Novel Gels: An Emerging Approach for Delivering of Therapeutic Molecules and Recent Trends
Abstract
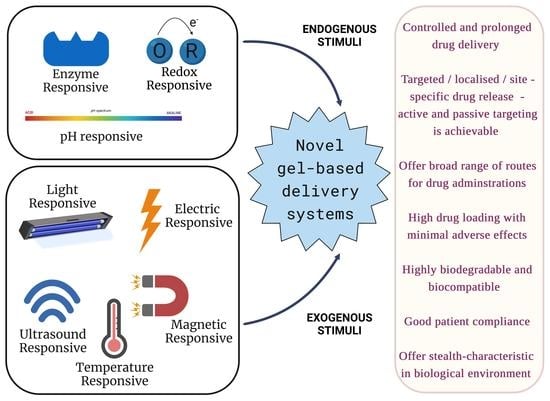
:1. Introduction
2. Advances in Novel Gel-Based Delivery Systems
2.1. Intelligent Hydrogels
2.2. In Situ Gels
2.3. Emulsion Gels
2.4. Microgels
2.5. Nanogels
2.6. Vesicular Gels
2.6.1. Liposomal Gels
2.6.2. Niosomal Gels
2.6.3. Transferosome Gels
3. Novel Gel-Based Delivery Approaches for Delivering Therapeutic Molecules—Recent Trends
3.1. Hydrogel Systems
3.2. Thermosensitive Hydrogels
3.3. Light Stimuli Hydrogels
3.4. pH Stimuli Hydrogels
4. Descriptive Patents Established for Novel Gel-Based Delivery Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iriventi, P.; Gupta, N.V.; Osmani, R.A.M.; Balamuralidhara, V. Design & development of nanosponge loaded topical gel of curcumin and caffeine mixture for augmented treatment of psoriasis. DARU J. Pharm. Sci. 2020, 28, 489–506. [Google Scholar] [CrossRef]
- Uzunalli, G.; Guler, M.O. Peptide gels for controlled release of proteins. Ther. Deliv. 2020, 11, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, M.; Schneider, J.P. Peptide hydrogels for affinity-controlled release of therapeutic cargo: Current and potential strategies. J. Pept. Sci. 2021, 28, e3377. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tiwari, S. A review on biomacromolecular hydrogel classification and its applications. Int. J. Biol. Macromol. 2020, 162, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Micale, N.; Citarella, A.; Molonia, M.S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Hydrogels for the Delivery of Plant-Derived (Poly)Phenols. Molecules 2020, 25, 3254. [Google Scholar] [CrossRef] [PubMed]
- Op’t Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F.A.D.T.G. Design Considerations for Hydrogel Wound Dressings: Strategic and Molecular Advances. Tissue Eng. Part B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Du, W.; Zong, Q.; Guo, R.; Ling, G.; Zhang, P. Injectable Nanocomposite Hydrogels for Cancer Therapy. Macromol. Biosci. 2021, 21, 2100186. [Google Scholar] [CrossRef]
- Chen, T.; Hou, K.; Ren, Q.; Chen, G.; Wei, P.; Zhu, M. Nanoparticle-Polymer Synergies in Nanocomposite Hydrogels: From Design to Application. Macromol. Rapid Commun. 2018, 39, e1800337. [Google Scholar] [CrossRef]
- Jiménez, G.; Venkateswaran, S.; López-Ruiz, E.; Peran, M.; Pernagallo, S.; Díaz-Monchón, J.J.; Canadas, R.; Antich, C.; Oliveira, J.M.; Callanan, A.; et al. A soft 3D polyacrylate hydrogel recapitulates the cartilage niche and allows growth-factor free tissue engineering of human articular cartilage. Acta Biomater. 2019, 90, 146–156. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.-K.; Ren, K.; Zuo, J.; Ding, J.; Chen, X. Thermosensitive Hydrogels as Scaffolds for Cartilage Tissue Engineering. Biomacromolecules 2019, 20, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Barouti, G.; Liow, S.S.; Dou, Q.; Ye, H.; Orione, C.; Guillaume, S.M.; Loh, X.J. New Linear and Star-Shaped Thermogelling Poly([R]-3-hydroxybutyrate) Copolymers. Chem. A Eur. J. 2016, 22, 10501–10512. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cheng, Y.; Tong, J.; Zhang, L.; Wei, Y.; Tian, M. Recent advances in thermo-sensitive hydrogels for drug delivery. J. Mater. Chem. B 2021, 9, 2979–2992. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, B.; Taranejoo, S.; Monemian, S.A.; Moghaddam, Z.S.; Daliri, K.; Derakhshankhah, H.; Derakhshani, Z. Classification of stimuli–responsive polymers as anticancer drug delivery systems. Drug Deliv. 2015, 22, 145–155. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Stimuli-responsive hydrogels for intratumoral drug delivery. Drug Discov. Today 2021, 26, 2397–2405. [Google Scholar] [CrossRef]
- Khan, S.; Akhtar, N.; Minhas, M.U.; Badshah, S.F. pH/Thermo-Dual Responsive Tunable In Situ Cross-Linkable Depot Injectable Hydrogels Based on Poly(N-Isopropylacrylamide)/Carboxymethyl Chitosan with Potential of Controlled Localized and Systemic Drug Delivery. AAPS PharmSciTech 2019, 20, 119. [Google Scholar] [CrossRef]
- Oliva, N.; Conde, J.; Wang, K.; Artzi, N. Designing Hydrogels for On-Demand Therapy. Acc. Chem. Res. 2017, 50, 669–679. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Chen, L.; Shen, T.; Wu, D. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of hydrogels: Linking the nano to the microscale. Adv. Colloid Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef]
- Azeera, M.; Vaidevi, S.; Ruckmani, K. Characterization Techniques of Hydrogel and Its Applications; Springer: Cham, Switzerland, 2018; pp. 1–24. [Google Scholar] [CrossRef]
- Alexander, A.; Ajazuddin; Khan, J.; Saraf, S.; Saraf, S. Polyethylene glycol (PEG)–Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammadi, S.; Aminabad, N.S.; Sabzi, A.; Zarebkohan, A.; Razavi, M.; Vosough, M.; Bodaghi, M.; Maleki, H. Smart and Biomimetic 3D and 4D Printed Composite Hydrogels: Opportunities for Different Biomedical Applications. Biomedicines 2021, 9, 1537. [Google Scholar] [CrossRef] [PubMed]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart Hydrogels—Synthetic Stimuli-Responsive Antitumor Drug Release Systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef]
