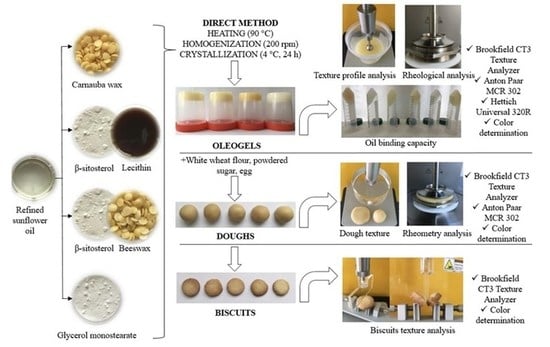
Evaluation of Structural Behavior in the Process Dynamics of Oleogel-Based Tender Dough Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. Oleogel Characterization
2.1.1. Textural Profile Analysis (TPA)
2.1.2. Oil Binding Capacity
2.1.3. Oleogel Rheology
2.1.4. Color Determination of Oleogel Samples
| Parameters | OG_GM | OG_CRW | OG_BS:BW | OG_BS:LEC | MR |
|---|---|---|---|---|---|
| Texture profile analysis | |||||
| Hardness (N) | 2.46 B ± 0.25 | 2.54 B ± 0.63 | 6.37 A ± 0.18 | 2.93 B ± 0.25 | 3.58 B ± 0.32 |
| Adhesiveness (mJ) | 0.95 AB ± 0.49 | 0.55 B ± 0.21 | 1.20 AB ± 0.28 | 1.55 AB ± 0.49 | 2.10 A ± 0.00 |
| Cohesiveness | 0.08 B ± 0.01 | 0.12 B ± 0.01 | 0.05 B ± 0.03 | 0.21 B ± 0.12 | 0.59 A ± 0.00 |
| Color | |||||
| L* | 77.16 AB ± 5.47 | 75.70 AB ± 4.92 | 79.28 AB ± 3.50 | 73.16 B ± 2.47 | 84.18 A ± 2.66 |
| a* | 22.09 B ± 3.20 | 24.74 B ± 3.66 | 26.32 AB ± 5.65 | 32.85 A ± 2.97 | 11.66 C ± 2.09 |
| b* | 21.23 C ± 2.30 | 31.13 AB ± 1.87 | 30.37 B ± 1.65 | 33.91 A ± 0.64 | 19.15 C ± 0.81 |
| ΔE | 12.74 | 19.66 | 19.10 | 28.08 | - |
| Oil binding capacity | |||||
| Oil loss (%) | 2.53 A ± 0.36 | 3.15 A ± 0.37 | 0.05 A ± 0.00 | 2.77 A ± 1.55 | - |
2.2. Dough Texture, Rheometric Analysis, and Color Determination
2.3. Biscuits Texture Analysis and Color Determination
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Oleogel Preparation
4.3. Oleogel Characterization
4.3.1. Texture Profile Analysis (TPA)
4.3.2. Oil Binding Capacity (OBC)
4.3.3. Oleogel Rheology
4.4. Applicability of Oleogels in Tender Dough Products
4.4.1. Dough and Biscuits Preparation
4.4.2. Dough Texture Analysis
4.4.3. Dynamic Viscoelastic Measurement of Dough
4.4.4. Biscuits Texture Analysis
4.5. Color Determination
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bascuas, S.; Morell, P.; Hernando, I.; Quiles, A. Recent trends in oil structuring using hydrocolloids. Food Hydrocoll. 2021, 118, 106612. [Google Scholar] [CrossRef]
- Hwang, H.-S. A critical review on structures, health effects, oxidative stability, and sensory properties of oleogels. Biocatal. Agric. Biotechnol. 2020, 26, 101657. [Google Scholar] [CrossRef]
- Naeli, M.H.; Milani, J.M.; Farmani, J.; Zargaraan, A. Development of innovative ethyl cellulose-hydroxypropyl methylcellulose biopolymer oleogels as low saturation fat replacers: Physical, rheological and microstructural characteristics. Int. J. Biol. Macromol. 2020, 156, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the European Union. COMMISSION REGULATION (EU) 2019/649 of 24 April 2019 Amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as Regards Trans Fat, Other than Trans Fat Naturally Occurring in Fat of Animal Origin; 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R0649 (accessed on 13 May 2022).
