Extrusion-Based 3D Printing for Highly Porous Alginate Materials Production
Abstract
:1. Introduction
2. Results and Discussion
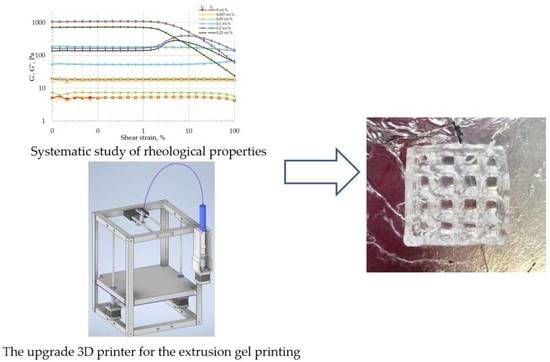
2.1. Rheological Study
2.2. 3D Printing Process
2.3. Aerogel Preparation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Gel-like Material
4.3. 3D Printing Process
4.4. Preparation of the Aerogel
4.5. Supercritical Drying
4.6. Characterization
Author Contributions
Funding
Conflicts of Interest
References
- Daminabo, S.C.; Goel, S.; Grammatikos, S.A.; Nezhad, H.Y.; Thakur, V.K. Fused deposition modeling-based additive manufacturing (3D printing): Techniques for polymer material systems. Mater. Today Chem. 2020, 16, 100248. [Google Scholar] [CrossRef]
- DebRoy, T.; Mukherjee, T.; Milewski, J.; Elmer, J.; Ribic, B.; Blecher, J.; Zhang, W. Scientific, technological and economic issues in metal printing and their solutions. Nat. Mater. 2019, 18, 1026–1032. [Google Scholar] [CrossRef]
- Haider, M.S.; Ahmad, T.; Yang, M.; Hu, C.; Hahn, L.; Stahlhut, P.; Groll, J.; Luxenhofer, R. Tuning the Thermogelation and Rheology of Poly (2-Oxazoline)/Poly (2-Oxazine)s Based Thermosensitive Hydrogels for 3D Bioprinting. Gels 2021, 7, 78. [Google Scholar] [CrossRef]
- Woern, A.L.; Byard, D.J.; Oakley, R.B.; Fiedler, M.J.; Snabes, S.L.; Pearce, J.M. Fused particle fabrication 3-D printing: Recycled materials’ optimization and mechanical properties. Materials 2018, 11, 1413. [Google Scholar] [CrossRef] [Green Version]
- Valino, A.D.; Dizon, J.R.C.; Espera Jr, A.H.; Chen, Q.; Messman, J.; Advincula, R.C. Advances in 3D printing of thermoplastic polymer composites and nanocomposites. Prog. Polym. Sci. 2019, 98, 101162. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Manapat, J.Z.; Chen, Q.; Ye, P.; Advincula, R.C. 3D printing of polymer nanocomposites via stereolithography. Macromol. Mater. Eng. 2017, 302, 1600553. [Google Scholar] [CrossRef]
- Karakurt, I.; Lin, L. 3D printing technologies: Techniques, materials, and post-processing. Curr. Opin. Chem. Eng. 2020, 28, 134–143. [Google Scholar] [CrossRef]
- Placone, J.K.; Engler, A.J. Recent advances in extrusion-based 3D printing for biomedical applications. Adv. Healc. Mater. 2018, 7, 1701161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Zhou, C.; Chen, Y. Rapid manufacturing in minutes: The development of a mask projection stereolithography process for high-speed fabrication. In Proceedings of the International Manufacturing Science and Engineering Conference, Notre Dame, IN, USA, 4–8 June 2012; pp. 405–414. [Google Scholar]
- Baldacchini, T.; Saksena, J.; Sklare, S.C.; Vinson, B.T.; Huang, Y.; Chrisey, D.B.; Narayan, R.J. Translation of laser-based three-dimensional printing technologies. MRS Bull. 2021, 46, 174–185. [Google Scholar] [CrossRef]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D printing in pharmaceutical and medical applications–recent achievements and challenges. Pharm. Res. 2018, 35, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, S.; McGee, K.; Morris, J.; Alexander, A.; Kuhlmann, J.; Vrieze, T.; McCollough, C.H.; Matsumoto, J. Anatomic modeling using 3D printing: Quality assurance and optimization. 3D Print. Med. 2017, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Durfee, W.K.; Iaizzo, P.A. Medical applications of 3D printing. In Engineering in Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 527–543. [Google Scholar]
- Stergar, J.; Maver, U. Review of aerogel-based materials in biomedical applications. J. Sol-Gel Sci. Technol. 2016, 77, 738–752. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer aerogels and foams: Chemistry, properties, and applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel based systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef]
- Maleki, H.; Hüsing, N. Current status, opportunities and challenges in catalytic and photocatalytic applications of aerogels: Environmental protection aspects. Appl. Catal. B Environ. 2018, 221, 530–555. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Koebel, M.; Rigacci, A.; Achard, P. Aerogel-based thermal superinsulation: An overview. J. Sol-Gel Sci. Technol. 2012, 63, 315–339. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Su, B.-L.; Xia, H.; Zhao, S.; Gao, C.; Wang, L.; Ogbeide, O.; Feng, J.; Hasan, T. Printed aerogels: Chemistry, processing, and applications. Chem. Soc. Rev. 2021, 50, 3842–3888. [Google Scholar] [CrossRef]
