Current Progress of Si/Graphene Nanocomposites for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Si and Graphene in Electrodes
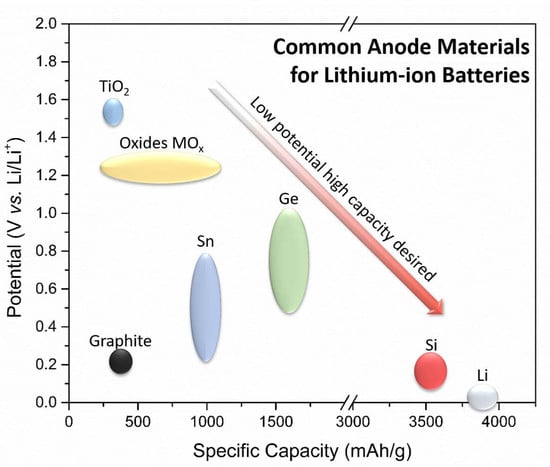
2.1. Si Electrochemistry
2.2. Graphene in Electrodes
3. Si/Graphene Anodes
3.1. Various Synthesis Methods to Form Si/Graphene Composite Anode
3.2. Composition of Si/Graphene Composite Anode
3.3. Graphene Quality
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tawfik, H.; Hung, Y.; Mahajan, D. Metal bipolar plates for PEM fuel cell—A review. J. Power Sources 2007, 163, 755–767. [Google Scholar] [CrossRef]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y. A hole-conductor–free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, M.; Wang, H.; Fukuda, K.; Hara, Y.; Adachi, Y. Effect of Carbon Coating on Electrochemical Performance of Treated Natural Graphite as Lithium-Ion Battery Anode Material. J. Electrochem. Soc. 2000, 147, 1245–1250. [Google Scholar] [CrossRef]
- Tsumura, T.; Kojitani, N.; Izumi, I.; Iwashita, N.; Toyoda, M.; Inagaki, M. Carbon coating of anatase-type TiO2 and photoactivity. J. Mater. Chem. 2002, 12, 1391–1396. [Google Scholar] [CrossRef]
- Yang, W.; Araki, H.; Tang, C.; Thaveethavorn, S.; Kohyama, A.; Suzuki, H.; Noda, T. Single-Crystal SiC Nanowires with a Thin Carbon Coating for Stronger and Tougher Ceramic Composites. Adv. Mater. 2005, 17, 1519–1523. [Google Scholar] [CrossRef]
- Show, Y. Electrically conductive amorphous carbon coating on metal bipolar plates for PEFC. Surf. Coat. Technol. 2007, 202, 1252–1255. [Google Scholar] [CrossRef]
- Nagaura, T.; Tozawa, K. Lithium ion rechargeable battery. Prog. Batter. Sol. Cells 1990, 9, 209. [Google Scholar]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Ji, L.W.; Lin, Z.; Alcoutlabi, M.; Zhang, X.W. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation—Approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Shi, H.; Barker, J.; Saidi, M.Y.; Koksbang, R. Structure and lithium intercalation properties of synthetic and natural graphite. J. Electrochem. Soc. 1996, 143, 3466–3472. [Google Scholar] [CrossRef]
- Weydanz, W.; Wohlfahrt-Mehrens, M.; Huggins, R.A. A room temperature study of the binary lithium–silicon and the ternary lithium–chromium–silicon system for use in rechargeable lithium batteries. J. Power Sources 1999, 81, 237–242. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.; Chen, L.; Wu, Z.; Liang, Y. A high capacity nano Si composite anode material for lithium rechargeable batteries. Electrochem. Solid-State Lett. 1999, 2, 547–549. [Google Scholar] [CrossRef]
- Huggins, R.A. Lithium alloy negative electrodes. J. Power Sources 1999, 81, 13–19. [Google Scholar] [CrossRef]
- Reece, S.Y.; Hamel, J.A.; Sung, K.; Jarvi, T.D.; Esswein, A.J.; Pijpers, J.J.; Nocera, D.G. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 2011, 334, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Obrovac, M.; Christensen, L. Structural changes in silicon anodes during lithium insertion/extraction. Electrochem. Solid-State Lett. 2004, 7, A93–A96. [Google Scholar] [CrossRef]
- Wang, G.; Ahn, J.; Yao, J.; Bewlay, S.; Liu, H. Nanostructured Si–C composite anodes for lithium-ion batteries. Electrochem. Commun. 2004, 6, 689–692. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.F.; Yang, Y.; Hsu, C.M.; Cui, Y. Carbon–Silicon Core–Shell Nanowires as High Capacity Electrode for Lithium Ion Batteries. Nano Lett. 2009, 9, 3370–3374. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhu, Y.; Langrock, A.; Manivannan, A.; Ehrman, S.H.; Wang, C. Graphene-Bonded and-Encapsulated Si Nanoparticles for Lithium Ion Battery Anodes. Small 2013, 9, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Smith, K.B.; Hayner, C.M.; Kung, H.H. Silicon nanoparticles-graphene paper composites for Li ion battery anodes. Chem. Commun. (Camb.) 2010, 46, 2025–2027. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Rong, J.; Fang, X.; Zhou, C. Porous doped silicon nanowires for lithium ion battery anode with long cycle life. Nano Lett. 2012, 12, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Magasinski, A.; Dixon, P.; Hertzberg, B.; Kvit, A.; Ayala, J.; Yushin, G. High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nat. Mater. 2010, 9, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Limthongkul, P.; Jang, Y.-I.; Dudney, N.J.; Chiang, Y.-M. Electrochemically-driven solid-state amorphization in lithium-silicon alloys and implications for lithium storage. Acta Mater. 2003, 51, 1103–1113. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zheng, J.; Zheng, H.; Mei, Z.; Du, X.; Li, H. Electrochemical performances and volume variation of nano-textured silicon thin films as anodes for lithium-ion batteries. Nanotechnology 2013, 24, 424011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Y.; Xiao, R.; Li, H.; Aifantis, K.E.; Huang, X. Investigation of crack patterns and cyclic performance of Ti–Si nanocomposite thin film anodes for lithium ion batteries. J. Power Sources 2012, 202, 236–245. [Google Scholar] [CrossRef]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano-and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Guo, Z.; Milin, E.; Wang, J.; Chen, J.; Liu, H.-K. Silicon/disordered carbon nanocomposites for lithium-ion battery anodes. J. Electrochem. Soc. 2005, 152, A2211–A2216. [Google Scholar] [CrossRef]
- Ng, S.H.; Wang, J.; Wexler, D.; Chew, S.Y.; Liu, H.K. Amorphous carbon-coated silicon nanocomposites: A low-temperature synthesis via spray pyrolysis and their application as high-capacity anodes for lithium-ion batteries. J. Phys. Chem. C 2007, 111, 11131–11138. [Google Scholar] [CrossRef]
- Wang, D.; Gao, M.; Pan, H.; Wang, J.; Liu, Y. High performance amorphous-Si@SiOx/C composite anode materials for Li-ion batteries derived from ball-milling and in situ carbonization. J. Power Sources 2014, 256, 190–199. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kim, J.W.; Sung, Y.-E.; Oh, S.M. Failure modes of silicon powder negative electrode in lithium secondary batteries. Electrochem. Solid-State Lett. 2004, 7, A306–A309. [Google Scholar] [CrossRef]
- Radvanyi, E.; Porcher, W.; De Vito, E.; Montani, A.; Franger, S.; Larbi, S.J.S. Failure mechanisms of nano-silicon anodes upon cycling: An electrode porosity evolution model. Phys. Chem. Chem. Phys. 2014, 16, 17142–17153. [Google Scholar] [CrossRef] [PubMed]
- Oumellal, Y.; Delpuech, N.; Mazouzi, D.; Dupre, N.; Gaubicher, J.; Moreau, P.; Soudan, P.; Lestriez, B.; Guyomard, D. The failure mechanism of nano-sized Si-based negative electrodes for lithium ion batteries. J. Mater. Chem. 2011, 21, 6201–6208. [Google Scholar] [CrossRef]
- Luo, F.; Liu, B.; Zheng, J.; Chu, G.; Zhong, K.; Li, H.; Huang, X.; Chen, L. Review—Nano-Silicon/Carbon Composite Anode Materials Towards Practical Application for Next Generation Li-Ion Batteries. J. Electrochem. Soc. 2015, 162, A2509–A2528. [Google Scholar] [CrossRef]
- Lee, K.T.; Cho, J. Roles of nanosize in lithium reactive nanomaterials for lithium ion batteries. Nano Today 2011, 6, 28–41. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.A.; Grigorieva, I.; Firsov, A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Müller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D. Mechanically strong, electrically conductive, and biocompatible graphene paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fang, M.; Wu, F.; Wu, H.; Wang, L.; Chen, G. Preparation of graphene by exfoliation of graphite using wet ball milling. J. Mater. Chem. 2010, 20, 5817–5819. [Google Scholar] [CrossRef]