- Guadalupe, G.A.; Lerma-García, M.J.; Fuentes, A.; Barat, J.M.; Bas, M.d.C.; Fernández-Segovia, I. Presence of palm oil in foodstuffs: Consumers’ perception. Br. Food J. 2019, 121, 2148–2162. [Google Scholar] [CrossRef]
- Mba, O.I.; Dumont, M.-J.; Ngadi, M. Palm oil: Processing, characterization and utilization in the food industry—A review. Food Biosci. 2015, 10, 26–41. [Google Scholar] [CrossRef]
- Zbikowska, A.; Onacik-Gur, S.; Kowalska, M.; Rutkowska, J. trans Fatty Acids in Polish Pastry. J. Food Prot. 2019, 82, 1028–1033. [Google Scholar] [CrossRef]
- Reddy, S.Y.; Jeyarani, T. Trans-free bakery shortenings from mango kernel and mahua fats by fractionation and blending. J. Am. Oil Chem. Soc. 2001, 78, 635–640. [Google Scholar] [CrossRef]
- Rios, R.V.; Pessanha, M.D.F.; Almeida, P.F.d.; Viana, C.L.; Lannes, S.C.d.S. Application of fats in some food products. Food Sci. Technol. 2014, 34, 3–15. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, R.D. Shortenings: Types and Formulations; Bailey’s Industrial Oil and Fat Products: Hoboken, NJ, USA, 2005. [Google Scholar]
- Muresan, V. Oleogelifierea—Tehnologii Disponibile și Aplicabilitate în Produse Alimentare; Editura MEGA: Cluj-Napoca, Romania, 2019; ISBN 978-606-020-098-7. [Google Scholar]
- Food and Drug Administration. Direct Food Substances Affirmed as Generally Recognized as Safe. Listing of Specific Substances Affirmed as GRAS. Carnauba Wax. 2022. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1978 (accessed on 13 May 2022).
- Food and Drug Administration. Direct Food Substances Affirmed as Generally Recognized as Safe. Listing of Specific Substances Affirmed as GRAS. Glyceryl Monostearate. 2022. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=184.1324 (accessed on 13 May 2022).
- EFSA. Beeswax (E 901) as a Glazing Agent and as Carrier for—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC); 2007. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/615 (accessed on 13 May 2022).
- Zhao, M.; Lan, Y.; Cui, L.; Monono, E.; Rao, J.; Chen, B. Formation, characterization, and potential food application of rice bran wax oleogels: Expeller-pressed corn germ oil versus refined corn oil. Food Chem. 2020, 309, 125704. [Google Scholar] [CrossRef]
- Mert, B.; Demirkesen, I. Reducing saturated fat with oleogel/shortening blends in a baked product. Food Chem. 2016, 199, 809–816. [Google Scholar] [CrossRef]
- Jang, A.; Bae, W.; Hwang, H.S.; Lee, H.G.; Lee, S. Evaluation of canola oil oleogels with candelilla wax as an alternative to shortening in baked goods. Food Chem. 2015, 187, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.K.; Amoah, C.; Lim, J.; Jeong, S.; Lee, S. Assessing the effectiveness of wax-based sunflower oil oleogels in cakes as a shortening replacer. LWT 2017, 86, 430–437. [Google Scholar] [CrossRef]
- Yilmaz, E.; Ogutcu, M. The texture, sensory properties and stability of cookies prepared with wax oleogels. Food Funct. 2015, 6, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.K.; Lee, S. Utilization of foam structured hydroxypropyl methylcellulose for oleogels and their application as a solid fat replacer in muffins. Food Hydrocoll. 2018, 77, 796–802. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lim, J.; Lee, J.; Hwang, H.S.; Lee, S. Utilization of Oleogels as a Replacement for Solid Fat in Aerated Baked Goods: Physicochemical, Rheological, and Tomographic Characterization. J. Food Sci. 2017, 82, 445–452. [Google Scholar] [CrossRef]