- Shi, J.; Yu, L.; Ding, J. PEG-based thermosensitive and biodegradable hydrogels. Acta Biomater. 2021, 128, 42–59. [Google Scholar] [CrossRef]
- Sun, Z.; Song, C.; Wang, C.; Hu, Y.; Wu, J. Hydrogel-Based Controlled Drug Delivery for Cancer Treatment: A Review. Mol. Pharm. 2020, 17, 373–391. [Google Scholar] [CrossRef]
- Le, T.M.D.; Duong, H.T.T.; Thambi, T.; Giang Phan, V.H.; Jeong, J.H.; Lee, D.S. Bioinspired pH- and Temperature-Responsive Injectable Adhesive Hydrogels with Polyplexes Promotes Skin Wound Healing. Biomacromolecules 2018, 19, 3536–3548. [Google Scholar] [CrossRef]
- Basu, S.; Pacelli, S.; Paul, A. Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery. Acta Biomater. 2020, 105, 159–169. [Google Scholar] [CrossRef]
- Rizzo, F.; Kehr, N.S. Recent Advances in Injectable Hydrogels for Controlled and Local Drug Delivery. Adv. Healthc. Mater. 2020, 10, 2001341. [Google Scholar] [CrossRef]
- Stayton, P.; El-Sayed, M.; Murthy, N.; Bulmuş, V.; Lackey, C.; Cheung, C.; Hoffman, A. ‘Smart’ delivery systems for biomolecular therapeutics. Orthod. Craniofacial Res. 2005, 8, 219–225. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Chen, F. pH-Responsive Drug-Delivery Systems. Chem. Asian J. 2015, 10, 284–305. [Google Scholar] [CrossRef] [PubMed]
- Chandrawati, R. Enzyme-responsive polymer hydrogels for therapeutic delivery. Exp. Biol. Med. 2016, 241, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-Responsive Hydrogels: Intelligent Materials for Biosensing and Drug Delivery. Acc. Chem. Res. 2017, 50, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Billah, S.M.R.; Mondal, I.H.; Somoal, S.H.; Pervez, M.N.; Haque, O. Enzyme-Responsive Hydrogels; Springer: Cham, Switzerland, 2019; pp. 309–330. [Google Scholar] [CrossRef]
- Rafael, D.; Melendres, M.M.R.; Andrade, F.; Montero, S.; Martinez-Trucharte, F.; Vilar-Hernandez, M.; Durán-Lara, E.F.; Schwartz, S.; Abasolo, I. Thermo-responsive hydrogels for cancer local therapy: Challenges and state-of-art. Int. J. Pharm. 2021, 606, 120954. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S., Jr. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Griffin, D.R.; Kasko, A.M. Photoselective Delivery of Model Therapeutics from Hydrogels. ACS Macro Lett. 2012, 1, 1330–1334. [Google Scholar] [CrossRef]
- Fakhari, A.; Subramony, J.A. Engineered in-situ depot-forming hydrogels for intratumoral drug delivery. J. Control. Release 2015, 220, 465–475. [Google Scholar] [CrossRef]
- Fang, G.; Yang, X.; Wang, Q.; Zhang, A.; Tang, B. Hydrogels-based ophthalmic drug delivery systems for treatment of ocular diseases. Mater. Sci. Eng. C 2021, 127, 112212. [Google Scholar] [CrossRef]
- Choi, B.; Loh, X.J.; Tan, A.; Loh, C.K.; Ye, E.; Joo, M.K.; Jeong, B. Introduction to in situ forming hydrogels for biomedical applications. In In-Situ Gelling Polymers; Springer: Singapore, 2015; pp. 5–35. [Google Scholar] [CrossRef]
- Loh, X.J. In-Situ Gelling Polymers: For Biomedical Applications; Springer: Singapore, 2015. [Google Scholar]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Shim, W.S.; Kim, J.-H.; Kim, K.; Kim, Y.-S.; Park, R.-W.; Kim, I.-S.; Kwon, I.C.; Lee, D.S. pH- and temperature-sensitive, injectable, biodegradable block copolymer hydrogels as carriers for paclitaxel. Int. J. Pharm. 2007, 331, 11–18. [Google Scholar] [CrossRef]
- Kang, J.H.; Turabee, H.; Lee, D.S.; Kwon, Y.J.; Ko, Y.T. Temperature and pH-responsive in situ hydrogels of gelatin derivatives to prevent the reoccurrence of brain tumor. Biomed. Pharmacother. 2021, 143, 112144. [Google Scholar] [CrossRef] [PubMed]
- Podual, K.; Doyle, F.J.; Peppas, N.A. Dynamic behavior of glucose oxidase-containing microparticles of poly(ethylene glycol)-grafted cationic hydrogels in an environment of changing pH. Biomaterials 2000, 21, 1439–1450. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Bomba, H.; Gu, Z. Stimuli-responsive delivery of therapeutics for diabetes treatment. Bioeng. Transl. Med. 2016, 1, 323–337. [Google Scholar] [CrossRef]
- Xu, X.; Shang, H.; Zhang, T.; Shu, P.; Liu, Y.; Xie, J.; Zhang, D.; Tan, H.; Li, J. A stimuli-responsive insulin delivery system based on reversible phenylboronate modified cyclodextrin with glucose triggered host-guest interaction. Int. J. Pharm. 2018, 548, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef]
- Jalalvandi, E.; Shavandi, A. In situ-forming and pH-responsive hydrogel based on chitosan for vaginal delivery of therapeutic agents. J. Mater. Sci. Mater. Med. 2018, 29, 158. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr. Polym. 2013, 92, 1685–1699. [Google Scholar] [CrossRef]
- Bhardwaj, T.R.; Kanwar, M.; Lal, R.; Gupta, A. Natural Gums and Modified Natural Gums as Sustained-Release Carriers. Drug Dev. Ind. Pharm. 2000, 26, 1025–1038. [Google Scholar] [CrossRef]
