A Review on the Synthesis and Characterization of Biomass-Derived Carbons for Adsorption of Emerging Contaminants from Water
Abstract
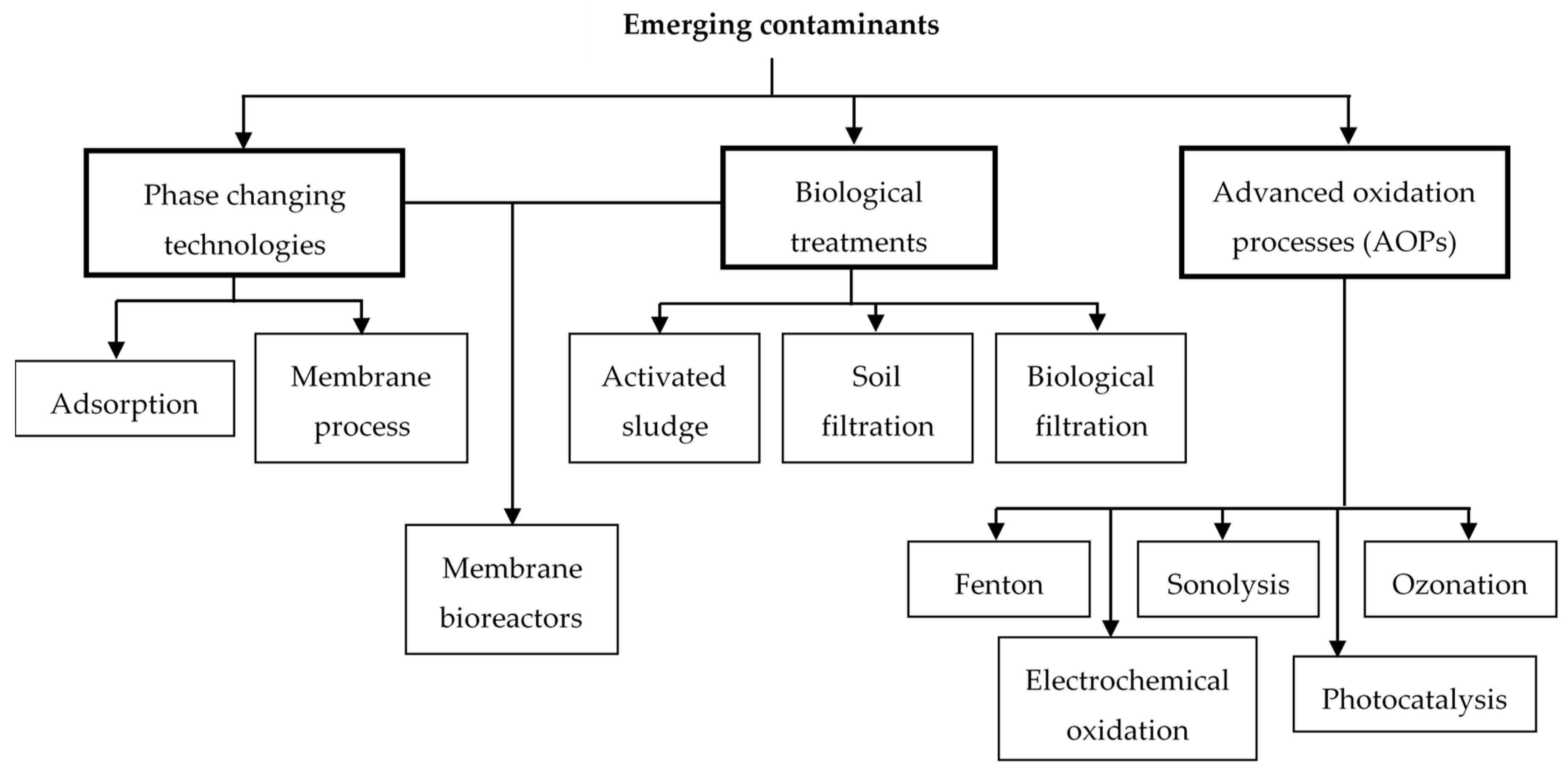
:1. Emerging Contaminants
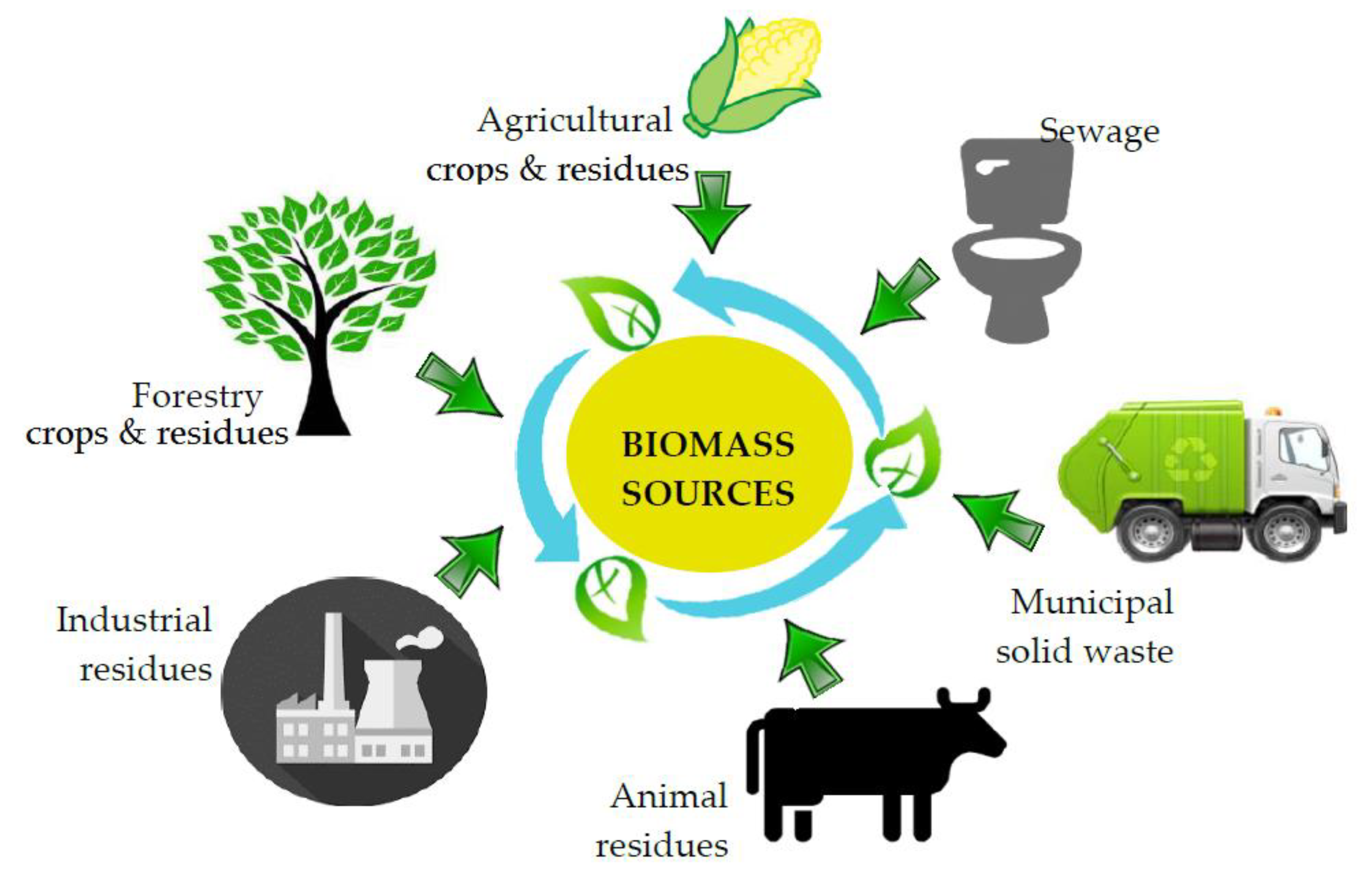
2. Biomass Feedstocks
2.1. Dedicated Energy Crops
2.2. Forest Residues
2.3. Agricultural Crop Residues
2.4. Algae
2.5. Municipal Solid Wastes (MSW)
2.6. Wet Waste
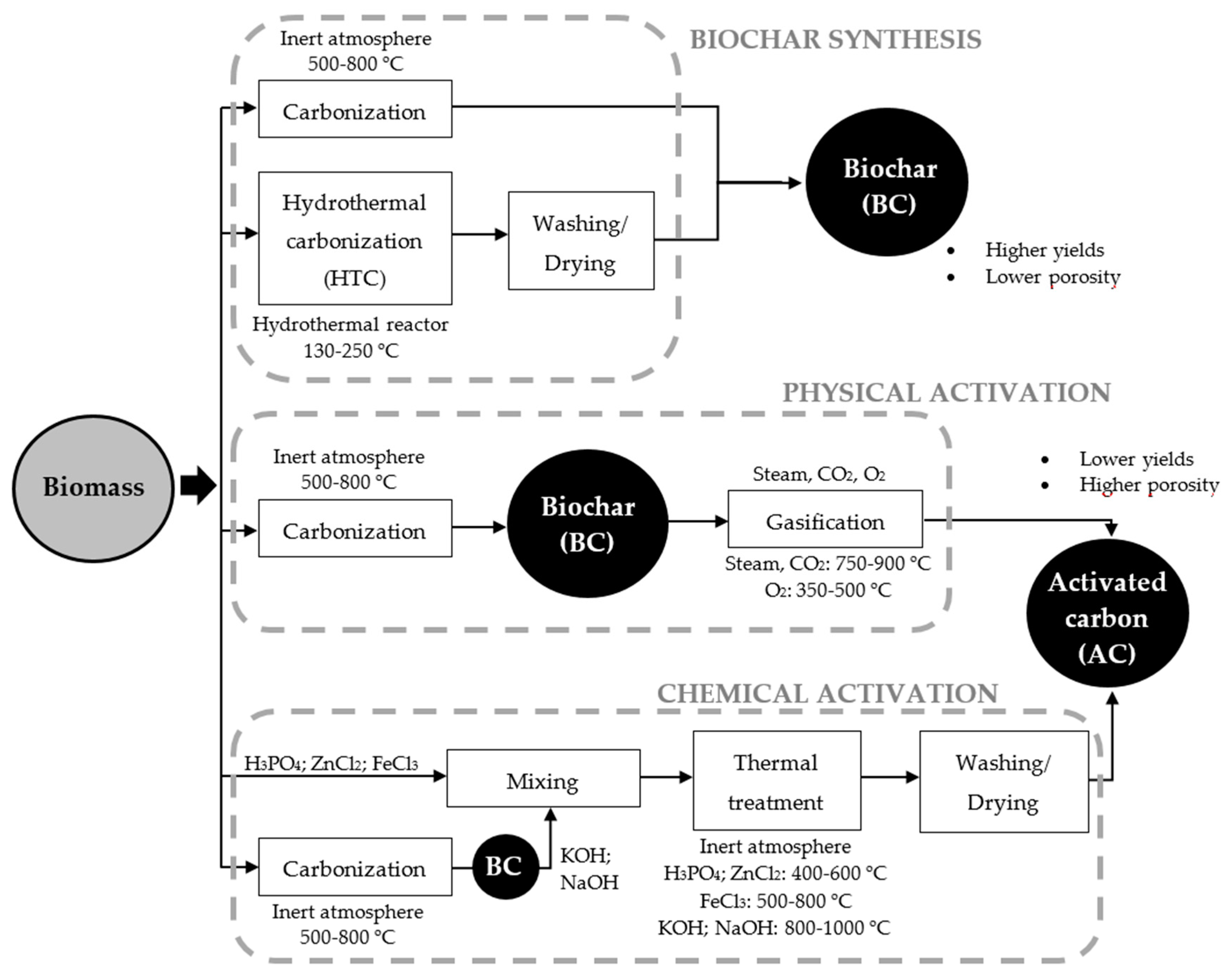
3. Synthesis of Biomass-Derived Carbons
3.1. Biochars
3.2. Activated Carbons
3.2.1. Physical Activation
3.2.2. Chemical Activation
4. Characterization Techniques
4.1. Textural Characterization
4.1.1. N2 Adsorption–Desorption at −196 °C
4.1.2. CO2 Adsorption at 0 °C
4.2. Surface Chemistry
4.2.1. Fourier Transform Infrared (FTIR) Spectroscopy
4.2.2. X-ray Photoelectron Spectroscopy (XPS)
4.2.3. Temperature Programmed Desorption (TPD)
4.2.4. Boehm Titration
4.2.5. Point of Zero Charge
5. Experimental Procedures for Adsorption Tests
5.1. Adsorption Equilibrium
5.2. Adsorption Kinetics
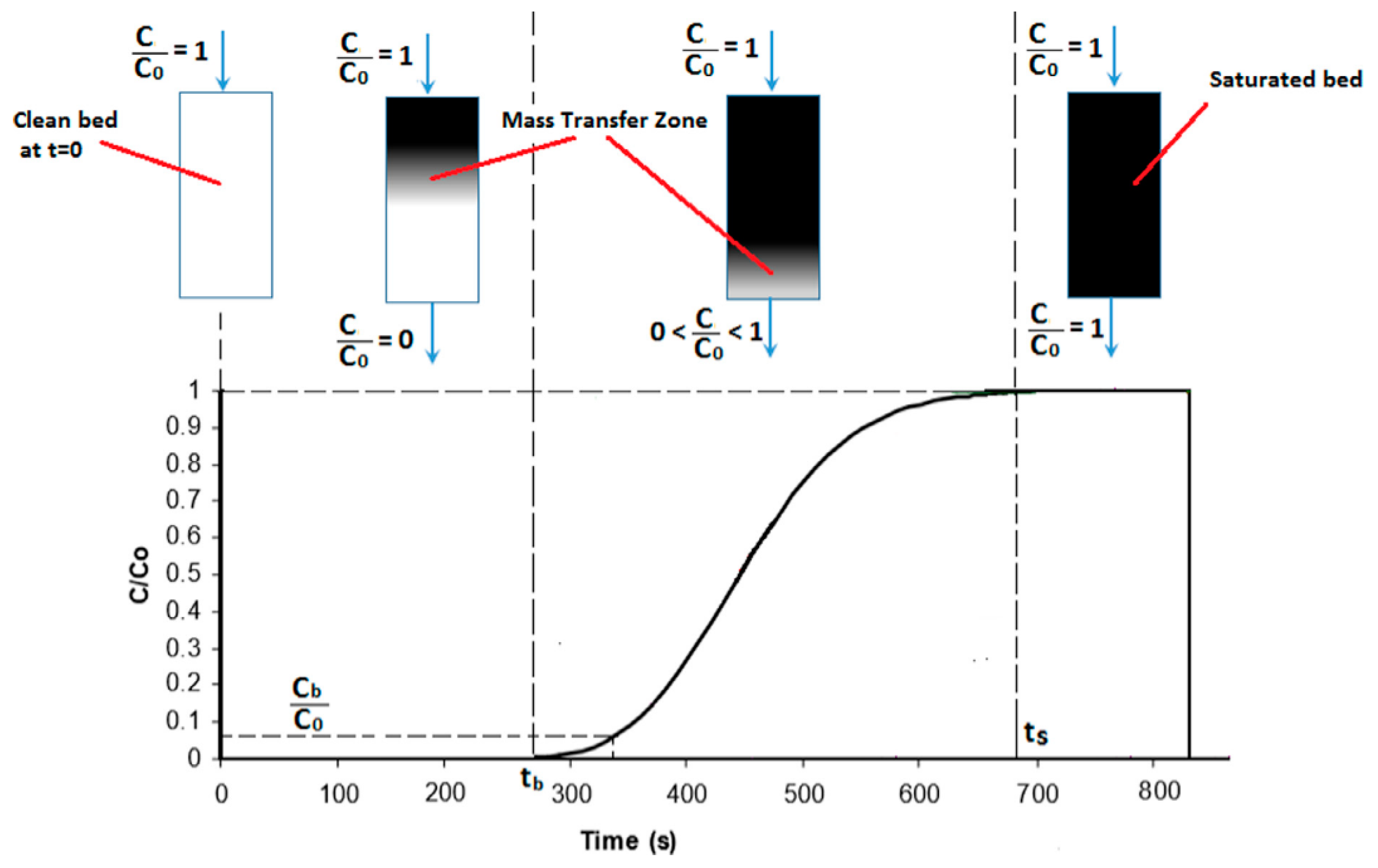
5.3. Adsorption Dynamics
6. Adsorption of Emerging Contaminants on Biomass-Derived Carbons
6.1. Adsorption on Biochars
6.2. Adsorption on Activated Carbons
7. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taheran, M.; Naghdi, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Emerging contaminants: Here today, there tomorrow! Environ. Nanotechno. Monit. Manag. 2018, 10, 122–126. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, J.; Sun, P.; Liu, J.; Ji, Y.; Nakada, N.; Qiao, Z.; Tanaka, H.; Yang, Y. Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: Systematic review and bibliometric analysis. Sci. Total Environ. 2018, 627, 1253–1263. [Google Scholar] [CrossRef]
- La Farré, M.; Pérez, S.; Kantiani, L. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal. Chem. 2008, 27, 991–1007. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Sipma, J.; Osuna, B.; Collado, N.; Monclús, H.; Ferrero, G.; Comas, J.; Rodriguez-Roda, I. Comparison of removal of pharmaceuticals in MBR and activated sludge systems. Desalination 2010, 250, 653–659. [Google Scholar] [CrossRef]
- Carr, D.L.; Morse, A.N.; Zak, J.C.; Anderson, T.A. Biological degradation of common pharmaceuticals and personal care products in soils with high water content. Water Air Soil Pollut. 2011, 217, 127–134. [Google Scholar] [CrossRef]
- Matamoros, V.; Sala, L.; Salvadó, V. Evaluation of a biologically-based filtration water reclamation plant for removing emerging contaminants: A pilot plant study. Bioresour. Technol. 2012, 104, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Huang, J.; Deng, S.; Chen, W.; Yu, G. Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in different biological wastewater treatment processes. Environ. Sci. Technol. 2011, 45, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-K.; Sheng, G.-P.; Shi, B.-J.; Li, W.-W.; Yu, H.-Q. A Novel electrochemical membrane bioreactor as a potential net energy producer for sustainable wastewater treatment. Sci. Rep. 2013, 3, 1864. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Z.; Mao, B.; Zhan, J.; Wu, Z. Electrochemical membrane bioreactors for sustainable wastewater treatment: Principles and challenges. Curr. Environ. Eng. 2015, 2, 38–49. [Google Scholar] [CrossRef]
- Żur, J.; Piński, A.; Marchlewicz, A.; Hupert-Kocurek, K.; Wojcieszyńska, D.; Guzik, U. Organic micropollutants paracetamol and ibuprofen—Toxicity, biodegradation, and genetic background of their utilization by bacteria. Environ. Sci. Pollut. Res. 2018, 25, 21498–21524. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Ali, I.; Gupta, V.K. Advances in water treatment by adsorption technology. Nat. Protoc. 2007, 1, 2661–2667. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Rachna Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244–245, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Jeirani, Z.; Niu, C.H.; Soltan, J. Adsorption of emerging pollutants on activated carbon. Rev. Chem. Eng. 2017, 33, 491–522. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N.D. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Freyria, F.; Geobaldo, F.; Bonelli, B.; Freyria, F.S.; Geobaldo, F.; Bonelli, B. Nanomaterials for the Abatement of Pharmaceuticals and Personal Care Products from Wastewater. Appl. Sci. 2018, 8, 170. [Google Scholar] [CrossRef]
- Krauskopf, L.G. Plasticizer structure/performance relationships. J. Vinyl Addit. Technol. 1993, 15, 140–147. [Google Scholar] [CrossRef]
- Rabaçal, M.; Ferreira, A.F.; Silva, C.A.M.; Costa, M. Biorefineries; Rabaçal, M., Ferreira, A.F., Silva, C.A.M., Costa, M., Eds.; Lecture Notes in Energy; Springer: Cham, Switzerland, 2017; Volume 57. [Google Scholar]
- Bhaskar, T.; Pandey, A.; Mohan, S.V.; Lee, D.J.; Khanal, S.K. Waste Biorefinery: Potential and Perspectives; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Shahbazali, E. Biorefinery: From biomass to chemicals and fuels. Green Process. Synth. 2013, 2, 87–88. [Google Scholar] [CrossRef]
- Demirbaş, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- De Wit, M.; Faaij, A. European biomass resource potential and costs. Biomass Bioenergy 2010, 34, 188–202. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Growing Dedicated Energy Crops on Marginal Lands and Ecosystem Services. Soil Sci. Soc. Am. J. 2016, 80, 845–858. [Google Scholar] [CrossRef]
- Glithero, N.J.; Wilson, P.; Ramsden, S.J. Optimal combinable and dedicated energy crop scenarios for marginal land. Appl. Energy 2015, 147, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Dessbesell, L.; Xu, C.; Pulkki, R.; Leitch, M.; Mahmood, N. Forest biomass supply chain optimization for a biorefinery aiming to produce high-value bio-based materials and chemicals from lignin and forestry residues: A review of literature. Can. J. For. Res. 2017, 47, 277–288. [Google Scholar] [CrossRef]