- Cai, M.; Thorpe, D.; Adamson, D.H.; Schniepp, H.C. Methods of graphite exfoliation. J. Mater. Chem. 2012, 22, 24992–25002. [Google Scholar] [CrossRef]
- Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Bruno, G. Graphene CVD growth on copper and nickel: Role of hydrogen in kinetics and structure. Phys. Chem. Chem. Phys. 2011, 13, 20836–20843. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of Graphene and Its Applications: A Review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Katsnelson, M.I. Graphene: Carbon in two dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, T.-T.; Girit, C.; Hao, Z.; Martin, M.C.; Zettl, A.; Crommie, M.F.; Shen, Y.R.; Wang, F. Direct observation of a widely tunable bandgap in bilayer graphene. Nature 2009, 459, 820. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Yang, H.B.; Sheng, Z.M.; Lu, Z.S.; Song, Q.L.; Li, C.M. Layered graphene/quantum dots for photovoltaic devices. Angew. Chem. Int. Ed. 2010, 49, 3014–3017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhao, S.Y.; Tian, X.N.; Zhao, X.S. Layered Graphene Oxide Nanostructures with Sandwiched Conducting Polymers as Supercapacitor Electrodes. Langmuir 2010, 26, 17624–17628. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.; Kudo, T.; Honma, I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.; Lu, J.; Wu, F.; Li, L.; Chen, J.; Tan, G.; Ye, Y.; Amine, K. Graphene-based three-dimensional hierarchical sandwich-type architecture for high-performance Li/S batteries. Nano Lett. 2013, 13, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Cen, Y.; Yao, Y.; Xu, Q.; Xia, Z.; Sisson, R.D.; Liang, J. Fabrication of TiO2-graphene composite for the enhanced performance of lithium batteries. RSC Adv. 2016, 6, 66971–66977. [Google Scholar] [CrossRef]
- Paek, S.-M.; Yoo, E.; Honma, I. Enhanced cyclic performance and lithium storage capacity of SnO2/graphene nanoporous electrodes with three-dimensionally delaminated flexible structure. Nano Lett. 2008, 9, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, S.P.; Wang, X. Microwave-assisted one-pot synthesis of metal/metal oxide nanoparticles on graphene and their electrochemical applications. Electrochim. Acta 2011, 56, 3338–3344. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, H.-K.; Ahn, D.-J.; Lee, S.-I.; Roh, K.C.; Kim, K.-B. Self-assembly of Si entrapped graphene architecture for high-performance Li-ion batteries. Electrochem. Commun. 2013, 34, 117–120. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Zhong, C.; Chou, S.-L.; Liu, H.-K. Flexible free-standing graphene-silicon composite film for lithium-ion batteries. Electrochem. Commun. 2010, 12, 1467–1470. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, Y.; Xiong, Q.; Cheng, J.; Zhang, Q.; Wang, X.; Gu, C.; Tu, J. Self-assembly silicon/porous reduced graphene oxide composite film as a binder-free and flexible anode for lithium-ion batteries. Electrochim. Acta 2015, 156, 86–93. [Google Scholar] [CrossRef]
- Tao, H.-C.; Fan, L.-Z.; Mei, Y.; Qu, X. Self-supporting Si/Reduced Graphene Oxide nanocomposite films as anode for lithium ion batteries. Electrochem. Commun. 2011, 13, 1332–1335. [Google Scholar] [CrossRef]
- Zhou, M.; Cai, T.; Pu, F.; Chen, H.; Wang, Z.; Zhang, H.; Guan, S. Graphene/carbon-coated Si nanoparticle hybrids as high-performance anode materials for Li-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 3449–3455. [Google Scholar] [CrossRef] [PubMed]
- Chabot, V.; Feng, K.; Park, H.W.; Hassan, F.M.; Elsayed, A.R.; Yu, A.; Xiao, X.; Chen, Z. Graphene wrapped silicon nanocomposites for enhanced electrochemical performance in lithium ion batteries. Electrochim. Acta 2014, 130, 127–134. [Google Scholar] [CrossRef]