- Guo, J.-H.; Skinner, G.W.; Harcum, W.W.; Barnum, P.E. Pharmaceutical applications of naturally occurring water-soluble polymers. Pharm. Sci. Technol. Today 1998, 1, 254–261. [Google Scholar] [CrossRef]
- Dodane, V.; Vilivalam, V.D. Pharmaceutical applications of chitosan. Pharm. Sci. Technol. Today 1998, 1, 246–253. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. An overview on preparation of emulsion-filled gels and emulsion particulate gels. Trends Food Sci. Technol. 2019, 86, 85–94. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Kumar, R.; Mishra, G.; Sharma, U.; Singh, G.; Ahalawat, S. Sol-Gel processing of silica nanoparticles and their applications. Adv. Colloid Interface Sci. 2014, 214, 17–37. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, B.; Sun, Z.; Liu, T.; Cai, Y.; Huang, L.; Deng, X.; Zhao, M.; Zhao, Q. A novel preparation strategy of emulsion gel solely stabilized by alkaline assisted steam-cooking treated insoluble soybean fiber. Food Hydrocoll. 2022, 129, 107646. [Google Scholar] [CrossRef]
- Smeets, N.M.B.; Hoare, T. Designing responsive microgels for drug delivery applications. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3027–3043. [Google Scholar] [CrossRef]
- Imaz, A.; Forcada, J. New Biocompatible Microgels. Macromol. Symp. 2009, 281, 85–88. [Google Scholar] [CrossRef]
- Martín-Molina, A.; Quesada-Pérez, M. A review of coarse-grained simulations of nanogel and microgel particles. J. Mol. Liq. 2019, 280, 374–381. [Google Scholar] [CrossRef]
- Sung, B.; Kim, C.; Kim, M.-H. Biodegradable colloidal microgels with tunable thermosensitive volume phase transitions for controllable drug delivery. J. Colloid Interface Sci. 2015, 450, 26–33. [Google Scholar] [CrossRef]
- Vinogradov, S.V. Colloidal Microgels in Drug Delivery Applications. Curr. Pharm. Des. 2006, 12, 4703–4712. [Google Scholar] [CrossRef] [Green Version]
- Kousalová, J.; Etrych, T. Polymeric Nanogels as Drug Delivery Systems. Physiol. Res. 2018, 67, S305–S317. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Taymouri, S.; Ghassami, E. Supramolecular Self-Assembled Nanogels a New Platform for Anticancer Drug Delivery. Curr. Pharm. Des. 2018, 23, 5242–5260. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-H.; Jiwpanich, S.; Chacko, R.; Bickerton, S.; Thayumanavan, S. Surface-Functionalizable Polymer Nanogels with Facile Hydrophobic Guest Encapsulation Capabilities. J. Am. Chem. Soc. 2010, 132, 8246–8247. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Rosenholm, J.M.; Minhas, M.U.; Badshah, S.F.; Naeem, A.; Khan, K.U.; Fahad, M. Nanogels as drug-delivery systems: A comprehensive overview. Ther. Deliv. 2019, 10, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Müller, R. Biodegradability of Polymers: Regulations and Methods for Testing. In Biopolymers Online; American Cancer Society: Atlanta, GA, USA, 2005. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Ahmed, S.; Alhareth, K.; Mignet, N. Advancement in nanogel formulations provides controlled drug release. Int. J. Pharm. 2020, 584, 119435. [Google Scholar] [CrossRef]
- Rajkumar, J.; Gv, R.; Sastri K, T.; Burada, S. Recent update on proniosomal gel as topical drug delivery system. Asian J. Pharm. Clin. Res. 2019, 12, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Brandl, M. Vesicular Phospholipid Gels: A Technology Platform. J. Liposome Res. 2007, 17, 15–26. [Google Scholar] [CrossRef]
- Tian, W.; Schulze, S.; Brandl, M.; Winter, G. Vesicular phospholipid gel-based depot formulations for pharmaceutical proteins: Development and in vitro evaluation. J. Control. Release 2010, 142, 319–325. [Google Scholar] [CrossRef]
- Breitsamer, M.; Winter, G. Vesicular phospholipid gels as drug delivery systems for small molecular weight drugs, peptides and proteins: State of the art review. Int. J. Pharm. 2018, 557, 1–8. [Google Scholar] [CrossRef]
- Fetih, G.; Fathalla, D.; El-Badry, M. Liposomal Gels for Site-Specific, Sustained Delivery of Celecoxib: In Vitro and In Vivo Evaluation. Drug Dev. Res. 2014, 75, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Pavelić, Ž.; Skalko-Basnet, N.; Schubert, R. Liposomal gels for vaginal drug delivery. Int. J. Pharm. 2001, 219, 139–149. [Google Scholar] [CrossRef]
- Pavelić, Z.; Skalko-Basnet, N.; Jalsenjak, I. Liposomal gel with chloramphenicol: Characterisation and in vitro release. Acta Pharm. 2004, 54, 319–330. [Google Scholar] [PubMed]
- Singh, S.; Vardhan, H.; Kotla, N.G.; Maddiboyina, B.; Sharma, D.; Webster, T.J. The role of surfactants in the formulation of elastic liposomal gels containing a synthetic opioid analgesic. Int. J. Nanomed. 2016, 11, 1475–1482. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.K.; Kumar, P.; Thakkar, H.P. Formulation of Niosomal Gel for Enhanced Transdermal Lopinavir Delivery and Its Comparative Evaluation with Ethosomal Gel. AAPS PharmSciTech 2012, 13, 1502–1510. [Google Scholar] [CrossRef] [Green Version]