- Gregg, J.S.; Smith, S.J. Global and regional potential for bioenergy from agricultural and forestry residue biomass. Mitig. Adapt. Strateg. Glob. Chang. 2010, 15, 241–262. [Google Scholar] [CrossRef]
- Scarlat, N.; Martinov, M.; Dallemand, J.-F. Assessment of the availability of agricultural crop residues in the European Union: Potential and limitations for bioenergy use. Waste Manag. 2010, 30, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Monforti, F.; Bódis, K.; Scarlat, N.; Dallemand, J.-F. The possible contribution of agricultural crop residues to renewable energy targets in Europe: A spatially explicit study. Renew. Sustain. Energy Rev. 2013, 19, 666–677. [Google Scholar] [CrossRef]
- Ward, A.J.; Lewis, D.M.; Green, F.B. Anaerobic digestion of algae biomass: A review. Algal Res. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Chinnasamy, S.; Singh, M.; Das, K.C. Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl. Energy 2011, 88, 3425–3431. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, Y. Municipal solid waste (MSW) as a renewable source of energy: Current and future practices in China. Bioresour. Technol. 2010, 101, 3816–3824. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.; Jess, A.; Kern, C.; Pöhlmann, F.; Glowienka, K.; Wasserscheid, P. Formic Acid-Based Fischer–Tropsch Synthesis for Green Fuel Production from Wet Waste Biomass and Renewable Excess Energy. ACS Sustain. Chem. Eng. 2016, 4, 5078–5086. [Google Scholar] [CrossRef]
- Albert, J.; Wasserscheid, P. Expanding the scope of biogenic substrates for the selective production of formic acid from water-insoluble and wet waste biomass. Green Chem. 2015, 17, 5164–5171. [Google Scholar] [CrossRef] [Green Version]
- Karakaş, C.; Özçimen, D.; İnan, B. Potential use of olive stone biochar as a hydroponic growing medium. J. Anal. Appl. Pyrolysis 2017, 125, 17–23. [Google Scholar] [CrossRef]
- Kula, I.; Uğurlu, M.; Karaoğlu, H.; Çelik, A. Adsorption of Cd(II) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl2 activation. Bioresour. Technol. 2008, 99, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Ubago-Pérez, R.; Carrasco-Marín, F.; Fairén-Jiménez, D.; Moreno-Castilla, C. Granular and monolithic activated carbons from KOH-activation of olive stones. Microporous Mesoporous Mater. 2006, 92, 64–70. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Y.; Wang, Z.; Li, Y.; Wang, L.; Ding, L.; Gao, X.; Ma, Y.; Guo, Y. Application studies of activated carbon derived from rice husks produced by chemical-thermal process—A review. Adv. Colloid Interface Sci. 2011, 163, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhai, S.-R.; Xiao, Z.-Y.; Song, Y.; An, Q.-D.; Song, X.-W. Dye adsorption of mesoporous activated carbons produced from NaOH-pretreated rice husks. Bioresour. Technol. 2013, 136, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-T.; Jiang, T.-J. Mesoporous activated carbon produced from coconut shell using a single-step physical activation process. Biomass Convers. Biorefinery 2018, 8, 711–718. [Google Scholar] [CrossRef]
- Wu, W.; Li, J.; Lan, T.; Müller, K.; Niazi, N.K.; Chen, X.; Xu, S.; Zheng, L.; Chu, Y.; Li, J.; et al. Unraveling sorption of lead in aqueous solutions by chemically modified biochar derived from coconut fiber: A microscopic and spectroscopic investigation. Sci. Total Environ. 2017, 576, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Production of high surface area mesoporous activated carbons from waste biomass using hydrogen peroxide-mediated hydrothermal treatment for adsorption applications. Chem. Eng. J. 2015, 273, 622–629. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Yao, C.; Liu, Y.; Zhang, C.; Jia, L.; Li, D.; Fu, Z.; Sun, D.; Robert Kirk, S.; Yin, D. Bamboo-derived porous biochar for efficient adsorption removal of dibenzothiophene from model fuel. Fuel 2018, 211, 121–129. [Google Scholar] [CrossRef]
- Hameed, B.H.; Din, A.T.M.; Ahmad, A.L. Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies. J. Hazard. Mater. 2007, 141, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Bedia, J.; Rodríguez-Mirasol, J.; Cordero, T. Water vapour adsorption on lignin-based activated carbons. J. Chem. Technol. Biotechnol. 2007, 82, 548–557. [Google Scholar] [CrossRef]
- Cordero, T.; Rodríguez-Mirasol, J.; Bedia, J.; Rodríguez, J.J. Preparation of carbon materials from lignin. Opt. Pura y Apl. 2007, 40, 161–168. [Google Scholar]
- Rodríguez-Mirasol, J.; Bedia, J.; Cordero, T.; Rodríguez, J. Influence of water vapor on the adsorption of VOCs on lignin-based activated carbons. Sep. Sci. Technol. 2005, 40, 3113–3135. [Google Scholar] [CrossRef]
- Gonzalez-Serrano, E.; Cordero, T.; Rodriguez-Mirasol, J.; Cotoruelo, L.; Rodriguez, J.J. Removal of water pollutants with activated carbons prepared from H3PO4 activation of lignin from kraft black liquors. Water Res. 2004, 38, 3043–3050. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Yoshida, T.; Hidaka, A.; Oshima, T.; Baba, Y. Preparation of activated carbons with high specific surface areas using lignin and crab shells, and their use in methane adsorption. Carbon 2015, 93, 1080. [Google Scholar] [CrossRef]
- Bedia, J.; Belver, C.; Ponce, S.; Rodriguez, J.J.; Rodriguez, J.J. Adsorption of antipyrine by activated carbons from FeCl3-activation of Tara gum. Chem. Eng. J. 2018, 333, 58–65. [Google Scholar] [CrossRef]
