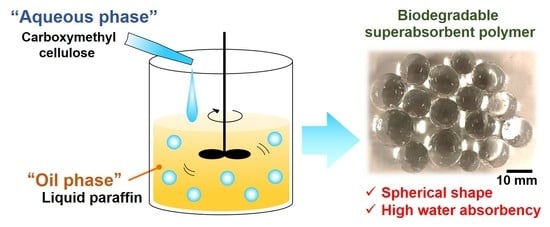
Preparation and Enzyme Degradability of Spherical and Water-Absorbent Gels from Sodium Carboxymethyl Cellulose
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of the Spherical CMCG
2.1.1. Optimization of the Initial CMC Concentration
2.1.2. Effect of the Feed Amount of the Crosslinking Agent
2.2. Water-Holding Capacity
2.3. Cellulase Degradability
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of CMCG
4.3. Water Absorbency
4.4. Determination of Particle Size
4.5. Water-Holding Capacity
4.6. Cellulase Degradation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omidian, H.; Hashemi, S.A.; Sammes, P.G.; Meldrum, I. A model for the swelling of superabsorbent polymers. Polymer 1998, 39, 6697–6704. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Li, A.; Wang, A. Preparation, swelling behaviors, and slow-release properties of a poly(acrylic acid-co-acrylamide)/sodium humate superabsorbent composite. Ind. Eng. Chem. Res. 2006, 45, 48–53. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Raffa, P.; Picchioni, F.; Koning, C.E. Superabsorbent polymers: From long-established, microplastics generating systems, to sustainable, biodegradable and future proof alternatives. Prog. Polym. Sci. 2022, 125, 101475. [Google Scholar] [CrossRef]
- Kono, H.; Fujita, S.; Oeda, I. Comparative study of homogeneous solvents for the esterification crosslinking of cellulose with 1,2,3,4-butanetetracarboxylic dianhydride and water absorbency of the reaction products. J. Appl. Polym. Sci. 2012, 5, 478–486. [Google Scholar] [CrossRef]
- Liu, T.G.; Wang, Y.T.; Li, B.; Deng, H.B.; Huang, Z.L.; Qian, L.W.; Wang, X. Urea free synthesis of chitin-based acrylate superabsorbent polymers under homogeneous conditions: Effects of the degree of deacetylation and the molecular weight. Carbohydr. Polym. 2017, 174, 464–473. [Google Scholar] [CrossRef]
- Kono, H.; Oeda, I.; Nakamura, T. The preparation, swelling characteristics, and albumin adsorption and release behaviors of a novel chitosan-based polyampholyte hydrogel. React. Funct. Polym. 2013, 73, 97–107. [Google Scholar] [CrossRef]
- Sami, A.J.; Khalid, M.; Jamil, T.; Aftab, S.; Mangat, S.A.; Shakoori, A.R.; Iqbal, S. Formulation of novel chitosan guargum based hydrogels for sustained drug release of paracetamol. Int. J. Biol. Macromol. 2018, 108, 324–332. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; El-Wahab, H.A.; Ismail, M.A.; Naser, A.M.; Abdelhai, F.; El-Damhougy, B.K.; Nady, N.; Meganid, A.S.; Alkhursani, S.A. Characterization of starch-based three components of gamma-ray cross-linked hydrogels to be used as a soil conditioner. Mater. Sci. Eng. B 2020, 260, 114645. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, G.; Kumar, A.; AlGani, T.S.; Naushad, M.; Alothman, Z.A.; Stadler, F. Adsorption of cationic dyes onto carrageenan and itaconic acid-based superabsorbent hydrogel: Synthesis, characterization and isotherm analysis. J. Hazard. Mater. 2022, 421, 126729. [Google Scholar] [CrossRef]
- Kono, H. Carboxymethyl cellulose-based hydrogels. In Cellulose and Cellulose Derivatives: Synthesis, Modification and Applications, 1st ed.; Mondal, M.I.H., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2015; pp. 243–258. ISBN 9781634831277. [Google Scholar]