- Muzzalupo, R.; Tavano, L. Niosomal drug delivery for transdermal targeting: Recent advances. Res. Rep. Transdermal Drug Deliv. 2015, 4, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Lehr, C.-M.; Kok, W.; Junginger, H.E.; Verhoef, J.C.; Bouwstra, J.A. Niosomes for oral delivery of peptide drugs. J. Control. Release 1992, 21, 145–153. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Das, B.; Sen, S.O.; Maji, R.; Nayak, A.K.; Sen, K.K. Transferosomal gel for transdermal delivery of risperidone: Formulation optimization and ex vivo permeation. J. Drug Deliv. Sci. Technol. 2017, 38, 59–71. [Google Scholar] [CrossRef]
- Cevc, G.; Blume, G.; Schätzlein, A. Transfersomes-mediated transepidermal delivery improves the regio-specificity and biological activity of corticosteroids in vivo1Dedicated to the late Dr. Henri Ernest Bodde.1. J. Control. Release 1997, 45, 211–226. [Google Scholar] [CrossRef]
- Malakar, J.; Sen, S.O.; Nayak, A.K.; Sen, K.K. Formulation, optimization and evaluation of transferosomal gel for transdermal insulin delivery. Saudi Pharm. J. 2012, 20, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, H.A.E. Transfersomes for transdermal drug delivery. Expert Opin. Drug Deliv. 2006, 3, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomás, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Godbey, W.T.; McPherson, G.L.; John, V.T. Microgel, nanogel and hydrogel–hydrogel semi-IPN composites for biomedical applications: Synthesis and characterization. Colloid Polym. Sci. 2006, 284, 1121–1129. [Google Scholar] [CrossRef]
- Thambi, T.; Phan, V.H.G.; Lee, D.S. Stimuli-Sensitive Injectable Hydrogels Based on Polysaccharides and Their Biomedical Applications. Macromol. Rapid Commun. 2016, 37, 1881–1896. [Google Scholar] [CrossRef]
- Yoshida, R.; Uchida, K.; Kaneko, Y.; Sakai, K.; Kikuchi, A.; Sakurai, Y.; Okano, T. Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature 1995, 374, 240–242. [Google Scholar] [CrossRef]
- Meyer, D.E.; Chilkoti, A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat. Biotechnol. 1999, 17, 1112–1115. [Google Scholar] [CrossRef]
- Li, Y.; Lin, T.-Y.; Luo, Y.; Liu, Q.; Xiao, W.; Guo, W.; Lac, D.; Zhang, H.; Feng, C.; Wachsmann-Hogiu, S.; et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat. Commun. 2014, 5, 4712. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.; Deng, X.; Wu, Q.; Cao, X.; Ye, T.; Wang, S. Liposome-loaded thermo-sensitive hydrogel for stabilization of SN-38 via intratumoral injection: Optimization, characterization, and antitumor activity. Pharm. Dev. Technol. 2018, 23, 106–115. [Google Scholar] [CrossRef]
- Mirrahimi, M.; Abed, Z.; Beik, J.; Shiri, I.; Dezfuli, A.S.; Mahabadi, V.P.; Kamrava, S.K.; Ghaznavi, H.; Shakeri-Zadeh, A. A thermo-responsive alginate nanogel platform co-loaded with gold nanoparticles and cisplatin for combined cancer chemo-photothermal therapy. Pharmacol. Res. 2019, 143, 178–185. [Google Scholar] [CrossRef]
- Chen, L.; Deng, J.; Yu, A.; Hu, Y.; Jin, B.; Du, P.; Zhou, J.; Lei, L.; Wang, Y.; Vakal, S.; et al. Drug-peptide supramolecular hydrogel boosting transcorneal permeability and pharmacological activity via ligand-receptor interaction. Bioact. Mater. 2022, 10, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jiang, L.; Shi, H.; Xu, C.; Liu, M.; Li, Q.; Zheng, L.; Chi, H.; Wang, M.; Liu, Z.; et al. Effectiveness of an ocular adhesive polyhedral oligomeric silsesquioxane hybrid thermo-responsive FK506 hydrogel in a murine model of dry eye. Bioact. Mater. 2022, 9, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; He, C.; Quan, F.; Yu, S.; Chen, X. DOX/IL-2/IFN-γ co-loaded thermo-sensitive polypeptide hydrogel for efficient melanoma treatment. Bioact. Mater. 2018, 3, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Song, H.; Zhou, X.; Chen, Y.; Liu, Q.; Gao, X.; Zhu, X.; Chen, D. Novel facile thermosensitive hydrogel as sustained and controllable gene release vehicle for breast cancer treatment. Eur. J. Pharm. Sci. 2019, 134, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Xue, B.; Jia, Y.; Yuan, L.; Han, R.; Yang, F.; Peng, J.; Qian, Z. Sustained co-delivery of gemcitabine and cis-platinum via biodegradable thermo-sensitive hydrogel for synergistic combination therapy of pancreatic cancer. Nano Res. 2019, 12, 1389–1399. [Google Scholar] [CrossRef]
- Zhao, M.; Bozzato, E.; Joudiou, N.; Ghiassinejad, S.; Danhier, F.; Gallez, B.; Préat, V. Codelivery of paclitaxel and temozolomide through a photopolymerizable hydrogel prevents glioblastoma recurrence after surgical resection. J. Control. Release 2019, 309, 72–81. [Google Scholar] [CrossRef]
- Chakole, C.M.; Sahoo, P.K.; Pandey, J.; Chauhan, M.K. A green chemistry approach towards synthesizing hydrogel for sustained ocular delivery of brinzolamide: In vitro and ex vivo evaluation. J. Indian Chem. Soc. 2021, 99, 100323. [Google Scholar] [CrossRef]
- Asfour, M.H.; El-Alim, S.H.A.; Awad, G.E.A.; Kassem, A.A. Chitosan/β-glycerophosphate in situ forming thermo-sensitive hydrogel for improved ocular delivery of moxifloxacin hydrochloride. Eur. J. Pharm. Sci. 2021, 167, 106041. [Google Scholar] [CrossRef]