- Kazmierczak-Razna, J.; Nowicki, P.; Pietrzak, R. Coniferous Wood Sawdust-based Activated Carbons as Adsorbents Obtained with the Use of Microwave Radiation. J. Wood Chem. Technol. 2018, 38, 286–299. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gómez-Serrano, V.; Gong, H. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Yang, K.; Peng, J.; Srinivasakannan, C.; Zhang, L.; Xia, H.; Duan, X. Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour. Technol. 2010, 101, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Yuen, F.K.; Hameed, B.H. Recent developments in the preparation and regeneration of activated carbons by microwaves. Adv. Colloid Interface Sci. 2009, 149, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J. Application of agricultural based activated carbons by microwave and conventional activations for basic dye adsorption: Review. J. Environ. Chem. Eng. 2016, 4, 89–99. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.-H.; Antonietti, M.; Titirici, M.-M. Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Xu, C.-Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.; Chen, W.; Yang, H.; Chen, H. The structure evolution of biochar from biomass pyrolysis and its correlation with gas pollutant adsorption performance. Bioresour. Technol. 2017, 246, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cordero, D.; Heras, F.; Alonso-Morales, N.; Gilarranz, M.A.; Rodriguez, J.J. Porous structure and morphology of granular chars from flash and conventional pyrolysis of grape seeds. Biomass Bioenergy 2013, 54, 123–132. [Google Scholar] [CrossRef]
- Wafiq, A.; Reichel, D.; Hanafy, M. Pressure influence on pyrolysis product properties of raw and torrefied Miscanthus: Role of particle structure. Fuel 2016, 179, 156–167. [Google Scholar] [CrossRef]
- Cetin, E.; Moghtaderi, B. Effect of pyrolysis pressure and heating rate on radiata pine char structure and apparent gasification reactivity. Fuel 2005, 84, 1328–1334. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Xiang, J.; Sun, L.; Li, P.; Zhang, J.; Zheng, C. Pyrolysis of Maize Stalk on the Characterization of Chars Formed under Different Devolatilization Conditions. Energy Fuels 2009, 23, 4605–4611. [Google Scholar] [CrossRef]
- Burhenne, L.; Damiani, M.; Aicher, T. Effect of feedstock water content and pyrolysis temperature on the structure and reactivity of spruce wood char produced in fixed bed pyrolysis. Fuel 2013, 107, 836–847. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Wang, X.; Zhang, S.; Chen, H. Biomass-based pyrolytic polygeneration system on cotton stalk pyrolysis: Influence of temperature. Bioresour. Technol. 2012, 107, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, J.-Y.; Cho, T.-S.; Choi, J.W. Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). Bioresour. Technol. 2012, 118, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Yang, H.; Chen, Y.; Yang, M.; Chen, L.; Wang, X.; Chen, H. Chemical structure evolution of char during the pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 2015, 116, 263–271. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Rodríguez-Reinoso, F.; Molina-Sabio, M. Activated carbons from lignocellulosic materials by chemical and/or physical activation: An overview. Carbon 1992, 30, 1111–1118. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Carrasco-Marı́n, F.; López-Ramón, M.V.; Alvarez-Merino, M.A. Chemical and physical activation of olive-mill waste water to produce activated carbons. Carbon 2001, 39, 1415–1420. [Google Scholar] [CrossRef]
- Yang, T.; Lua, A.C. Characteristics of activated carbons prepared from pistachio-nut shells by physical activation. J. Colloid Interface Sci. 2003, 267, 408–417. [Google Scholar] [CrossRef]
- Bouchelta, C.; Medjram, M.S.; Bertrand, O.; Bellat, J.-P. Preparation and characterization of activated carbon from date stones by physical activation with steam. J. Anal. Appl. Pyrolysis 2008, 82, 70–77. [Google Scholar] [CrossRef]
- Bansal, R.C.; Donnet, J.-B.; Stoeckli, F. Active Carbon; M. Dekker: New York, NY, USA, 1988. [Google Scholar]
- Gergova, K.; Galushko, A.; Petrov, N.; Minkova, V. Investigation of the porous structure of activated carbons prepared by pyrolysis of agricultural by-products in a stream of water vapor. Carbon 1992, 30, 721–727. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Do, D.D. The preparation of activated carbon from macadamia nutshell by chemical activation. Carbon 1997, 35, 1723–1732. [Google Scholar] [CrossRef]
- Hayashi, J.; Kazehaya, A.; Muroyama, K.; Watkinson, A.P. Preparation of activated carbon from lignin by chemical activation. Carbon 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- Rosas, J.M.; Bedia, J.; Rodríguez-Mirasol, J.; Cordero, T. HEMP-derived activated carbon fibers by chemical activation with phosphoric acid. Fuel 2009, 88, 19–26. [Google Scholar] [CrossRef]
- Rosas, J.M.; Bedia, J.; Rodríguez-Mirasol, J.; Cordero, T. Preparation of Hemp-Derived Activated Carbon Monoliths. Adsorption of Water Vapor. Ind. Eng. Chem. Res. 2008, 47, 1288–1296. [Google Scholar] [CrossRef]
- Girgis, B.S.; El-Hendawy, A.-N.A. Porosity development in activated carbons obtained from date pits under chemical activation with phosphoric acid. Microporous Mesoporous Mater. 2002, 52, 105–117. [Google Scholar] [CrossRef]
- Martin, M.J.; Artola, A.; Balaguer, M.D.; Rigola, M. Activated carbons developed from surplus sewage sludge for the removal of dyes from dilute aqueous solutions. Chem. Eng. J. 2003, 94, 231–239. [Google Scholar] [CrossRef]
- Hasar, H. Adsorption of nickel(II) from aqueous solution onto activated carbon prepared from almond husk. J. Hazard. Mater. 2003, 97, 49–57. [Google Scholar] [CrossRef]