- Jeoung, D.; Joo, S.W.; Hu, Y.; Shinde, V.V.; Cho, E.; Jung, S. Carboxymethyl cellulose-based superabsorbent hydrogels containing carboxymehtyl β-cyclodextrin for enhanced mechanical strength and effective drug delivery. Eur. Polym. J. 2018, 105, 17–25. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, X.; Zhen, J.; Lei, Z. Preparation of superabsorbent resin with fast water absorption rate based on hydroxymethyl cellulose sodium and its application. Carbohydr. Polym. 2019, 225, 115214. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, Y.; Liu, L.; Yao, J. Synthesis and characterization of a novel cellulose-g-poly(acrylic acid-co-acrylamide) superabsorbent composite based on flax yarn waste. Carbohydr. Polym. 2012, 87, 2519–2525. [Google Scholar] [CrossRef]
- Lim, D.-W.; Song, K.-G.; Yoon, K.-J.; Ko, S.-W. Synthesis of acrylic acid-based superabsorbent interpenetrated with sodium PVA sulfate using inverse-emulsion polymerization. Eur. Polym. J. 2002, 38, 579–586. [Google Scholar] [CrossRef]
- Benda, D.; Šňupárek, J.; Čermák, V. Inverse emulsion polymerization of acrylamide and salts of acrylic acid. Eur. Polym. J. 1997, 33, 1345–1352. [Google Scholar] [CrossRef]
- Calvo, P.; Remon-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Afshar, M.; Dini, G.; Vaezifar, S.; Mehdikhani, M.; Movahedi, B. Preparation and characterization of sodium alginate/polyvinyl alcohol hydrogel containing drug-loaded chitosan nanoparticles as a drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 56, 101530. [Google Scholar] [CrossRef]
- Mousaviasl, S.; Saleh, T.; Shojaosadati, S.A.; Boddohi, S. Synthesis and characterization of schizophyllan nanogels via inverse emulsion using biobased materials. Int. J. Biol. Macromol. 2018, 120, 468–474. [Google Scholar] [CrossRef]
- Milašinović, N.; Čalijac, B.; Vidović, B.; Sakač, M.C.; Vujić, Z.; Knežević-Jugović, Z. Sustained release of α-lipoic acid from chitosan microbeads synthetized by inverse emulsion method. J. Taiwan Inst. Chem. Eng. 2016, 60, 106–112. [Google Scholar] [CrossRef]
- Riegger, B.R.; Bäurer, B.; Mirzayeva, A.; Tovar, G.E.M.; Bach, M. A systematic approach of chitosan nanoparticle preparation via emulsion crosslinking as potential adsorbent in wastewater treatment. Carbohydr. Polym. 2018, 180, 46–54. [Google Scholar] [CrossRef]
- Yaghoobi, M.; Zaheri, P.; Mousavi, S.H.; Ardehali, B.A.; Yousefi, T. Evaluation of mean diameter and drop size distribution of an emulsion liquid membrane system in a horizontal mixer-settler. Chem. Eng. Res. Des. 2021, 167, 231–241. [Google Scholar] [CrossRef]
- Bąk, A.; Podgórska, W. Investigation of drop breakage and coalescence in the liquid–liquid system with nonionic surfactants Tween 20 and Tween 80. Chem. Eng. Sci. 2012, 74, 181–191. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiao, X. Influences of cation valence on water absorbency of crosslinked carboxymethyl cellulose. Int. J. Biol. Macromol. 2021, 177, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kono, H. Characterization and properties of carboxymethyl cellulose hydrogels crosslinked by polyethylene glycol. Carbohydr. Polym. 2014, 106, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Su, H.; Fang, L.; Tan, T. Superabsorbent hydrogels from poly(aspartic acid) with salt-, temperature- and pH-responsiveness properties. Polymer 2005, 46, 5368–5376. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, M.; Ren, J.; Wang, J.; Fan, L.; Xu, Q. Preparation and swelling properties of graphene oxide/poly(acrylic acid-co-acrylamide) super-absorbent hydrogel nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 97–106. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, S.; Li, Y.; Hu, S.; Yang, P. Swelling characterization of ionic responsive superabsorbent resin containing carboxylate sodium groups. React. Funct. Polym. 2022, 170, 105144. [Google Scholar] [CrossRef]
- Asad, S.A.; Tabassum, A.; Hameed, A.; Hassan, F.U.; Afzal, A.; Khan, S.A.; Ahmed, R.; Shahzad, M. Determination of lytic enzyme activities of indigenous Trichoderma isolates from Pakistan. Braz. J. Microbiol. 2015, 46, 1053–1064. [Google Scholar] [CrossRef] [Green Version]
- Kono, H.; Hara, H.; Hashimoto, H.; Shimizu, Y. Nonionic gelation agents prepared from hydroxypropyl guar gum. Carbohydr. Polym. 2015, 117, 636–643. [Google Scholar] [CrossRef]
- Kono, H.; Fujita, S. Biodegradable superabsorbent hydrogels derived from cellulose by esterification crosslinking with 1,2,3,4-butanetetracarboxylic dianhydride. Carbohydr. Polym. 2012, 87, 2582–2588. [Google Scholar] [CrossRef]
- Kono, H.; Onishi, K.; Nakamura, T. Characterization and bisphenol A adsorption capacity of β-cyclodextrin-carboxymethylcellulose-based hydrogels. Carbohydr. Polym. 2013, 98, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Yamanaka, T.; Koizumi, A.; Sakamoto, M.; Aita, R.; Endo, H.; Yachi, Y.; Yamauchi, N.; Otsubo, T.; Ikeda, K.; et al. Application of novel sialoglyco particulates enhances the detection sensitivity of the equine influenza virus by real-time reverse transcriptase polymerase chain reaction. ACS Appl. Bio Mater. 2019, 2, 1255–1261. [Google Scholar] [CrossRef] [PubMed]











| Sample a | Initial Feed Amount | CMC Concentration (wt%) c | Feed Mass Ratio of EGDE/CMC | Yields d | |
|---|---|---|---|---|---|
| CMC (AGU b) | EGDE | ||||
| CMCG2.5,0.4 | 1.0 g (4.6 mmol) | 0.4 g (2.3 mmol) | 2.5 | 0.4 | - |
| CMCG5,0.4 | 2.0 g (9.2 mmol) | 0.8 g (4.6 mmol) | 5 | 0.4 | - |
| CMCG7.5,0.4 | 3.0 g (13.8 mmol) | 1.2 g (6.9 mmol) | 7.5 | 0.4 | 2.5 g (58%) |
| CMCG10,0.4 | 4.0 g (18.3 mmol) | 1.6 g (9.2 mmol) | 10 | 0.4 | 5.3 g (90%) |
| CMCG15,0.4 | 6.0 g (27.5 mmol) | 2.4 g (13.8 mmol) | 15 | 0.4 | 5.6 g (65%) |
| CMCG10,0.2 | 4.0 g (18.3 mmol) | 0.8 g (4.6 mmol) | 10 | 0.2 | 4.3 g (86%) |
| CMCG10,0.6 | 4.0 g (18.3 mmol) | 2.4 g (13.8 mmol) | 10 | 0.6 | 4.0 g (60%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, S.; Tazawa, T.; Kono, H. Preparation and Enzyme Degradability of Spherical and Water-Absorbent Gels from Sodium Carboxymethyl Cellulose. Gels 2022, 8, 321. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050321
Fujita S, Tazawa T, Kono H. Preparation and Enzyme Degradability of Spherical and Water-Absorbent Gels from Sodium Carboxymethyl Cellulose. Gels. 2022; 8(5):321. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050321
Chicago/Turabian StyleFujita, Sayaka, Toshiaki Tazawa, and Hiroyuki Kono. 2022. "Preparation and Enzyme Degradability of Spherical and Water-Absorbent Gels from Sodium Carboxymethyl Cellulose" Gels 8, no. 5: 321. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8050321