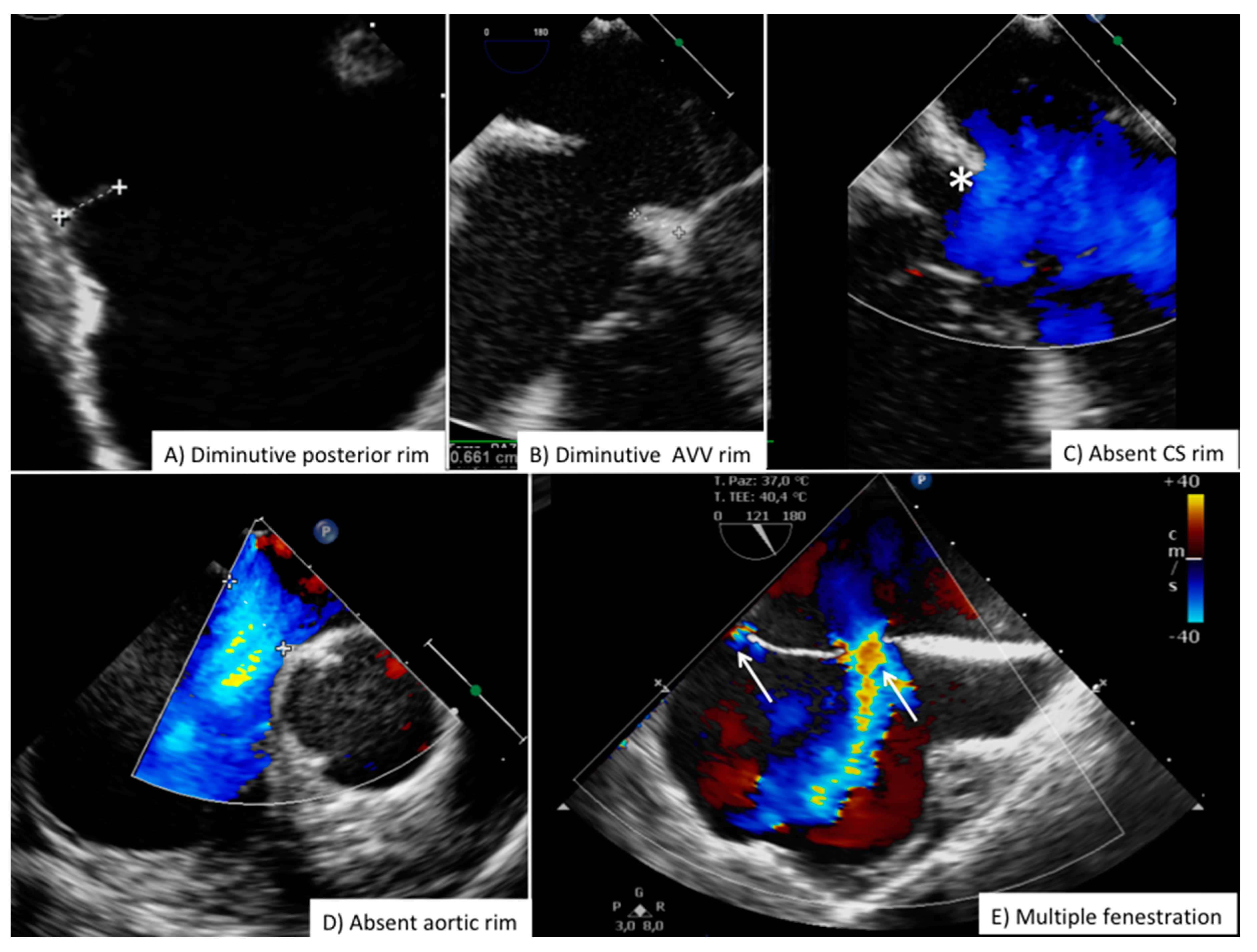
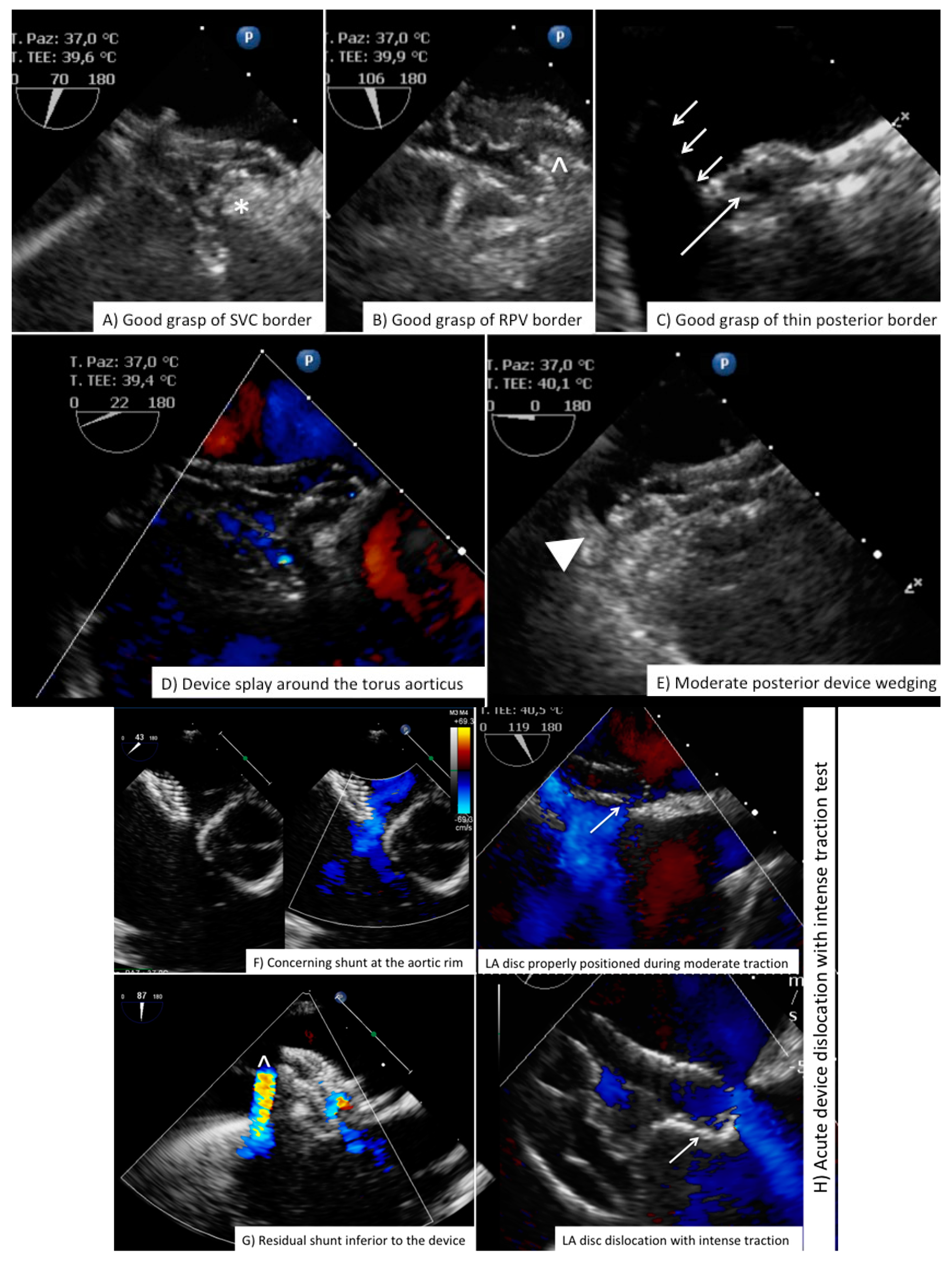
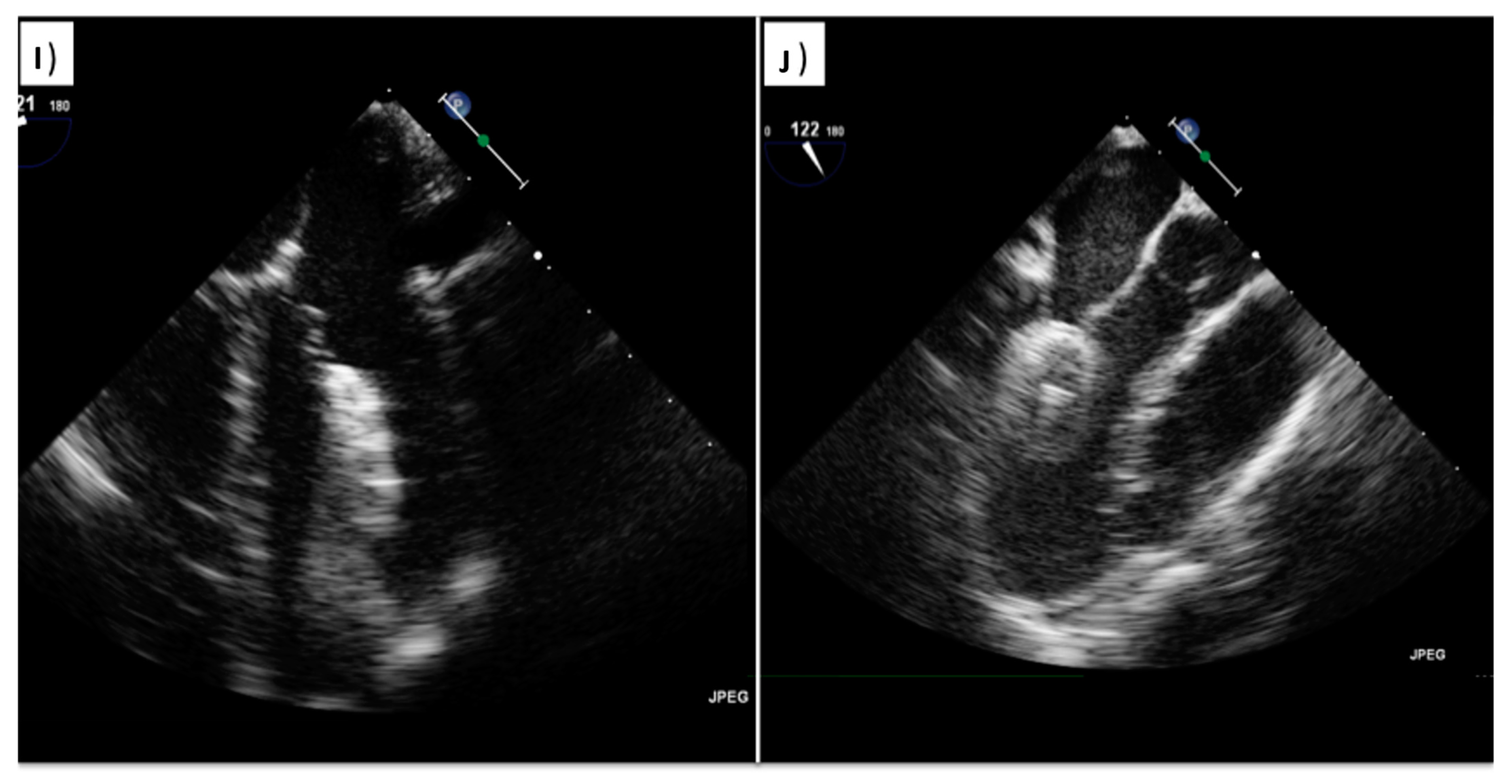
| View | Atrial Septal Anatomy | Procedural Assessment | Suggested Multiplane Angles | Esophageal Position |
|---|---|---|---|---|
| Basal transverse | SVC, superior aortic, RUPV | Device relationship in atrial roof | 0°, 15°, 30°, 45° | Mid- to upper esophagus |
| Four-chamber | Posterior and AVV rims, maximal ASD diameter | Device relationship to AV valves | 0°, 15°, 30°, | Mid-esophagus |
| Short-axis | Posterior and aortic rims, maximal ASD diameter, PFO tunnel and atrial anterior-posterior distance | Device relationship to AoV and posterior atrial wall | 30°, 45°, 60°, 75° | Mid- to upper esophagus |
| Bicaval | IVC and SVC rims, maximal ASD diameter, PFO amplitude and lenght | Device relationship to RA roof/dome | 90°, 105°, 120° | Mid-to upper esophagus and deep transgastric |
| Long-axis | Dome/roof of LA | Device relationship to LA dome/roof | 120°, 135°, 150° | Mid- to upper esophagus |