- Limpimwong, W.; Kumrungsee, T.; Kato, N.; Yanaka, N.; Thongngam, M. Rice bran wax oleogel: A potential margarine replacement and its digestibility effect in rats fed a high-fat diet. J. Funct. Foods 2017, 39, 250–256. [Google Scholar] [CrossRef]
- Pehlivanoglu, H.; Demirci, M.; Toker, O.S. Rheological properties of wax oleogels rich in high oleic acid. Int. J. Food Prop. 2018, 20, S2856–S2867. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Li, L.; Li, B.; Zhao, L.; Liu, G.-q.; Liu, X.; Wang, X. Structure and Physical Properties of Organogels Developed by Sitosterol and Lecithin with Sunflower Oil. J. Am. Oil Chem. Soc. 2014, 91, 1783–1792. [Google Scholar] [CrossRef]
- Kupiec, M.; Zbikowska, A.; Marciniak-Lukasiak, K.; Kowalska, M. Rapeseed Oil in New Application: Assessment of Structure of Oleogels Based on their Physicochemical Properties and Microscopic Observations. Agriculture 2020, 10, 211. [Google Scholar] [CrossRef]
- Pandolsook, S.; Kupongsak, S. Influence of bleached rice bran wax on the physicochemical properties of organogels and water-in-oil emulsions. J. Food Eng. 2017, 214, 182–192. [Google Scholar] [CrossRef]
- Ye, X.; Li, P.; Lo, Y.M.; Fu, H.; Cao, Y. Development of Novel Shortenings Structured by Ethylcellulose Oleogels. J. Food Sci. 2019, 84, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Hwang, H.-S.; Lee, S. Oil-structuring characterization of natural waxes in canola oil oleogels: Rheological, thermal, and oxidative properties. Appl. Biol. Chem. 2016, 60, 17–22. [Google Scholar] [CrossRef]
- Okuro, P.K.; Tavernier, I.; Bin Sintang, M.D.; Skirtach, A.G.; Vicente, A.A.; Dewettinck, K.; Cunha, R.L. Synergistic interactions between lecithin and fruit wax in oleogel formation. Food Funct. 2018, 9, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.D.; Patel, A.R.; Tavernier, I.; De Clercq, N.; Van Raemdonck, K.; Van de Walle, D.; Delbaere, C.; Dewettinck, K. The feasibility of wax-based oleogel as a potential co-structurant with palm oil in low-saturated fat confectionery fillings. Eur. J. Lipid Sci. Technol. 2016, 118, 1903–1914. [Google Scholar] [CrossRef]
- Sun, H.; Xu, J.; Lu, X.; Xu, Y.; Regenstein, J.M.; Zhang, Y.; Wang, F. Development and characterization of monoglyceride oleogels prepared with crude and refined walnut oil. LWT 2022, 154, 112769. [Google Scholar] [CrossRef]
- Yang, S.; Yang, G.; Chen, X.; Chen, J.; Liu, W. Interaction of monopalmitate and carnauba wax on the properties and crystallization behavior of soybean oleogel. Grain Oil Sci. Technol. 2020, 3, 49–56. [Google Scholar] [CrossRef]
- Thakur, D.; Singh, A.; Prabhakar, P.K.; Meghwal, M.; Upadhyay, A. Optimization and characterization of soybean oil-carnauba wax oleogel. LWT 2022, 157, 113108. [Google Scholar] [CrossRef]
- Kaushik, I. Organogelation: It’s Food Application. MOJ Food Process. Technol. 2017, 4, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Wijarnprecha, K.; Aryusuk, K.; Santiwattana, P.; Sonwai, S.; Rousseau, D. Structure and rheology of oleogels made from rice bran wax and rice bran oil. Food Res. Int. 2018, 112, 199–208. [Google Scholar] [CrossRef]
- Martins, A.J.; Cerqueira, M.A.; Cunha, R.L.; Vicente, A.A. Fortified beeswax oleogels: Effect of beta-carotene on the gel structure and oxidative stability. Food Funct. 2017, 8, 4241–4250. [Google Scholar] [CrossRef]
- Paciulli, M.; Littardi, P.; Carini, E.; Paradiso, V.M.; Castellino, M.; Chiavaro, E. Inulin-based emulsion filled gel as fat replacer in shortbread cookies: Effects during storage. LWT 2020, 133, 109888. [Google Scholar] [CrossRef]