- Pakzad, Y.; Fathi, M.; Omidi, Y.; Mozafari, M.; Zamanian, A. Synthesis and characterization of timolol maleate-loaded quaternized chitosan-based thermosensitive hydrogel: A transparent topical ocular delivery system for the treatment of glaucoma. Int. J. Biol. Macromol. 2020, 159, 117–128. [Google Scholar] [CrossRef]
- Bao, Z.; Yu, A.; Shi, H.; Hu, Y.; Jin, B.; Lin, D.; Dai, M.; Lei, L.; Li, X.; Wang, Y. Glycol chitosan/oxidized hyaluronic acid hydrogel film for topical ocular delivery of dexamethasone and levofloxacin. Int. J. Biol. Macromol. 2021, 167, 659–666. [Google Scholar] [CrossRef]
- Wang, L.; Pan, H.; Gu, D.; Li, P.; Su, Y.; Pan, W. A composite System Combining Self-Targeted Carbon Dots and Thermosensitive Hydrogels for Challenging Ocular Drug Delivery. J. Pharm. Sci. 2022, 111, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Bártolo, P.J. Photopolymerizable hydrogels in regenerative medicine and drug delivery. In Hot Topics in Biomaterials; Future Science Ltd.: London, UK, 2014; pp. 6–28. [Google Scholar] [CrossRef]
- Lim, H.L.; Hwang, Y.; Kar, M.; Varghese, S. Smart hydrogels as functional biomimetic systems. Biomater. Sci. 2014, 2, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Fourniols, T.; Randolph, L.D.; Staub, A.; Vanvarenberg, K.; Leprince, J.G.; Préat, V.; des Rieux, A.; Danhier, F. Temozolomide-loaded photopolymerizable PEG-DMA-based hydrogel for the treatment of glioblastoma. J. Control. Release 2015, 210, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.; Parker, S.G.; Zhang, X.; Gooding, J.J.; Ru, Y.; Liu, Y.; Zhou, Y. Light-Induced Hydrogel Based on Tumor-Targeting Mesoporous Silica Nanoparticles as a Theranostic Platform for Sustained Cancer Treatment. ACS Appl. Mater. Interfaces 2016, 8, 15857–15863. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel concept of the smart NIR-light–controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojugo, A.S.E.; McSheehy, P.M.J.; McIntyre, D.J.O.; McCoy, C.; Stubbs, M.; Leach, M.O.; Judson, I.R.; Griffiths, J.R. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: A comparison of exogenous19F and31P probes. NMR Biomed. 1999, 12, 495–504. [Google Scholar] [CrossRef]
- van Dyke, R.W. Acidification of Lysosomes and Endosomes; Springer: Boston, MA, USA, 1996; pp. 331–360. [Google Scholar]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef]
- Raza, F.; Zhu, Y.; Chen, L.; You, X.; Zhang, J.; Khan, A.; Khan, M.W.; Hasnat, M.; Zafar, H.; Wu, J.; et al. Paclitaxel-loaded pH responsive hydrogel based on self-assembled peptides for tumor targeting. Biomater. Sci. 2019, 7, 2023–2036. [Google Scholar] [CrossRef]
- Xu, C.; Yan, Y.; Tan, J.; Yang, D.; Jia, X.; Wang, L.; Xu, Y.; Cao, S.; Sun, S. Biodegradable Nanoparticles of Polyacrylic Acid–Stabilized Amorphous CaCO3 for Tunable pH-Responsive Drug Delivery and Enhanced Tumor Inhibition. Adv. Funct. Mater. 2019, 29, 1808146. [Google Scholar] [CrossRef]
- Ren, Y.; Li, X.; Han, B.; Zhao, N.; Mu, M.; Wang, C.; Du, Y.; Wang, Y.; Tong, A.; Liu, Y.; et al. Improved anti-colorectal carcinomatosis effect of tannic acid co-loaded with oxaliplatin in nanoparticles encapsulated in thermosensitive hydrogel. Eur. J. Pharm. Sci. 2019, 128, 279–289. [Google Scholar] [CrossRef]
- Makhmalzadeh, B.S.; Molavi, O.; Vakili, M.R.; Zhang, H.-F.; Solimani, A.; Abyaneh, H.S.; Loebenberg, R.; Lai, R.; Lavasanifar, A. Functionalized Caprolactone-Polyethylene Glycol Based Thermo-Responsive Hydrogels of Silibinin for the Treatment of Malignant Melanoma. J. Pharm. Pharm. Sci. 2018, 21, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Song, L.; Chen, L.; Zhang, W.; Chen, Y.; Zang, F.; Chen, H.; Ma, M.; Gu, N.; Zhang, Y. Injectable magnetic supramolecular hydrogel with magnetocaloric liquid-conformal property prevents post-operative recurrence in a breast cancer model. Acta Biomater. 2018, 74, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Park, M.H.; Jo, G.; Kim, S.Y.; Chun, H.J.; Yang, D.H. Photo-Cured Glycol Chitosan Hydrogel for Ovarian Cancer Drug Delivery. Mar. Drugs 2019, 17, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Wei, S.; Liu, L.; Qi, D.; Wang, J.; Chen, G.; He, W.; He, C.; Chen, X.; Gu, Z. Enhanced local cancer therapy using a CA4P and CDDP co-loaded polypeptide gel depot. Biomater. Sci. 2019, 7, 860–866. [Google Scholar] [CrossRef]
- Permana, A.D.; Utami, R.N.; Layadi, P.; Himawan, A.; Juniarti, N.; Anjani, Q.K.; Utomo, E.; Mardikasari, S.A.; Arjuna, A.; Donnelly, R.F. Thermosensitive and mucoadhesive in situ ocular gel for effective local delivery and antifungal activity of itraconazole nanocrystal in the treatment of fungal keratitis. Int. J. Pharm. 2021, 602, 120623. [Google Scholar] [CrossRef]
- Chaudhari, P.; Naik, R.; Mallela, L.S.; Roy, S.; Birangal, S.; Ghate, V.; Kunhanna, S.B.; Lewis, S.A. A supramolecular thermosensitive gel of ketoconazole for ocular applications: In silico, in vitro, and ex vivo studies. Int. J. Pharm. 2022, 613, 121409. [Google Scholar] [CrossRef]