- Tsai, W.; Chang, C.; Lin, M.; Chien, S.; Sun, H.; Hsieh, M. Adsorption of acid dye onto activated carbons prepared from agricultural waste bagasse by ZnCl2 activation. Chemosphere 2001, 45, 51–58. [Google Scholar] [CrossRef]
- Deng, H.; Yang, L.; Tao, G.; Dai, J. Preparation and characterization of activated carbon from cotton stalk by microwave assisted chemical activation—Application in methylene blue adsorption from aqueous solution. J. Hazard. Mater. 2009, 166, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Serrano, E.; Cordero, T.; Rodríguez-Mirasol, J.; Rodríguez, J.J. Development of Porosity upon Chemical Activation of Kraft Lignin with ZnCl2. Ind. Eng. Chem. Res. 1997, 36, 4832–4838. [Google Scholar] [CrossRef]
- Bedia, J.; Monsalvo, V.M.; Rodriguez, J.J.; Mohedano, A.F. Iron catalysts by chemical activation of sewage sludge with FeCl3 for CWPO. Chem. Eng. J. 2017, 318, 224–230. [Google Scholar] [CrossRef]
- Mohedano, A.F.; Monsalvo, V.M.; Bedia, J.; Lopez, J.; Rodriguez, J.J. Highly stable iron catalysts from sewage sludge for CWPO. J. Environ. Chem. Eng. 2014, 2, 2359–2364. [Google Scholar] [CrossRef]
- Zazo, J.A.; Bedia, J.; Fierro, C.M.; Pliego, G.; Casas, J.A.; Rodriguez, J.J. Highly stable Fe on activated carbon catalysts for CWPO upon FeCl3 activation of lignin from black liquors. Catal. Today 2012, 187, 115–121. [Google Scholar] [CrossRef]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Zhu, Z.; Lu, G.Q. A comparative study of chemical treatment by FeCl3, MgCl2, and ZnCl2 on microstructure, surface chemistry, and double-layercapacitance of carbons from waste biomass. J. Mater. Res. 2010, 25, 1451–1459. [Google Scholar] [CrossRef]
- Fu, K.; Yue, Q.; Gao, B.; Sun, Y.; Wang, Y.; Li, Q.; Zhao, P.; Chen, S. Physicochemical and adsorptive properties of activated carbons from Arundo donax Linn utilizing different iron salts as activating agents. J. Taiwan Inst. Chem. Eng. 2014, 45, 3007–3015. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.; Cazorla-Amorós, D.; Linares-Solano, A. Understanding chemical reactions between carbons and NaOH and KOH. Carbon 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A. Preparation of activated carbons from Spanish anthracite—II. Activation by NaOH. Carbon 2001, 39, 751–759. [Google Scholar] [CrossRef]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Jang, H.M.; Yoo, S.; Choi, Y.-K.; Park, S.; Kan, E. Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresour. Technol. 2018, 259, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Castelló, D.; Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Preparation of activated carbons from Spanish anthracite—I. Activation by KOH. Carbon 2001, 39, 741–749. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Microporous activated carbon from Siris seed pods by microwave-induced KOH activation for metronidazole adsorption. J. Anal. Appl. Pyrolysis 2013, 99, 101–109. [Google Scholar] [CrossRef]
- Salman, J.M.; Hameed, B.H. Effect of preparation conditions of oil palm fronds activated carbon on adsorption of Bentazon from aqueous solutions. J. Hazard. Mater. 2010, 175, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, C.; Bedia, J.; Bonal, P.; Rodriguez, J.J.; Gómez-Sainero, L.M. Chloroform conversion into ethane and propane by catalytic hydrodechlorination with Pd supported on activated carbons from lignin. Catal. Sci. Technol. 2018, 8, 3926–3935. [Google Scholar] [CrossRef]
- Tascón, J.M.D. Novel Carbon Adsorbents, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Rodríguez-Reinoso, F.; Molina-Sabio, M. Textural and chemical characterization of microporous carbons. Adv. Colloid Interface Sci. 1998, 76–77, 271–294. [Google Scholar] [CrossRef]
- Patrick, J.W. Porosity in Carbons: Characterization and Applications; Edward Arnold: London, UK, 1995. [Google Scholar]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the bet equation applicable to microporous adsorbents? In Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar]
- Kaneko, K.; Ishii, C. Superhigh surface area determination of microporous solids. Colloids Surf. 1992, 67, 203–212. [Google Scholar] [CrossRef]
- Lippens, B.C.; de Boer, J.H. Studies on pore systems in catalysts: V. The t method. J. Catal. 1965, 4, 319–323. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, and Porosity, 2nd ed.; Academic Press: London, UK; New York, NY, USA, 1982. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Horváth, G.; Kawazoe, K. Method for the calculation of effective pore size distribution in molecular sieve carbon. J. Chem. Eng. Jpn. 1983, 16, 470–475. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A. Usefulness of CO2 adsorption at 273 K for the characterization of porous carbons. In Carbon; Pergamon: Oxford, UK, 2004; Volume 42, pp. 1231–1236. [Google Scholar]
- Gaffney, J.S.; Marley, N.A.; Jones, D.E. Fourier Transform Infrared (FTIR) Spectroscopy. In Characterization of Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Barrios, V.A.E.; Méndez, J.R.R.; Aguilar, N.V.P.; Espinosa, G.A.; Rodríguez, J.L.D. Materials. In Infrared Spectroscopy—Materials Science, Engineering and Technology; InTech: Vienna, Austria, 2012. [Google Scholar]
- Fanning, P.E.; Vannice, M.A. A DRIFTS study of the formation of surface groups on carbon by oxidation. Carbon 1993, 31, 721–730. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Biniak, S.; Szymański, G.; Siedlewski, J.; Świątkowski, A. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; López-Ramón, M.V.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, J.; Liu, D.; Liu, M.; Zhang, H.; Zhao, P.; Zhang, C. Facile synthesis of porous nitrogen-doped carbon for aerobic oxidation of amines to imines. Microporous Mesoporous Mater. 2018, 266, 198–203. [Google Scholar] [CrossRef]