- Jung, D.; Oh, I.; Lee, J.; Lee, S. Utilization of butter and oleogel blends in sweet pan bread for saturated fat reduction: Dough rheology and baking performance. LWT 2020, 125, 109194. [Google Scholar] [CrossRef]
- Li, S.; Wu, G.; Li, X.; Jin, Q.; Wang, X.; Zhang, H. Roles of gelator type and gelation technology on texture and sensory properties of cookies prepared with oleogels. Food Chem. 2021, 356, 129667. [Google Scholar] [CrossRef] [PubMed]
- Jan, K.N.; Panesar, P.S.; Singh, S. Optimization of antioxidant activity, textural and sensory characteristics of gluten-free cookies made from whole indian quinoa flour. LWT 2018, 93, 573–582. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, P.; Kumar, V.; Panghal, A.; Kaur, J.; Gat, Y. Effect of addition of flaxseed flour on phytochemical, physicochemical, nutritional, and textural properties of cookies. J. Saudi Soc. Agric. Sci. 2019, 18, 372–377. [Google Scholar] [CrossRef]
- Onacik-Gur, S.; Zbikowska, A. Effect of high-oleic rapeseed oil oleogels on the quality of short-dough biscuits and fat migration. J. Food Sci. Technol. 2020, 57, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, L.; Wu, G.; Jin, Q.; Wang, X.; Zhang, H. Relationship between the microstructure and physical properties of emulsifier based oleogels and cookies quality. Food Chem. 2022, 377, 131966. [Google Scholar] [CrossRef] [PubMed]
- Keskin Uslu, E.; Yılmaz, E. Preparation and characterization of glycerol monostearate and polyglycerol stearate oleogels with selected amphiphiles. Food Struct. 2021, 28, 100192. [Google Scholar] [CrossRef]
- Pang, M.; Wang, X.; Cao, L.; Shi, Z.; Lei, Z.; Jiang, S. Structure and thermal properties of β-sitosterol-beeswax-sunflower oleogels. Int. J. Food Sci. Technol. 2019, 55, 1900–1908. [Google Scholar] [CrossRef]
- Wan, W.B.; Han, L.J.; Liu, G.Q.; Liu, X.Q. Effect of Storage Conditions on Apparent Viscosity of Oleogel Developed by β-Sitosterol and Lecithin with Sunflower Oil. Adv. Mater. Res. 2014, 1004–1005, 903–907. [Google Scholar] [CrossRef]
- Hwang, H.S.; Singh, M.; Lee, S. Properties of Cookies Made with Natural Wax-Vegetable Oil Organogels. J. Food Sci. 2016, 81, C1045–C1054. [Google Scholar] [CrossRef] [PubMed]
- Mert, B.; Demirkesen, I. Evaluation of highly unsaturated oleogels as shortening replacer in a short dough product. LWT-Food Sci. Technol. 2016, 68, 477–484. [Google Scholar] [CrossRef]

 OG_CRW,
OG_CRW,  OG_BS:BW,
OG_BS:BW,  OG_BS:LEC,
OG_BS:LEC,  OG_GM. (b) Frequency sweep at a strain of 0.01% and 20 °C.
OG_GM. (b) Frequency sweep at a strain of 0.01% and 20 °C.  OG_CRW,
OG_CRW,  OG_BS:BW,
OG_BS:BW,  OG_BS:LEC,
OG_BS:LEC,  OG_GM.
OG_GM.
 OG_CRW,
OG_CRW,  OG_BS:BW,
OG_BS:BW,  OG_BS:LEC,
OG_BS:LEC,  OG_GM. (b) Frequency sweep at a strain of 0.01% and 20 °C.
OG_GM. (b) Frequency sweep at a strain of 0.01% and 20 °C.  OG_CRW,
OG_CRW,  OG_BS:BW,
OG_BS:BW,  OG_BS:LEC,
OG_BS:LEC,  OG_GM.
OG_GM.
 D_CRW,
D_CRW,  D_BS:BW,
D_BS:BW,  D_BS:LEC,
D_BS:LEC,  D_GM,
D_GM,  D_MR. (b) Changes in the dynamic viscoelastic properties of oleogel and margarine doughs: tan δ.
D_MR. (b) Changes in the dynamic viscoelastic properties of oleogel and margarine doughs: tan δ.  D_CRW,
D_CRW,  D_BS:BW,
D_BS:BW,  D_BS:LEC,
D_BS:LEC,  D_GM,
D_GM,  D_MR.
D_MR.
 D_CRW,
D_CRW,  D_BS:BW,
D_BS:BW,  D_BS:LEC,
D_BS:LEC,  D_GM,
D_GM,  D_MR. (b) Changes in the dynamic viscoelastic properties of oleogel and margarine doughs: tan δ.