- Alkholief, M.; Kalam, M.A.; Almomen, A.; Alshememry, A.; Alshamsan, A. Thermoresponsive sol-gel improves ocular bioavailability of Dipivefrin hydrochloride and potentially reduces the elevated intraocular pressure in vivo. Saudi Pharm. J. 2020, 28, 1019–1029. [Google Scholar] [CrossRef]
- Mahboobian, M.M.; Mohammadi, M.; Mansouri, Z. Development of thermosensitive in situ gel nanoemulsions for ocular delivery of acyclovir. J. Drug Deliv. Sci. Technol. 2020, 55, 101400. [Google Scholar] [CrossRef]
- Cucina, A.; Filali, S.; Risler, A.; Febvay, C.; Salmon, D.; Pivot, C.; Pelandakis, M.; Pirot, F. Dual 0.02% chlorhexidine digluconate—0.1% disodium EDTA loaded thermosensitive ocular gel for Acanthamoeba keratitis treatment. Int. J. Pharm. 2019, 556, 330–337. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, P.; Mariño, C.; Vázquez, J.A.; Caeiro-Rey, J.R.; Landin, M. Targeting joint inflammation for osteoarthritis management through stimulus-sensitive hyaluronic acid based intra-articular hydrogels. Mater. Sci. Eng. C 2021, 128, 112254. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, S.; Dou, H.; Liu, Q.; Shu, G.; Lin, J.; Zhang, W.; Peng, G.; Zhong, Z.; Fu, H. Novel glucosamine-loaded thermosensitive hydrogels based on poloxamers for osteoarthritis therapy by intra-articular injection. Mater. Sci. Eng. C 2021, 118, 111352. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.H.M.D.; Ambrosio, F.N.; Ferraraz, D.C.; Windisch-Neto, H.; Querobino, S.M.; Nascimento-Sales, M.; Alberto-Silva, C.; Christoffolete, M.A.; Franco, M.K.K.D.; Kent, B.; et al. Sulforaphane-loaded hyaluronic acid-poloxamer hybrid hydrogel enhances cartilage protection in osteoarthritis models. Mater. Sci. Eng. C 2021, 128, 112345. [Google Scholar] [CrossRef] [PubMed]
- GhavamiNejad, A.; Samarikhalaj, M.; Aguilar, L.E.; Park, C.H.; Kim, C.S. pH/NIR Light-Controlled Multidrug Release via a Mussel-Inspired Nanocomposite Hydrogel for Chemo-Photothermal Cancer Therapy. Sci. Rep. 2016, 6, 33594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, C.; Liu, C.; Hu, H.; Guo, X.-L.; Jiang, B.-P.; Liang, H.; Shen, X.-C. NIR-II light-modulated thermosensitive hydrogel for light-triggered cisplatin release and repeatable chemo-photothermal therapy. Chem. Sci. 2019, 10, 4699–4706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Zhang, H.; Wei, Y.; Wei, Y.; Xie, Y.; Cong, F. Reduction- and pH-Sensitive Hyaluronan Nanoparticles for Delivery of Iridium(III) Anticancer Drugs. Biomacromolecules 2017, 18, 2102–2117. [Google Scholar] [CrossRef] [PubMed]
- Malekimusavi, H.; Ghaemi, A.; Masoudi, G.; Chogan, F.; Rashedi, H.; Yazdian, F.; Omidi, M.; Javadi, S.; Haghiralsadat, B.F.; Teimouri, M.; et al. Graphene oxide- l -arginine nanogel: A pH-sensitive fluorouracil nanocarrier. Biotechnol. Appl. Biochem. 2019, 66, 772–780. [Google Scholar] [CrossRef]
- Zhang, F.; Gong, S.; Wu, J.; Li, H.; Oupicky, D.; Sun, M. CXCR4-Targeted and Redox Responsive Dextrin Nanogel for Metastatic Breast Cancer Therapy. Biomacromolecules 2017, 18, 1793–1802. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Han, X.; Liang, C.; Liu, J.; Song, X.; Ge, Z.; Liu, Z. Redox-Sensitive Nanoscale Coordination Polymers for Drug Delivery and Cancer Theranostics. ACS Appl. Mater. Interfaces 2017, 9, 23555–23563. [Google Scholar] [CrossRef]
- Kumar, P.; Wasim, L.; Chopra, M.; Chhikara, A. Co-delivery of Vorinostat and Etoposide Via Disulfide Cross-Linked Biodegradable Polymeric Nanogels: Synthesis, Characterization, Biodegradation, and Anticancer Activity. AAPS PharmSciTech 2018, 19, 634–647. [Google Scholar] [CrossRef]
- Park, H.; Choi, Y.; Jeena, M.T.; Ahn, E.; Choi, Y.; Kang, M.-G.; Lee, C.G.; Kwon, T.-H.; Rhee, H.-W.; Ryu, J.-H.; et al. Reduction-Triggered Self-Cross-Linked Hyperbranched Polyglycerol Nanogels for Intracellular Delivery of Drugs and Proteins. Macromol. Biosci. 2018, 18, 1700356. [Google Scholar] [CrossRef]
- Li, F.; Yang, H.; Bie, N.; Xu, Q.; Yong, T.; Wang, Q.; Gan, L.; Yang, X. Zwitterionic Temperature/Redox-Sensitive Nanogels for Near-Infrared Light-Triggered Synergistic Thermo-Chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 23564–23573. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xie, W.; Miao, Y.; Wang, D.; Guo, Z.; Ghosal, A.; Li, Y.; Wei, Y.; Feng, S.; Zhao, L.; et al. Magnetic Hydrogel with Optimally Adaptive Functions for Breast Cancer Recurrence Prevention. Adv. Healthc. Mater. 2019, 8, 1900203. [Google Scholar] [CrossRef] [PubMed]
- Parisi, O.I.; Morelli, C.; Scrivano, L.; Sinicropi, M.S.; Cesario, M.G.; Candamano, S.; Puoci, F.; Sisci, D. Controlled release of sunitinib in targeted cancer therapy: Smart magnetically responsive hydrogels as restricted access materials. RSC Adv. 2015, 5, 65308–65315. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Hao, J.; Dong, S. Magnetic Fullerene-DNA/Hyaluronic Acid Nanovehicles with Magnetism/Reduction Dual-Responsive Triggered Release. Biomacromolecules 2017, 18, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Aboali, F.A.; Habib, D.A.; Elbedaiwy, H.M.; Farid, R.M. Curcumin-loaded proniosomal gel as a biofreindly alternative for treatment of ocular inflammation: In-vitro and in-vivo assessment. Int. J. Pharm. 2020, 589, 119835. [Google Scholar] [CrossRef]