| Procedure | Imaging Modality | Outpatient Clinic Setting | Pre-Procedural Evaluation | Intra-Procedural Evaluation | Quantitative Data | Complexity of Training | Conscious Sedation, Anesthesia Support | Proposed Sequence in Diagnostic Algorithm |
|---|---|---|---|---|---|---|---|---|
| PFO closure | Neuroimaging modality | No | Yes | No | No | High | No * | 1 |
| TCD | Yes | Yes | No | Yes: Shunt grading | Mild | No | 2 | |
| TTE | Yes | Yes | No | Yes | Moderate | No | 3 | |
| TEE | Yes | Yes | Yes | Yes: Amplitude and length of PFO tunnel | High | Yes | 4 | |
| ICE | No | No | Yes | No | High | No | 4 § | |
| ASD closure | TTE | Yes | Yes | No | Yes: RV size (RV dilation if RV EDA > 12.6 cm2/m2 in men, 11.5 cm2/m2 in women) (17) | Moderate | No | 1 |
| CMR † | Yes | Yes | No | Yes: RV size (RV dilation if RV EDV > 91 mL2/m2 in men, 80 mL2/m2 in men) (18) | High | No * | 2 † | |
| TEE | Yes | No ‡ | Yes | Yes: ASD border analysis and balloon sizing | High | Yes | 3 | |
| ICE | No | No | Yes | No | High | No | 4 § |