- Szymański, G.S.; Karpiński, Z.; Biniak, S.; Świa̧tkowski, A. The effect of the gradual thermal decomposition of surface oxygen species on the chemical and catalytic properties of oxidized activated carbon. Carbon 2002, 40, 2627–2639. [Google Scholar] [CrossRef]
- Haydar, S.; Moreno-Castilla, C.; Ferro-Garcı́a, M.A.; Carrasco-Marı́n, F.; Rivera-Utrilla, J.; Perrard, A.; Joly, J.P. Regularities in the temperature-programmed desorption spectra of CO2 and CO from activated carbons. Carbon 2000, 38, 1297–1308. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Schönherr, J.; Buchheim, J.; Scholz, P.; Adelhelm, P.; Schönherr, J.; Buchheim, J.R.; Scholz, P.; Adelhelm, P. Boehm Titration Revisited (Part I): Practical Aspects for Achieving a High Precision in Quantifying Oxygen-Containing Surface Groups on Carbon Materials. Carbon 2018, 4, 21. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Faria, P.C.; Órfão, J.J.; Pereira, M.F. Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res. 2004, 38, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Worch, E. Adsorption Technology in Water Treatment; De Gruyter: Berlin, Germany; Boston, MA, USA, 2012. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The Equation of the Characteristic Curve of Activated Charcoal. Proc. Acad. Sci. Phys. Chem. Sect. 1947, 55, 331–333. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A Useful Adsorption Isotherm. J. Phys. Chem. 1959, 63, 1024–1024. [Google Scholar] [CrossRef]
- Toth, J. State Equations of the Solid-gas Interface Layers. Acta Chim. Acad. Sci. Hung. 1971, 69, 311–328. [Google Scholar]
- Dubinin, M.M.; Astakhov, V.A. Development of Ideas of Volume Filling of Micropores during Adsorption of Gases and Vapours by Microporous Adsorbents 2. General Fundamentals of Theory of Gas and Vapour Adsorption on Zeolites. Izv. Akad. Nauk. SSSR-Ser. Khim. 1971, 11. [Google Scholar]
- Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E. (Eds.) Springer: Cham, Switzerland, 2017. [Google Scholar]
- Leyva-Ramos, R.; Geankoplis, C.J. Model simulation and analysis of surface diffusion of liquids in porous solids. Chem. Eng. Sci. 1985, 40, 799–807. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Low, M.J.D. Kinetics of Chemisorption of Gases on Solids. Chem. Rev. 1960, 60, 267–312. [Google Scholar] [CrossRef]
- Eckenfelder, W.W.; William, W. Industrial Water Pollution Control; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Noll, K.E.; Gounaris, V.; Hou, W. Adsorption Technology for Air and Water Pollution Control; Lewis Publishers: Boca Raton, FL, USA, 1992. [Google Scholar]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Najafi Nobar, S.; Farooq, S. Experimental and modeling study of adsorption and diffusion of gases in Cu-BTC. Chem. Eng. Sci. 2012, 84, 801–813. [Google Scholar] [CrossRef]
- Chu, K. Improved fixed bed models for metal biosorption. Chem. Eng. J. 2004, 97, 233–239. [Google Scholar] [CrossRef]
- Bohart, G.S.; Adams, E.Q. Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc. 1920, 42, 523–544. [Google Scholar] [CrossRef]
- Cooney, D.O. Adsorption Design for Wastewater Treatment; Lewis Publishers: Boca Raton, FL, USA, 1999. [Google Scholar]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of Gas Adsorption Kinetics I. A Theoretical Model for Respirator Cartridge Service Life. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Wolborska, A. Adsorption on activated carbon of p-nitrophenol from aqueous solution. Water Res. 1989, 23, 85–91. [Google Scholar] [CrossRef]
- Hamdaoui, O. Removal of copper(II) from aqueous phase by Purolite C100-MB cation exchange resin in fixed bed columns: Modeling. J. Hazard. Mater. 2009, 161, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Reguyal, F.; Sarmah, A.K. Adsorption of sulfamethoxazole by magnetic biochar: Effects of pH, ionic strength, natural organic matter and 17α-ethinylestradiol. Sci. Total Environ. 2018, 628–629, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, Z.; Zhao, J.; Herbert, S.; Xing, B. Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures. Environ. Pollut. 2013, 181, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Chen, W.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of sulfonamides to demineralized pine wood biochars prepared under different thermochemical conditions. Environ. Pollut. 2014, 186, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.-J.; Jiang, H.; Chen, J.-J.; Li, W.-W.; Yu, H.-Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.-R.; Wang, Y.-Y.; Liu, W.-J.; Wang, Y.-K.; Jiang, H. Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem. Eng. J. 2014, 248, 168–174. [Google Scholar] [CrossRef]
- Chen, T.; Luo, L.; Deng, S.; Shi, G.; Zhang, S.; Zhang, Y.; Deng, O.; Wang, L.; Zhang, J.; Wei, L. Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour. Technol. 2018, 267, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J.; Ren, X.; Ye, S.; Peng, B.; Feng, H. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Wang, X.; Liu, N.; Liu, Y.; Jiang, L.; Zeng, G.; Tan, X.; Liu, S.; Yin, Z.; Tian, S.; Li, J.; et al. Adsorption Removal of 17β-Estradiol from Water by Rice Straw-Derived Biochar with Special Attention to Pyrolysis Temperature and Background Chemistry. Int. J. Environ. Res. Public Health 2017, 14, 1213. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; He, L.; Hu, H.; Liu, N.; Gao, S.; Piao, Y. Removal of 17β-estradiol by using highly adsorptive magnetic biochar nanoparticles from aqueous solution. Chem. Eng. J. 2018, 352, 371–379. [Google Scholar] [CrossRef]