- Shukr, M.H.; Ismail, S.; El-Hossary, G.G.; El-Shazly, A.H. Design and evaluation of mucoadhesive in situ liposomal gel for sustained ocular delivery of travoprost using two steps factorial design. J. Drug Deliv. Sci. Technol. 2021, 61, 102333. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.S.M.; Dijkstra, P.J.; van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran–hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials 2010, 31, 3103–3113. [Google Scholar] [CrossRef]
- Wei, J.; Ran, P.; Li, Q.; Lu, J.; Zhao, L.; Liu, Y.; Li, X. Hierarchically structured injectable hydrogels with loaded cell spheroids for cartilage repairing and osteoarthritis treatment. Chem. Eng. J. 2022, 430, 132211. [Google Scholar] [CrossRef]
- Zhou, T.; Xiong, H.; Wang, S.Q.; Zhang, H.L.; Zheng, W.W.; Gou, Z.R.; Fan, C.Y.; Gao, C.Y. An injectable hydrogel dotted with dexamethasone acetate-encapsulated reactive oxygen species-scavenging micelles for combinatorial therapy of osteoarthritis. Mater. Today Nano 2022, 17, 100164. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Zhao, W.; Wang, H.; Sun, Y.; Chen, Y.; Luo, J.; Deng, L.; Xu, X.; Cui, W.; et al. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact. Mater. 2021, 6, 3596–3607. [Google Scholar] [CrossRef]
- Seo, B.-B.; Kwon, Y.; Kim, J.; Hong, K.H.; Kim, S.-E.; Song, H.-R.; Kim, Y.-M.; Song, S.-C. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact. Mater. 2022, 7, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wang, X.; Liao, J.; Shen, J.; Li, Y.; Cai, Z.; Hu, N.; Luo, X.; Cui, W.; Huang, W. Shear-responsive boundary-lubricated hydrogels attenuate osteoarthritis. Bioact. Mater. 2022, 16, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Ocular Inserts with Analyte Capture and Release Agents. U.S. Patent 20,210,338,211, 3 May 2021. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US340278458&_cid=P20-L2LQFZ-64472-1 (accessed on 30 April 2022).
- Drug Delivery from Hydrogels. JP Patent 2,021,120,395, 11 May 2021. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=JP334768825&_cid=P20-L2LQIS-64737-1 (accessed on 30 April 2022).
- Injectable Hydrogels for Cell Delivery to the Vitreous. WO Patent 2,021,113,515, 10 June 2020. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021113515&_cid=P20-L2LQN0-65347-1 (accessed on 30 April 2022).
- Polymer Formulations for Nasolacrimal Stimulation. U.S. Patent 20,210,069,496, 11 September 2020. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US319902595&_cid=P20-L2LQOA-65557-1 (accessed on 30 April 2022).
- A Method for Obtaining Ion-Exchange Polymeric Hydrogels for Eye Treatment and Hydrogel Lenses Thereof. WO Patent 2,021,038,279, 4 March 2021. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021038279&_cid=P20-L2LQQ0-65789-1 (accessed on 30 April 2022).
- An Etoricoxib Loaded Nanosponge Hydrogel for Arthritis & Process to Prepare Thereof. IN Patent 202,121,042,889, 22 September 2021. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=IN340501612&_cid=P20-L2LQRJ-65914-1 (accessed on 30 April 2022).
- Protein Hydrogel for Topical Application and Formulation for Skin Regeneration/Wound Healing. IN Patent 202,031,000,910, 8 January 2020. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=IN334769630&_cid=P20-L2LQSP-66090-1 (accessed on 30 April 2022).
- Preparation Method of Conductive Adhesive Hydrogel. CN Patent 112,442,194, 4 September 2019. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=CN320288668&_cid=P20-L2LQU0-66236-1 (accessed on 30 April 2022).
- Thrombin-Responsive Hydrogels and Devices for Auto-Anticoagulant Regulation. U.S. Patent 20,210,023,121, 14 September 2020. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US316414241&_cid=P20-L2LQV8-66462-1 (accessed on 30 April 2022).
- Thermosensitive Hydrogel for Cancer Therapeutics and Methods of Preparation Thereof. U.S. Patent 20,210,393,780, 20 May 2021. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US345561289&_cid=P20-L2LQWJ-66715-1 (accessed on 30 April 2022).
- Tunable Extended Release Hydrogels. WO Patent 2,021,174,021, 2 September 2021. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2021174021&_cid=P20-L2LQXW-66853-1 (accessed on 30 April 2022).
- Novel Self-Assembling Peptides and Their Use in the Formaiion of Hydrogels. IN Patent 9758/CHENP/2012, 19 November 2012. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=IN211695000&_cid=P20-L2LQZK-67034-1 (accessed on 30 April 2022).
- Synthetic Diblock Copolypeptide Hydrogels for Use in the Central Nervous System. U.S. Patent 20,140,286,865, 18 February 2014. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US123273430&_cid=P20-L2LR0O-67168-1 (accessed on 30 April 2022).