| PFO Closure | ASD Closure | |
|---|---|---|
| Before vascular access | Free LA appendage Normal aortic and mitral valve No intracardiac mass Atrial septal aneurysm Eustachian valve Chiari Network Accessory fenestration Antero-posterior septal distance | Free LA appendage No significant mitral valve disease Atrial septal aneurysm Eustachian valve Chiari Network Assess border features Multifenestrated ASD Confirm normal pulmonary vein anatomy Bidimensional and color-based shortest and largest ASD diameter 3D-based shortest and largest ASD diameter |
| After vascular access | Confirm right-to-left shunt at intracardiac bubble study Confirm correct tunnel wiring Confirm wire position in the proper pulmonary vein PFO tunnel amplitude and length | Confirm wire position in the proper pulmonary vein Balloon sizing in stop-flow condition |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egidy Assenza, G.; Spinardi, L.; Mariucci, E.; Balducci, A.; Ragni, L.; Ciuca, C.; Formigari, R.; Angeli, E.; Vornetti, G.; Gargiulo, G.D.; et al. Transcatheter Closure of PFO and ASD: Multimodality Imaging for Patient Selection and Perioperative Guidance. J. Cardiovasc. Dev. Dis. 2021, 8, 78. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd8070078
Egidy Assenza G, Spinardi L, Mariucci E, Balducci A, Ragni L, Ciuca C, Formigari R, Angeli E, Vornetti G, Gargiulo GD, et al. Transcatheter Closure of PFO and ASD: Multimodality Imaging for Patient Selection and Perioperative Guidance. Journal of Cardiovascular Development and Disease. 2021; 8(7):78. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd8070078
Chicago/Turabian StyleEgidy Assenza, Gabriele, Luca Spinardi, Elisabetta Mariucci, Anna Balducci, Luca Ragni, Cristina Ciuca, Roberto Formigari, Emanuela Angeli, Gianfranco Vornetti, Gaetano Domenico Gargiulo, and et al. 2021. "Transcatheter Closure of PFO and ASD: Multimodality Imaging for Patient Selection and Perioperative Guidance" Journal of Cardiovascular Development and Disease 8, no. 7: 78. https://0-doi-org.brum.beds.ac.uk/10.3390/jcdd8070078