- Essandoh, M.; Kunwar, B.; Pittman, C.U.; Mohan, D.; Mlsna, T. Sorptive removal of salicylic acid and ibuprofen from aqueous solutions using pine wood fast pyrolysis biochar. Chem. Eng. J. 2015, 265, 219–227. [Google Scholar] [CrossRef]
- Salem, N.A.; Yakoot, S.M. Non-steroidal Anti-inflammatory Drug, Ibuprofen Adsorption Using Rice Straw Based Biochar. Int. J. Pharmacol. 2016, 12, 729–736. [Google Scholar] [CrossRef]
- Lonappan, L.; Rouissi, T.; Kaur Brar, S.; Verma, M.; Surampalli, R.Y. An insight into the adsorption of diclofenac on different biochars: Mechanisms, surface chemistry, and thermodynamics. Bioresour. Technol. 2018, 249, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Mayer, B.K.; McNamara, P.J. Triclosan adsorption using wastewater biosolids-derived biochar. Environ. Sci. Water Res. Technol. 2016, 2, 761–768. [Google Scholar] [CrossRef]
- Mandal, A.; Singh, N.; Purakayastha, T.J. Characterization of pesticide sorption behaviour of slow pyrolysis biochars as low cost adsorbent for atrazine and imidacloprid removal. Sci. Total Environ. 2017, 577, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ouyang, W.; Hao, F.; Lin, C.; Wang, F.; Han, S.; Geng, X. Properties comparison of biochars from corn straw with different pretreatment and sorption behaviour of atrazine. Bioresour. Technol. 2013, 147, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, H.; Yu, L.; Sun, T. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars. J. Hazard. Mater. 2013, 244–245, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Charrua, A.B.; Weng, C.-H.; Yuan, X.; Ding, F. Characterization of biochars derived from agriculture wastes and their adsorptive removal of atrazine from aqueous solution: A comparative study. Bioresour. Technol. 2015, 198, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Sun, K.; Wu, F.; Gao, B.; Wang, Z.; Kang, M.; Bai, Y.; Zhao, Y.; Liu, X.; Xing, B. Single-solute and bi-solute sorption of phenanthrene and dibutyl phthalate by plant- and manure-derived biochars. Sci. Total Environ. 2014, 473–474, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, A.; Ghosh, S.; Li, F.; Dong, X.; Zhang, D.; Wu, M.; Li, H.; Pan, B. Effect of biochar aging on surface characteristics and adsorption behavior of dialkyl phthalates. Environ. Pollut. 2015, 206, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Greiner, B.G.; Shimabuku, K.K.; Summers, R.S. Influence of biochar thermal regeneration on sulfamethoxazole and dissolved organic matter adsorption. Environ. Sci. Water Res. Technol. 2018, 4, 169–174. [Google Scholar] [CrossRef]
- Han, X.; Liang, C.; Li, T.; Wang, K.; Huang, H.; Yang, X. Simultaneous removal of cadmium and sulfamethoxazole from aqueous solution by rice straw biochar. J. Zhejiang Univ. Sci. B 2013, 14, 640–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Han, X.; Liang, C.; Shohag, M.J.I.; Yang, X. Sorption of sulphamethoxazole by the biochars derived from rice straw and alligator flag. Environ. Technol. 2015, 36, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chu, Y.; Fang, C.; Huang, F.; Song, Y.; Xue, X. Sorption of tetracycline on biochar derived from rice straw under different temperatures. PLoS ONE 2017, 12, e0182776. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Chen, B.; Lin, Y.; Guan, Y. Aromatic and Hydrophobic Surfaces of Wood-derived Biochar Enhance Perchlorate Adsorption via Hydrogen Bonding to Oxygen-containing Organic Groups. Environ. Sci. Technol. 2014, 48, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Liu, F.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of Pharmaceutical Antibiotics on Template-Synthesized Ordered Micro- and Mesoporous Carbons. Environ. Sci. Technol. 2010, 44, 3116–3122. [Google Scholar] [CrossRef] [PubMed]
- Kimbell, L.K.; Tong, Y.; Mayer, B.K.; McNamara, P.J. Biosolids-Derived Biochar for Triclosan Removal from Wastewater. Environ. Eng. Sci. 2018, 35, 513–524. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Pignatello, J.J. π+-π Interactions between (hetero)aromatic amine cations and the graphitic surfaces of pyrogenic carbonaceous materials. Environ. Sci. Technol. 2015, 49, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Cederlund, H.; Börjesson, E.; Lundberg, D.; Stenström, J. Adsorption of Pesticides with Different Chemical Properties to a Wood Biochar Treated with Heat and Iron. Water Air Soil Pollut. 2016, 227, 203. [Google Scholar] [CrossRef]
- Sun, K.; Jin, J.; Keiluweit, M.; Kleber, M.; Wang, Z.; Pan, Z.; Xing, B. Polar and aliphatic domains regulate sorption of phthalic acid esters (PAEs) to biochars. Bioresour. Technol. 2012, 118, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Jonker, M.T.O.; Koelmans, A.A. Sorption of polycyclic aromatic hydrocarbons and polychlorinated biphenyls to soot and soot-like materials in the aqueous environment: Mechanistic considerations. Environ. Sci. Technol. 2002, 36, 3725–3734. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Pan, M.; Chen, J. Kinetic and isothermal adsorption-desorption of PAEs on biochars: Effect of biomass feedstock, pyrolysis temperature, and mechanism implication of desorption hysteresis. Environ. Sci. Pollut. Res. 2018, 25, 11493–11504. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, M.; Shi, C.; Huang, J.; Zhang, B. Adsorption of tetracycline and ciprofloxacin on activated carbon prepared from lignin with H3PO4 activation. Desalin. Water Treat. 2014, 52, 2678–2687. [Google Scholar] [CrossRef]