| Sno. | Types of Hydrogels | Composition | Drug Used | Disease | References |
|---|---|---|---|---|---|
| 1 | Thermo-stimuli Hydrogel | 7-ethyl-10-hydroxycamptothecin (SN-38) liposomal hydrogel | SN-38 | Hepatocellular carcinoma | [94] |
| 2 | alginate nanogel co-loaded with cisplatin and gold nanoparticles | cisplatin | colorectal cancer | [95] | |
| 3 | poly(γ-ethyl-L-glutamate)-poly(ethylene glycol)-poly(γ-ethyl-L-glutamate) (PELG-PEG-PELG) hydrogel | DOX/IL-2/IFN-γ | melanoma | [98] | |
| 4 | PHB-b-PDMAEMA | paclitaxel and Temozolomide | glioblastoma | [99] | |
| 5 | poly(3-caprolactone) (PCL)-10R5-PCL (PCLR) hydrogel | tannic acid/ oxaliplatin | colorectal cancer | [117] | |
| 6 | Caprolactone-Polyethylene Glycol | Silibinin | melanoma | [118] | |
| 7 | α-Cyclodextrin co-polymeric PEGylated iron oxide-based hydrogels | PTX/DOX | breast cancer | [119] | |
| 8 | β- cyclodextrin complexed glycol chitosan hydrogel | PTX | Ovarian Cancer | [120] | |
| 9 | mPEG-b-PELG | CA4P and cisplatin | colorectal cancer | [121] | |
| 10 | Pluronic F127, Pluronic F68, and Hydroxy Propyl Methyl Cellulose. | Itraconazole | Fungal Keratitis | [122] | |
| 11 | Sulfobutylether-β-cyclodextrin (SBE-β-CD) | Ketoconazole | Fungal Keratitis | [123] | |
| 12 | Poloxamers (P407 and P188), Carbopol-93 | Dipivefrin hydrochloride | Intraocular pressure | [124] | |
| 13 | Triacetin, Transcutol-P, Poloxamer 407, Poloxamer188 | Acyclovir | Ocular viral infections | [125] | |
| 14 | Poloxamer 407, disodium EDTA | Chlorhexidine digluconate | Acanthamoeba keratitis | [126] | |
| 15 | poloxamers, hyaluronic acid (HA), beta-lapachone (β Lap), | beta-lapachone (β Lap) | Restoring the synovial fluid | [127] | |
| 16 | Poloxamers, D—(+)-GlcN hydrochloride, papain, | Glucosamine (GlcN) | controlling inflammation and promoting cartilage re-generation | [128] | |
| 17 | Poloxamer, hyaluronic | Sulforaphane (SFN | SFN intra-articular release for OA treatment | [129] | |
| 18 | Photosensitive Hydrogels | azobenzene and α-cyclodextrin-functionalized hyaluronic acid with gold nanobipyramids | DOX | human epidermal keratinocyte | [110] |
| 19 | poly(N-isopropylacrylamide) hydrogel | Bortezomib and DOX | osteoblast | [130] | |
| 20 | Agarose based hydrogel | black phosphorus and DOX | breast cancer | [111] | |
| 21 | poly(N-phenylglycine)- poly (ethylene glycol) co-polymeric hydrogel | Cisplatin | breast cancer | [131] | |
| 23 | pH-stimuli Hydrogels | poly (acrylic acid) complexed with stabilized amorphous calcium carbonate | DOX | hepatocarcinoma | [116] |
| 24 | amphiphilic hyaluronan (HA)-and cystamin-pyrenyl | Organoiridium (III) | lung cancer | [132] | |
| 25 | Graphene oxide, L-arginine | 5-fluorouracil | breast cancer | [133] | |
| 26 | FER-8 peptide | PTX | hepatocarcinoma | [115] | |
| 27 | Dibenzaldehyde, poly (ethylene glycol) | DOX | hepatocarcinoma | [50] | |
| 28 | Redox- stimuli Hydrogels | dextrin nanogel | DOX | Breast Cancer | [134] |
| 29 | Polydopamine, poly (ethylene glycol) | DOX | breast cancer | [135] | |
| 30 | poly (ethylene glycol) monomethacrylate | Vorinostat and etoposide | cervical cancer | [136] | |
| 31 | polyglycerol nanogel | DOX | cervical cancer | [137] | |
| 33 | N-Isopropylacrylamide, Methacrylic acid, Benzalkonium chloride and poly (sulfobetaine methacrylate) | DOX and Indocyanine green | hepatocarcinoma | [138] | |
| 34 | Magnetism-Responsive Hydrogels | ferromagnetic vortex-domain iron oxide, chitosan and poly (ethylene glycol) | DOX | breast cancer | [139] |
| 35 | methacrylic acid, ethylene glycol dimethacrylate, 2,2′-azobisisobutyronitrile and glycidyl methacrylate | sunitinib | cervical cancer, breast cancer and Human Thyroid Tumor | [140] | |
| 36 | paramagnetic fullerene, DNA and Hyaluronic Acid | DOX | hepatocarcinoma | [141] | |
| 37 | Proniosomal gel | Surfactant, lecithin and cholesterol | Curcumin | Ocular Inflammation | [142] |
| 38 | Liposomal gel | Lecithin: cholesterol, Carbopol 934 | Travoprost | Glaucoma and ocular hypertension | [143] |
| 39 | Injectable hydrogel | horseradish peroxidase (HRP) and H2O, chitosan, hyaluronic acid (HA) | Dextrane Tyramine | cartilage tissue regeneration | [144] |
| 40 | bone marrow mesenchymal stem cell (MSC) spheroids, short fibre fillers, Kartogenin (KGN) | Celecoxib | cartilage regeneration, and inflammation removal | [145] | |
| 41 | poly (ethylene glycol)-b-polythioketal-b-poly(ethylene glycol), micelles | dexamethasone acetate | preventing cartilage extracellular matrix degeneration | [146] | |
| 42 | Gelatin, ulbecco’s phosphate buffered saline (DPBS), methacrylic anhydride. | diclofenac sodium | preventing the development of degenerative changes in OA via the synergistical treatment of enhanced lubrication (COF reduction) and sustained drug release (inflammation down-regulation) | [147] | |
| 43 | Hexachlorocyclotriphosphazene, Poly (dichlorophosphazene, Methoxy poly (ethylene glycol), | Triamcinolone acetonide | Effective prevention and long-term anti-OA treatment | [148] | |
| 44 | Shear—sensitive hydrogels | Hyaluronic acid, aldehyde groups, amino Groups, HSPC lipid, | Celecoxib | Minimizing shear-induced cartilage damage and inflammation | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sastri, T.K.; Gupta, V.N.; Chakraborty, S.; Madhusudhan, S.; Kumar, H.; Chand, P.; Jain, V.; Veeranna, B.; Gowda, D.V. Novel Gels: An Emerging Approach for Delivering of Therapeutic Molecules and Recent Trends. Gels 2022, 8, 316. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050316
Sastri TK, Gupta VN, Chakraborty S, Madhusudhan S, Kumar H, Chand P, Jain V, Veeranna B, Gowda DV. Novel Gels: An Emerging Approach for Delivering of Therapeutic Molecules and Recent Trends. Gels. 2022; 8(5):316. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050316
Chicago/Turabian StyleSastri, Trideva K., Vishal N. Gupta, Souvik Chakraborty, Sharadha Madhusudhan, Hitesh Kumar, Pallavi Chand, Vikas Jain, Balamuralidhara Veeranna, and Devegowda V. Gowda. 2022. "Novel Gels: An Emerging Approach for Delivering of Therapeutic Molecules and Recent Trends" Gels 8, no. 5: 316. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050316