- Chen, D.; Yi, R.; Chen, S.; Xu, T.; Gordin, M.L.; Wang, D. Facile synthesis of graphene–silicon nanocomposites with an advanced binder for high-performance lithium-ion battery anodes. Solid State Ion. 2014, 254, 65–71. [Google Scholar] [CrossRef]
- Su, M.; Wang, Z.; Guo, H.; Li, X.; Huang, S.; Xiao, W.; Gan, L. Enhancement of the Cyclability of a Si/Graphite@Graphene composite as anode for Lithium-ion batteries. Electrochim. Acta 2014, 116, 230–236. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, K.; Ji, G.; Lee, J.Y.; Zou, C.; Chen, X.; Wu, J. Graphene/nanosized silicon composites for lithium battery anodes with improved cycling stability. Carbon 2011, 49, 1787–1796. [Google Scholar] [CrossRef]
- Eoma, K.; Joshi, T.; Bordes, A.; Do, I.; Fuller, T. The design of a Li-ion full cell battery using a nano silicon and nano multi-layer graphene composite anode. J. Power Sources 2014, 249, 118–124. [Google Scholar] [CrossRef]
- Luo, J.; Zhao, X.; Wu, J.; Jang, H.D.; Kung, H.H.; Huang, J. Crumpled Graphene-Encapsulated Si Nanoparticles for Lithium Ion Battery Anodes. J. Phys. Chem. Lett. 2012, 3, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Zai, J.; Dai, F.; Gordin, M.L.; Wang, D. Dual Conductive Network-Enabled Graphene/Si–C Composite Anode with High Areal Capacity for Lithium-ion Batteries. Nano Energy 2014. [Google Scholar] [CrossRef]
- Xin, X.; Zhou, X.; Wang, F.; Yao, X.; Xu, X.; Zhu, Y.; Liu, Z. A 3D porous architecture of Si/graphene nanocomposite as high-performance anode materials for Li-ion batteries. J. Mater. Chem. 2012, 22, 7724–7730. [Google Scholar] [CrossRef]
- De Guzman, R.C.; Yang, J.; Cheng, M.M.-C.; Salley, S.O.; Simon Ng, K.Y. Effects of graphene and carbon coating modifications on electrochemical performance of silicon nanoparticle/graphene composite anode. J. Power Sources 2014, 246, 335–345. [Google Scholar] [CrossRef]
- Li, H.; Lu, C.; Zhang, B. A straightforward approach towards Si@C/graphene nanocomposite and its superior lithium storage performance. Electrochim. Acta 2014, 120, 96–101. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Xie, X.-L.; Rick, J.; Chang, F.-C.; Hwang, B.-J. Improved anode materials for lithium-ion batteries comprise non-covalently bonded graphene and silicon nanoparticles. J. Power Sources 2014, 247, 991–998. [Google Scholar] [CrossRef]
- Kannan, A.G.; Kim, S.H.; Yang, H.S.; Kim, D.-W. Silicon nanoparticles grown on a reduced graphene oxide surface as high-performance anode materials for lithium-ion batteries. RSC Adv. 2016, 6, 25159–25166. [Google Scholar] [CrossRef]
- Ji, J.; Ji, H.; Zhang, L.L.; Zhao, X.; Bai, X.; Fan, X.; Zhang, F.; Ruoff, R.S. Graphene-Encapsulated Si on Ultrathin-Graphite Foam as Anode for High Capacity Lithium-Ion Batteries. Adv. Mater. 2013, 25, 4673–4677. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jin, S.; Liao, Q.; Cui, H.; Wang, C.X. Encapsulated within graphene shell silicon nanoparticles anchored on vertically aligned graphene trees as lithium ion battery anodes. Nano Energy 2014, 5, 105–115. [Google Scholar] [CrossRef]
- Li, Z.F.; Zhang, H.; Liu, Q.; Liu, Y.; Stanciu, L.; Xie, J. Novel pyrolyzed polyaniline-grafted silicon nanoparticles encapsulated in graphene sheets as li-ion battery anodes. ACS Appl. Mater. Interfaces 2014, 6, 5996–6002. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.H.; Lee, Y.M.; Kong, B.-S.; Seo, J.-S.; Choi, J.W. Electrospun core–shell fibers for robust silicon nanoparticle-based lithium ion battery anodes. Nano Lett. 2012, 12, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yu, Y.; Kung, H.H.; Wang, B.; Jiang, J. Si@SiOx/graphene hydrogel composite anode for lithium-ion battery. J. Power Sources 2016, 306, 42–48. [Google Scholar] [CrossRef]