- Marzbali, M.H.; Esmaieli, M.; Abolghasemi, H.; Marzbali, M.H. Tetracycline adsorption by H3PO4-activated carbon produced from apricot nut shells: A batch study. Process Saf. Environ. Prot. 2016, 102, 700–709. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Wang, M.; Huang, J.; Huang, L. Preparation of activated carbons from Iris tectorum employing ferric nitrate as dopant for removal of tetracycline from aqueous solutions. Ecotoxicol. Environ. Saf. 2013, 98, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Sayğılı, H.; Güzel, F. Effective removal of tetracycline from aqueous solution using activated carbon prepared from tomato (Lycopersicon esculentum Mill.) industrial processing waste. Ecotoxicol. Environ. Saf. 2016, 131, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Güzel, F.; Sayğılı, H. Adsorptive efficacy analysis of novel carbonaceous sorbent derived from grape industrial processing wastes towards tetracycline in aqueous solution. J. Taiwan Inst. Chem. Eng. 2016, 60, 236–240. [Google Scholar] [CrossRef]
- Takdastan, A.; Mahvi, A.H.; Lima, E.C.; Shirmardi, M.; Babaei, A.A.; Goudarzi, G.; Neisi, A.; Heidari Farsani, M.; Vosoughi, M. Preparation, characterization, and application of activated carbon from low-cost material for the adsorption of tetracycline antibiotic from aqueous solutions. Water Sci. Technol. 2016, 74, 2349–2363. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-T.; Shi, L.-Q.; Shen, H.; Chen, X.; Xie, K.-P. Equilibrium, isotherm, kinetic and thermodynamic studies for removal of tetracycline antibiotics by adsorption onto hazelnut shell derived activated carbons from aqueous media. RSC Adv. 2016, 6, 109983–109991. [Google Scholar] [CrossRef]
- Oliveira, G.; Calisto, V.; Santos, S.M.; Otero, M.; Esteves, V.I. Paper pulp-based adsorbents for the removal of pharmaceuticals from wastewater: A novel approach towards diversification. Sci. Total Environ. 2018, 631–632, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Moura, F.C.C.; Rios, R.D.F.; Galvão, B.R.L. Emerging contaminants removal by granular activated carbon obtained from residual Macauba biomass. Environ. Sci. Pollut. Res. 2018, 25, 26482–26492. [Google Scholar] [CrossRef] [PubMed]
- To, M.-H.; Hadi, P.; Hui, C.-W.; Lin, C.S.K.; McKay, G. Mechanistic study of atenolol, acebutolol and carbamazepine adsorption on waste biomass derived activated carbon. J. Mol. Liq. 2017, 241, 386–398. [Google Scholar] [CrossRef]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 2012, 211–212, 310–317. [Google Scholar] [CrossRef]
- Chakraborty, P.; Banerjee, S.; Kumar, S.; Sadhukhan, S.; Halder, G. Elucidation of ibuprofen uptake capability of raw and steam activated biochar of Aegle marmelos shell: Isotherm, kinetics, thermodynamics and cost estimation. Process Saf. Environ. Prot. 2018, 118, 10–23. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Carvalho, A.P. Activated carbons for the adsorption of ibuprofen. Carbon 2007, 45, 1979–1988. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Ruiz-Rosas, R.; Marqués, M.D.; Cotoruelo, L.M.; Rodríguez-Mirasol, J.; Cordero, T. Removal of paracetamol on biomass-derived activated carbon: Modeling the fixed bed breakthrough curves using batch adsorption experiments. Chem. Eng. J. 2015, 279, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.; Lim, Y.; Ngadi, N.; Mat, R.; Hassan, O.; Inuwa, I.M.; Mohamed, N.B.; Low, J.H. Removal of acetaminophen by activated carbon synthesized from spent tea leaves: Equilibrium, kinetics and thermodynamics studies. Powder Technol. 2018, 338, 878–886. [Google Scholar] [CrossRef]
- Chen, D.; Xie, S.; Chen, C.; Quan, H.; Hua, L.; Luo, X.; Guo, L. Activated biochar derived from pomelo peel as a high-capacity sorbent for removal of carbamazepine from aqueous solution. RSC Adv. 2017, 7, 54969–54979. [Google Scholar] [CrossRef] [Green Version]
- Torrellas, S.Á.; García Lovera, R.; Escalona, N.; Sepúlveda, C.; Sotelo, J.L.; García, J. Chemical-activated carbons from peach stones for the adsorption of emerging contaminants in aqueous solutions. Chem. Eng. J. 2015, 279, 788–798. [Google Scholar] [CrossRef]
- Cunha, M.R.; Lima, E.C.; Cimirro, N.F.G.M.; Thue, P.S.; Dias, S.L.P.; Gelesky, M.A.; Dotto, G.L.; dos Reis, G.S.; Pavan, F.A. Conversion of Eragrostis plana Nees leaves to activated carbon by microwave-assisted pyrolysis for the removal of organic emerging contaminants from aqueous solutions. Environ. Sci. Pollut. Res. 2018, 25, 23315–23327. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, K.K.; Cazetta, A.L.; de Souza, P.S.C.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol. Environ. Saf. 2018, 147, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lupul, I.; Yperman, J.; Carleer, R.; Gryglewicz, G. Adsorption of atrazine on hemp stem-based activated carbons with different surface chemistry. Adsorption 2015, 21, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Chaparadza, A.; Hossenlopp, J.M. Adsorption kinetics, isotherms and thermodynamics of atrazine removal using a banana peel based sorbent. Water Sci. Technol. 2012, 65, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Mohsen Nourouzi, M.; Chuah, T.G.; Choong, T.S.Y. Adsorption of glyphosate onto activated carbon derived from waste newspaper. Desalin. Water Treat. 2010, 24, 321–326. [Google Scholar] [CrossRef]
- Salman, J.M. Batch Study for Insecticide Carbofuran Adsorption onto Palm-Oil-Fronds-Activated Carbon. J. Chem. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Al Bahri, M.; Calvo, L.; Gilarranz, M.A.; Rodriguez, J.J. Activated carbon from grape seeds upon chemical activation with phosphoric acid: Application to the adsorption of diuron from water. Chem. Eng. J. 2012, 203, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Bouhamidi, Y.; Kaouah, F.; Nouri, L.; Boumaza, S.; Trari, M.; Bendjama, Z. Kinetic, thermodynamic, and isosteric heat of dibutyl and diethyl phthalate removal onto activated carbon from Albizzia julibrissin pods. Part. Sci. Technol. 2018, 36, 235–243. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, L. Adsorption characteristics of dibutyl phthalate from aqueous solution using ginkgo leaves-activated carbon by chemical activation with zinc chloride. Desalin. Water Treat. 2015, 54, 1969–1980. [Google Scholar] [CrossRef]