D_MR. (b) Changes in the dynamic viscoelastic properties of oleogel and margarine doughs: tan δ.  D_CRW,
D_CRW,  D_BS:BW,
D_BS:BW,  D_BS:LEC,
D_BS:LEC,  D_GM,
D_GM,  D_MR.
D_MR.
| Parameters | D_GM | D_CRW | D_BS:BW | D_BS:LEC | D_MR |
|---|---|---|---|---|---|
| Texture profile analysis | |||||
| Hardness (N) | 41.84 B ± 2.41 | 92.49 A ± 10.40 | 29.27 BC ± 1.22 | 31.63 BC ± 2.12 | 21.80 C ± 1.41 |
| Adhesiveness (mJ) | 3.55 A ± 1.06 | 3.90 A ± 0.98 | 4.35 A ± 0.49 | 2.05 A ± 0.35 | 4.90 A ± 1.27 |
| Resilience | 0.01 A ± 0.00 | 0.02 A ± 0.01 | 0.01 A ± 0.00 | 0.01 A ± 0.00 | 0.01 A ± 0.00 |
| Cohesiveness | 0.08 B ± 0.01 | 0.07 B ± 0.02 | 0.10 AB ± 0.01 | 0.08 B ± 0.01 | 0.27 A ± 0.10 |
| Springiness index | 0.11 A ± 0.02 | 0.12 A ± 0.07 | 0.11 A ± 0.01 | 0.11 A ± 0.01 | 0.23 A ± 0.07 |
| Color | |||||
| L* | 66.04 A ± 1.39 | 58.03 B ± 3.15 | 65.43 A ± 3.31 | 70.28 A ± 0.91 | 68.01 A ± 2.56 |
| a* | 6.98 A ± 0.51 | 6.73 AB ± 0.70 | 6.12 AB ± 0.35 | 6.75 AB ± 0.58 | 5.70 B ± 0.19 |
| b* | 22.11 BC ± 0.50 | 20.89 C ± 0.72 | 20.89 C ± 0.57 | 22.87 AB ± 0.62 | 24.22 A ± 0.74 |
| ΔE | 3.16 | 10.57 | 4.23 | 2.84 | - |
| Parameters | B_GM | B_CRW | B_BS:BW | B_BS:LEC | B_MR |
|---|---|---|---|---|---|
| Texture profile analysis | |||||
| Hardness (N) | 20.97 B ± 0.39 | 13.20 CD ± 0.40 | 11.22 D ± 0.37 | 14.05 C ± 0.16 | 28.74 A ± 1.18 |
| Fracturability (N) | 20.97 B ± 0.39 | 3.87 E ± 0.88 | 11.22 D ± 0.37 | 14.05 C ± 0.16 | 28.74 A ± 1.18 |
| Color | |||||
| L* | 68.21 A ± 2.35 | 68.99 A ± 4.38 | 68.93 A ± 3.31 | 69.50 A ± 1.98 | 73.62 A ± 1.72 |
| a* | 13.40 BC ± 2.00 | 17.30 AB ± 3.41 | 18.18 A ± 1.94 | 16.12 ABC ± 1.42 | 12.50 C ± 1.10 |
| b* | 25.85 B ± 1.20 | 27.35 AB ± 2.33 | 29.53 A ± 0.62 | 29.08 A ± 0.61 | 28.77 A ± 0.85 |
| ΔE | 6.21 | 6.82 | 7.41 | 5.49 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanislav, A.E.; Pușcaș, A.; Păucean, A.; Mureșan, A.E.; Semeniuc, C.A.; Mureșan, V.; Mudura, E. Evaluation of Structural Behavior in the Process Dynamics of Oleogel-Based Tender Dough Products. Gels 2022, 8, 317. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050317
Tanislav AE, Pușcaș A, Păucean A, Mureșan AE, Semeniuc CA, Mureșan V, Mudura E. Evaluation of Structural Behavior in the Process Dynamics of Oleogel-Based Tender Dough Products. Gels. 2022; 8(5):317. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050317
Chicago/Turabian StyleTanislav, Anda E., Andreea Pușcaș, Adriana Păucean, Andruța E. Mureșan, Cristina A. Semeniuc, Vlad Mureșan, and Elena Mudura. 2022. "Evaluation of Structural Behavior in the Process Dynamics of Oleogel-Based Tender Dough Products" Gels 8, no. 5: 317. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050317