- Güneş, F. A direct synthesis of Si-nanowires on 3D porous graphene as a high performance anode material for Li-ion batteries. RSC Adv. 2016, 6, 1678–1685. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, J.; Sim, D.; Shi, W.; Tay, Y.Y.; Ma, J.; Hng, H.H.; Yan, Q. In situ growth of Si nanowires on graphene sheets for Li-ion storage. Electrochim. Acta 2012, 74, 176–181. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Zhang, X.; Luo, B.; Jin, M.; Liang, M.; Dayeh, S.; Picraux, S.T.; Zhi, L. Adaptable Silicon-Carbon Nanocables Sandwiched between Reduced Graphene Oxide Sheets as Lithium Ion Battery Anodes. ACS Nano 2013, 7, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Striebel, K.A. Effect of electrode density on cycle performance and irreversible capacity loss for natural graphite anode in lithium-ion batteries. J. Power Sources 2003, 119, 934–937. [Google Scholar] [CrossRef]
- Smekens, J.; Gopalakrishnan, R.; Van den Steen, N.; Omar, N.; Hegazy, O.; Hubin, A.; Van Mierlo, J. Influence of electrode density on the performance of Li-ion batteries: Experimental and simulation results. Energies 2016, 9, 104. [Google Scholar] [CrossRef]
- Pribat, D. Rechargeable Batteries—Materials, Technologies and New Trends; Zhang, Z., Zhang, S.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Nitta, N.; Yushin, G. High-capacity anode materials for lithium-ion batteries: Choice of elements and structures for active particles. Part. Part. Syst. Charact. 2014, 31, 317–336. [Google Scholar] [CrossRef]
- Chae, S.; Ko, M.; Kim, K.; Ahn, K.; Cho, J. Confronting Issues of the Practical Implementation of Si Anode in High-Energy Lithium-Ion Batteries. Joule 2017, 1, 47–60. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2009, 114, 832–842. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L.; Bangal, P.R. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 2010, 48, 1146–1152. [Google Scholar] [CrossRef]
- Some, S.; Kim, Y.; Yoon, Y.; Yoo, H.; Lee, S.; Park, Y.; Lee, H. High-quality reduced graphene oxide by a dual-function chemical reduction and healing process. Sci. Rep. 2013, 3, 1929. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.Q.; Cen, Y.J.; Sisson, R.D.; Liang, J.Y. A Synthesize Protocol for Graphene Nanosheets. In Proceedings of the 4th Asia Conference on Mechanical and Materials Engineering, Kuala Lumpur, Malaysia, 14–18 July 2016; pp. 3–6. [Google Scholar]
- Chen, J.; Bie, L.; Sun, J.; Xu, F. Enhanced electrochemical performances of silicon nanotube bundles anode coated with graphene layers. Mater. Res. Bull. 2016, 73, 394–400. [Google Scholar] [CrossRef]
- Ni, Z.H.; Yu, T.; Lu, Y.H.; Wang, Y.Y.; Feng, Y.P.; Shen, Z.X. Uniaxial strain on graphene: Raman spectroscopy study and band-gap opening. ACS Nano 2008, 2, 2301–2305. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Meyer, J.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.; Roth, S. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Ni, Z.H.; Yu, T.; Shen, Z.X.; Wang, H.M.; Wu, Y.H.; Chen, W.; Shen Wee, A.T. Raman studies of monolayer graphene: The substrate effect. J. Phys. Chem. C 2008, 112, 10637–10640. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Cançado, L.; Jorio, A.; Pimenta, M. Measuring the absolute Raman cross section of nanographites as a function of laser energy and crystallite size. Phys. Rev. B 2007, 76, 064304. [Google Scholar] [CrossRef]