- Hensleigh, R.M.; Cui, H.; Oakdale, J.S.; Jianchao, C.Y.; Campbell, P.G.; Duoss, E.B.; Spadaccini, C.M.; Zheng, X.; Worsley, M.A. Additive manufacturing of complex micro-architected graphene aerogels. Mater. Horiz. 2018, 5, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Wright, C.J.; Molino, B.Z.; Chung, J.H.Y.; Pannell, J.T.; Kuester, M.; Molino, P.J.; Hanks, T.W. Synthesis and 3D Printing of Conducting Alginate–Polypyrrole Ionomers. Gels 2020, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Kyle, S.; Jessop, Z.M.; Al-Sabah, A.; Whitaker, I.S. ‘Printability’of candidate biomaterials for extrusion based 3D printing: State-of-the-art. Adv. Healc. Mater. 2017, 6, 1700264. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Liu, Y.; Wang, Z.; Li, Y.; Jiang, G.; Mo, X.; Zhou, G. Three-dimensional printed electrospun fiber-based scaffold for cartilage regeneration. Mater. Des. 2019, 179, 107886. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Mamba, G.; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Lin, Y.; Chen, X.; Zhao, B.; Zhang, J. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Ajdary, R.; Huan, S.; Zanjanizadeh Ezazi, N.; Xiang, W.; Grande, R.; Santos, H.l.A.; Rojas, O.J. Acetylated nanocellulose for single-component bioinks and cell proliferation on 3D-printed scaffolds. Biomacromolecules 2019, 20, 2770–2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middelkoop, V.; Slater, T.; Florea, M.; Neațu, F.; Danaci, S.; Onyenkeadi, V.; Boonen, K.; Saha, B.; Baragau, I.-A.; Kellici, S. Next frontiers in cleaner synthesis: 3D printed graphene-supported CeZrLa mixed-oxide nanocatalyst for CO2 utilisation and direct propylene carbonate production. J. Clean. Prod. 2019, 214, 606–614. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, F.; Xu, X.; Zhou, C.; Lin, D. Three-dimensional printing hollow polymer template-mediated graphene lattices with tailorable architectures and multifunctional properties. ACS Nano 2018, 12, 1096–1106. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Alnaief, M.; Alzaitoun, M.; García-González, C.; Smirnova, I. Preparation of biodegradable nanoporous microspherical aerogel based on alginate. Carbohydr. Polym. 2011, 84, 1011–1018. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Bernard, I.; Marin, M.A.; Ward, K.R.; McHugh, M.A. Superabsorbent alginate aerogels. J. Supercrit. Fluids 2013, 79, 202–208. [Google Scholar] [CrossRef]
- Quignard, F.; Valentin, R.; Di Renzo, F. Aerogel materials from marine polysaccharides. New J. Chem. 2008, 32, 1300–1310. [Google Scholar] [CrossRef]
- Menshutina, N.; Tsygankov, P.; Khudeev, I.; Lebedev, A. Intensification methods of supercritical drying for aerogels production. Dry. Technol. 2020, 39, 1–14. [Google Scholar] [CrossRef]
- Tsygankov, P.Y.; Khudeev, I.I.; Lebedev, A.E.; Lebedev, E.A.; Menshutina, N.V. Lab scale high-pressure equipment for supercritical drying. Chem. Eng. Trans. 2018, 70, 877–882. [Google Scholar]










| Calcium Chloride Concentration, wt% | 0 | 0.025 | 0.05 | 0.1 | 0.2 | 0.25 |
| Viscosity, Pa∗s | 2.9 | 3.4 | 8.3 | 2552.1 | 1680.9 | 1283.4 |
| Calcium Chloride Concentration, wt% | 0 | 0.025 | 0.05 | 0.1 | 0.2 | 0.25 |
| Power-Law Index | 0.9131 | 0.9252 | 0.6089 | 0.2952 | 0.3074 | 0.1673 |
| Nozzle Diameter, mm | Length, mm | High, mm | Thickness, mm |
|---|---|---|---|
| 0.84 | 21.4 | 7.3 | 2.7 |
| 0.61 | 21.0 | 9.2 | 2.2 |
| 0.51 | 20.7 | 9.5 | 2.1 |
| 0.41 | 20.2 | 10.1 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menshutina, N.; Abramov, A.; Tsygankov, P.; Lovskaya, D. Extrusion-Based 3D Printing for Highly Porous Alginate Materials Production. Gels 2021, 7, 92. https://0-doi-org.brum.beds.ac.uk/10.3390/gels7030092
Menshutina N, Abramov A, Tsygankov P, Lovskaya D. Extrusion-Based 3D Printing for Highly Porous Alginate Materials Production. Gels. 2021; 7(3):92. https://0-doi-org.brum.beds.ac.uk/10.3390/gels7030092
Chicago/Turabian StyleMenshutina, Natalia, Andrey Abramov, Pavel Tsygankov, and Daria Lovskaya. 2021. "Extrusion-Based 3D Printing for Highly Porous Alginate Materials Production" Gels 7, no. 3: 92. https://0-doi-org.brum.beds.ac.uk/10.3390/gels7030092