- Ferreira, E.M.; Moutinho, M.V.; Stavale, F.; Lucchese, M.; Capaz, R.B.; Achete, C.; Jorio, A. Evolution of the Raman spectra from single-, few-, and many-layer graphene with increasing disorder. Phys. Rev. B 2010, 82, 125429. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Z. Graphene in biomedicine: Opportunities and challenges. Nanomedicine 2011, 6, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Badioli, M.; Alonso-González, P.; Thongrattanasiri, S.; Huth, F.; Osmond, J.; Spasenović, M.; Centeno, A.; Pesquera, A.; Godignon, P. Optical nano-imaging of gate-tunable graphene plasmons. Nature 2012, 487, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y. Si/C Nanocomposites for Li-Ion Battery Anode. Ph.D. Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2017. [Google Scholar]



| Carbon Form | 0-D [15] | 1-D [21] | 2-D [22] | 3-D [25] |
|---|---|---|---|---|
| Carbon Black | CNFs | Graphene | Hierarchical Carbon Frame | |
| Si Source | Si NPs | a-Si | Si NPs | Si NPs |
| Si Loading | 60% | 75% | 70% | 50% |
| Initial Capacity (mAh/g) | ~3000 | ~2000 | ~2300 | ~2000 |
| Cycling Performance (mAh/g) | ~1500 | ~1500 | ~1700 | ~1600 |
| 22 cycles | 55 cycles | 120 cycles | 100 cycles |
| Anode | Li Metal [26] | Graphite | Silicon |
|---|---|---|---|
| Potential vs. Lithium | 0 | ~0.15 | ~0.3 |
| Theoretical Capacity (mAh/g) | 3860 | 372 (LiC6) | 3578 (Li15Si4) |
| Typical Charging Rate | >1 C | >1 C | 1/10 C |
| Reported Cycle Life | 300 | >1000 | 100~200 |
| Ion Storage Mechanism | Plating/Stripping | Insertion/Extraction | Alloying/De-alloying |
| Current status | Research | Commercialized | Research |
| Si Source | Graphene Synthesis Method | Graphene Weight Ratio | Electrode Loading Density (mg/cm2) | Initial Reversible Capacity (mAh/g) | First Cycle Efficiency | Best Capacity Retention with Current Rate | Ref. with Similar Si/Graphene Structure |
|---|---|---|---|---|---|---|---|
| Si NPs | Graphite Oxidation + Thermal Reduction | ~40% | 2 | ~2050 | ~96% | 300th, ~56%, 1000 mA/g | [23,60] |
| Si NPs | Modified Hummers Method + Chemical Reduction | 73.6% | NR | ~1000 | ~41% | 100th, ~70.8%, 50 mA/g | [61,62] |
| Si NPs | Hummers Method + Thermal Reduction | 66.7% | NR | 1040 | 63% | 30th, 94%, 50 mA/g | [63,64,65,66,67] |
| Si NPs | Thermal Expansion | ~33% | NR | 2753 | ~80% | 30th, ~91%, 300 mA/g | [68,69] |
| Aerosol Droplets Si NPs | Crumpled Reduction | 40% | 0.2 | 1175 | ~95% | 250th, ~86%, 1000 mA/g | [70,71] |
| 3-D Porous Si by Magnesiothermic | Hummers Method + Thermal Reduction | ~40% | NR | 1100 | ~79% | 100th, ~50%, 5000 mA/g | [72,73,74,75,76] |
| Si NPs on graphite Foam | Hummers Method + Thermal Reduction | 15.1% | 1.5 | 1000 | 62.5% | 100th, ~37%, 400 mA/g | [77] |
| Si NPs on 3-D tree-like GNS | Microwave Plasma CVD | 19% | NR | 2731 | ~56% | 160th, ~67%, 150 mA/g | [78] |
| (PANI)-Si NPs | Modified Hummers Method + Pyrolysis Method | 26% | 0.3 | ~1500 | ~70% | 300th, ~76%, 2000 mA/g | [79] |
| Si NPs on Electrospunn CNFs | Chemical Method + Thermal Reduction | 0.6% | NR | 1270 | 71.2% | 50th, ~91%, 100 mA/g | [80] |
| Si NPs on Graphene Hydrogel | Modified Hummers Method Ascorbic Acid + Thermal Reduction | 29% | NR | 2250 | 53% | 150th, ~50%, 100 mA/g | [81] |
| 3D Si NWs | CVD + Plasma enhanced CVD | NR | ~0.5 | ~2600 | 97% | 100th, ~29%, 500 mA/g | [82] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cen, Y.; Sisson, R.D.; Qin, Q.; Liang, J. Current Progress of Si/Graphene Nanocomposites for Lithium-Ion Batteries. C 2018, 4, 18. https://0-doi-org.brum.beds.ac.uk/10.3390/c4010018
Cen Y, Sisson RD, Qin Q, Liang J. Current Progress of Si/Graphene Nanocomposites for Lithium-Ion Batteries. C. 2018; 4(1):18. https://0-doi-org.brum.beds.ac.uk/10.3390/c4010018
Chicago/Turabian StyleCen, Yinjie, Richard D. Sisson, Qingwei Qin, and Jianyu Liang. 2018. "Current Progress of Si/Graphene Nanocomposites for Lithium-Ion Batteries" C 4, no. 1: 18. https://0-doi-org.brum.beds.ac.uk/10.3390/c4010018