- Fang, Z.Q.; Huang, H.J. Adsorption of Di-N-butyl Phthalate onto Nutshell-Based Activated Carbon. Equilibrium, Kinetics and Thermodynamics. Adsorpt. Sci. Technol. 2009, 27, 685–700. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Johir, M.A.H.; Sornalingam, K. Sorptive removal of phenolic endocrine disruptors by functionalized biochar: Competitive interaction mechanism, removal efficacy and application in wastewater. Chem. Eng. J. 2018, 335, 801–811. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Parra, J.B.; Carvalho, A.P.; Ania, C.O. Waste-derived activated carbons for removal of ibuprofen from solution: Role of surface chemistry and pore structure. Bioresour. Technol. 2009, 100, 1720–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, S.; Bobde, K.; Aikat, K.; Halder, G. Biosorptive uptake of ibuprofen by steam activated biochar derived from mung bean husk: Equilibrium, kinetics, thermodynamics, modeling and eco-toxicological studies. J. Environ. Manag. 2016, 182, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Kinetic study of the decomposition of 2-butanol on carbon-based acid catalyst. AIChE J. 2010, 56, 1557–1568. [Google Scholar] [CrossRef]
- Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. A kinetic study of 2-propanol dehydration on carbon acid catalysts. J. Catal. 2010, 271, 33–42. [Google Scholar] [CrossRef]
- Sarıcı-Özdemir, Ç.; Önal, Y. Synthesis of new activated carbons produced from polymer waste. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 451–457. [Google Scholar] [CrossRef]
- Chang, K.L.; Shih, Y.H.; Tseng, C.H.; Chen, S.T.; Chen, C.C. Adsorption Studies on the Removal of an Herbicide (Atrazine) Using Activated Carbons Prepared from Agricultural Waste Sugarcane Bagasse. Appl. Mech. Mater. 2012, 251, 378–382. [Google Scholar] [CrossRef]
- Jung, C.; Park, J.; Lim, K.H.; Park, S.; Heo, J.; Her, N.; Oh, J.; Yun, S.; Yoon, Y. Adsorption of selected endocrine disrupting compounds and pharmaceuticals on activated biochars. J. Hazard. Mater. 2013, 263, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Toledo, I.; Ferro-García, M.A.; Rivera-Utrilla, J.; Moreno-Castilla, C.; Fernández, F.J.V. Bisphenol A Removal from Water by Activated Carbon. Effects of Carbon Characteristics and Solution Chemistry. Environ. Sci. Technol. 2005, 39, 6246–6250. [Google Scholar] [CrossRef] [PubMed]
- Stoeckli, F.; Hugi-Cleary, D. On the mechanisms of phenol adsorption by carbons. Russ. Chem. Bull. 2001, 50, 2060–2063. [Google Scholar] [CrossRef]
- Radovic, L.R.; Silva, I.F.; Ume, J.I.; Menéndez, J.A.; Leon, C.A.L.Y.; Scaroni, A.W. An experimental and theoretical study of the adsorption of aromatics possessing electron-withdrawing and electron-donating functional groups by chemically modified activated carbons. Carbon 1997, 35, 1339–1348. [Google Scholar] [CrossRef]
- Radovic, L.R. Chemistry and Physics of Carbon: A Series of Advances; Marcel Dekker, Inc.: New York, NY, USA, 2001; Volume 27. [Google Scholar]
- Fang, Z.; Smith, R.L. Production of Biofuels and Chemicals from Lignin; Springer: Singapore, 2016. [Google Scholar]
- Rodríguez, J.J.; Cordero, T.; Rodríguez-Mirasol, J. Carbon Materials from Lignin and Their Applications. In Production of Biofuels and Chemicals from Lignin; Springer: Singapore, 2016; pp. 217–262. [Google Scholar]
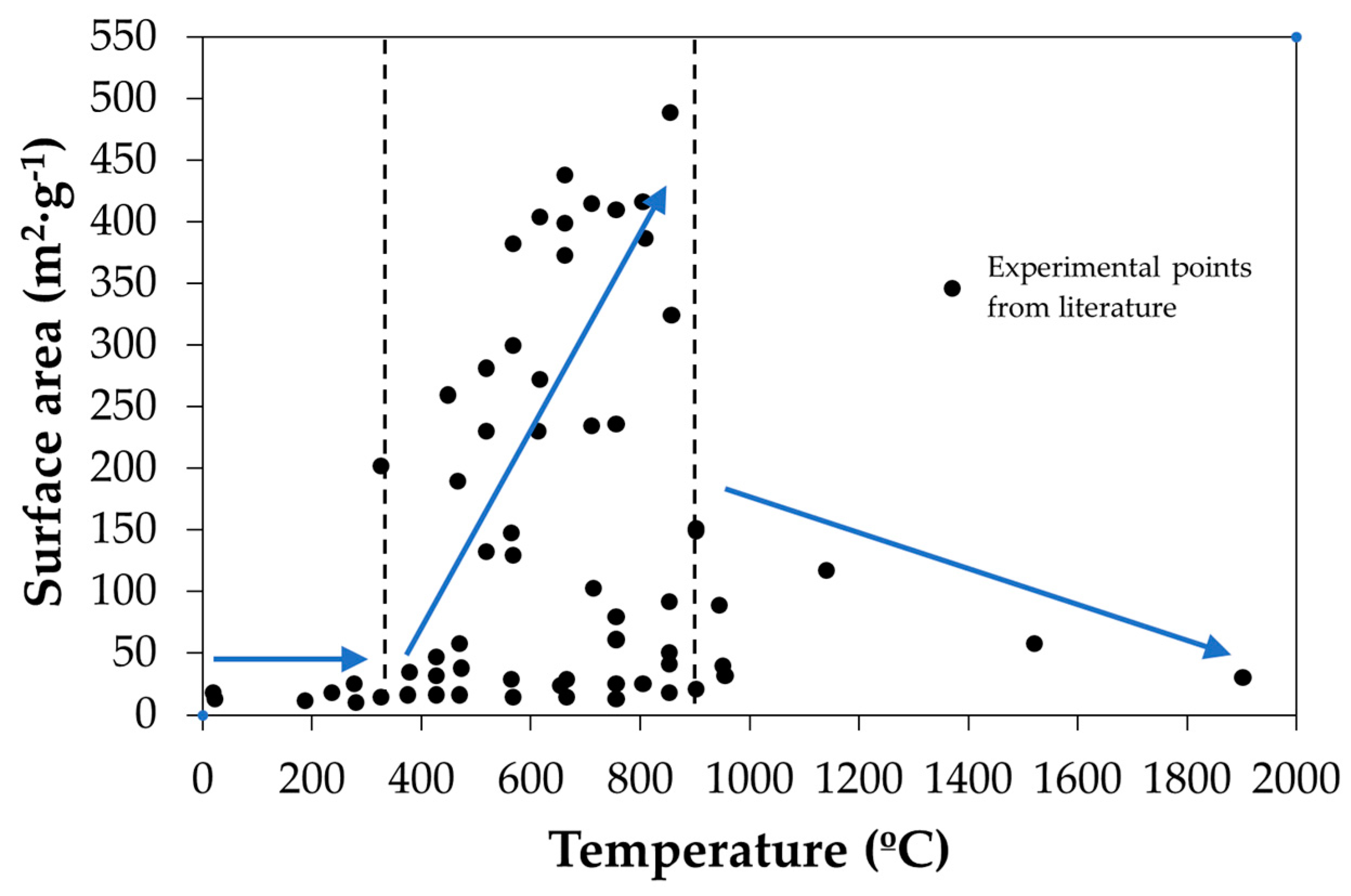

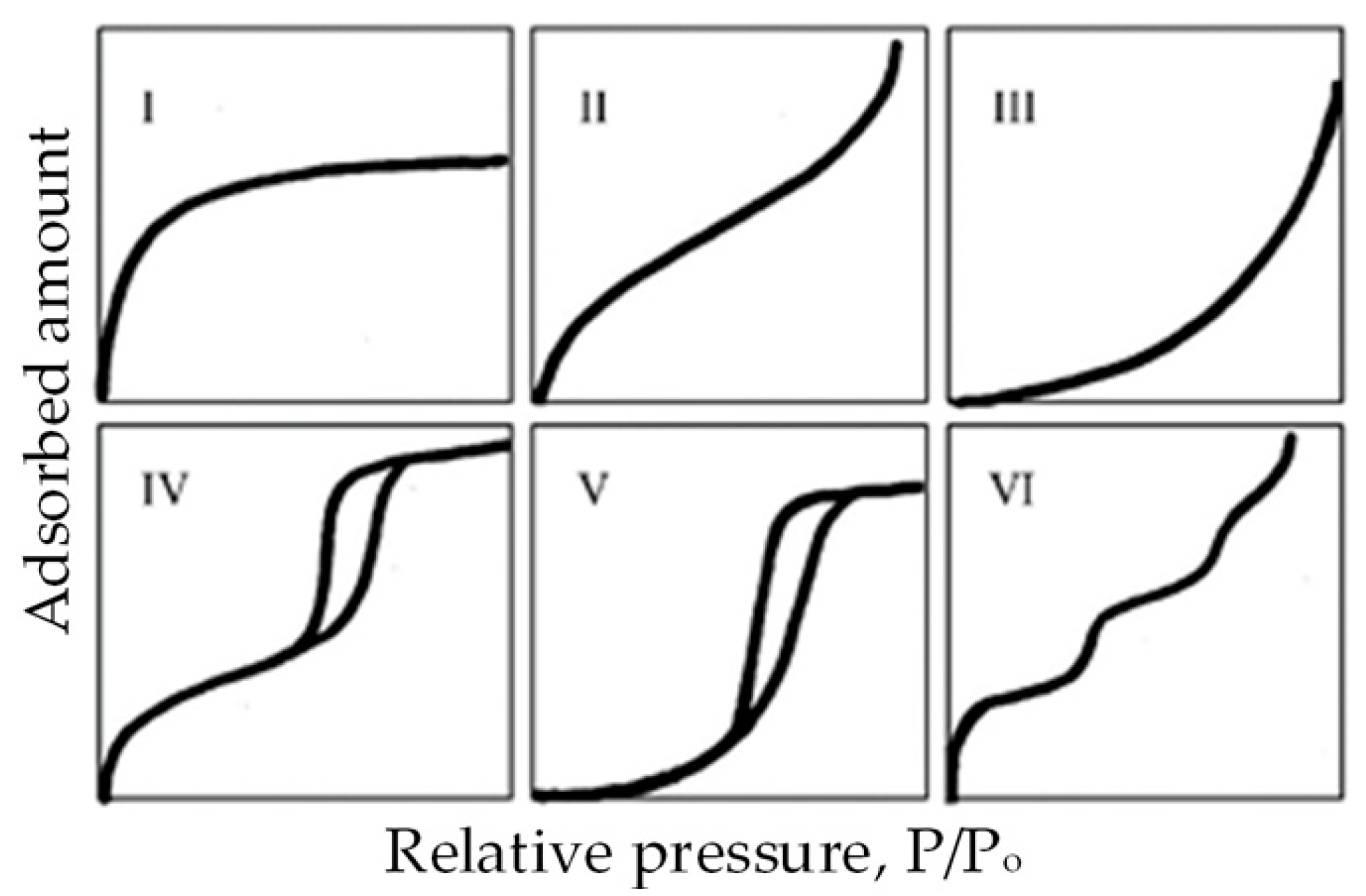
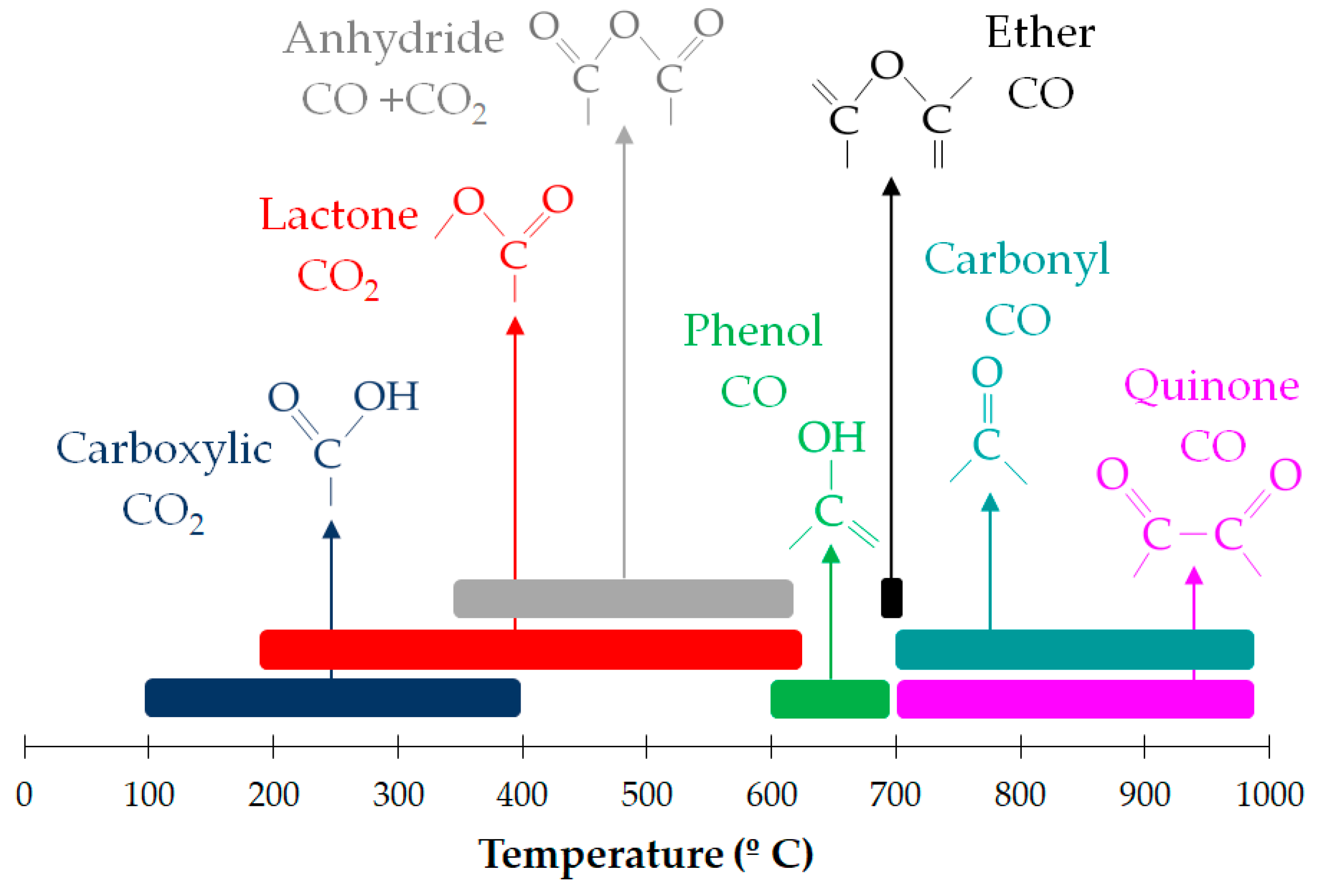
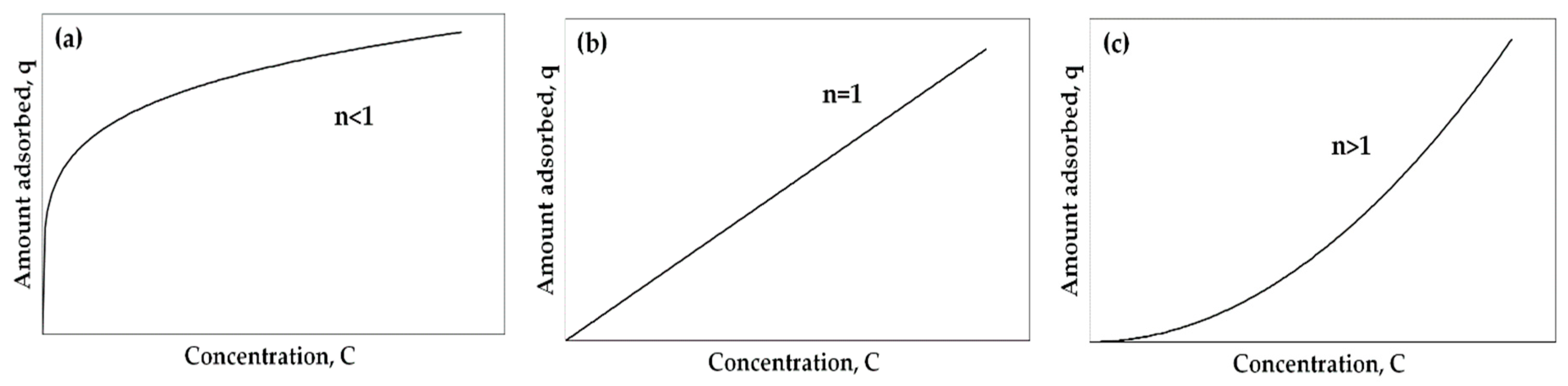
| Main Categories | Family | Most Representative Examples |
|---|---|---|
| Drugs and pharmaceuticals | antibiotics/antibacterials | tetracycline, sulfamethoxazole, amoxicillin, ofloxacin |
| steroids | 17β-estradiol, testosterone | |
| β-blocker | propranolol, salbutamol, atenolol | |
| nonsteroidal anti-inflammatory drugs (NSAID) | ibuprofen, naproxen, ketoprofen, diclofenac | |
| antiepileptic/anticonvulsants | gabapentin, carbamazepine | |
| antidepressant/hypnotic | diazepam, venlafaxine, amitriptyline, dosulepin, meprobanate | |
| analgesic | morphine, antipyrine, paracetamol | |
| hypertension | valsartan | |
| lipid regulation | bezafibrate, simvastatin, clofibric acid | |
| erectile dysfunction | sildenafil | |
| Stimulant and generally illegal drugs | hallucinogen | 3,4-methylenedioxymethamphetamine (MDMA), 3,4-methylenedioxy-N-ethylamphetamine (MDEA), 3,4-methylenedioxyamphetamine (MDA) |
| stimulant | amphetamine, cocaine, benzylpiperazine | |
| human indicator | caffeine, nicotine | |
| Personal care products | preservative | methylparaben, propylparaben |
| sunscreen agent | 1-benzophenone, homosalate | |
| disinfectants/antiseptic | chloramines, chlorine, chlorine dioxide, chlorhexidine digluconate, triclosan | |
| fragrances | musk xylol, tonalide | |
| Pesticides/herbicides | organohalogenated compounds | dichlorodiphenyltrichloroethane (DDT), lindane, vinclozolin, clopyralid |
| nitrogen containing | simazine, phenylurea, atrazine, glyphosate, diuron | |
| Plasticizers [22] | general purpose | phthalates |
| low volatility | trimetallites | |
| flame-resistant | epoxides |
| σ (cm−1) | Assignment | Designation |
|---|---|---|
| 3700 | υ (OH) | Free OH groups |
| 3400 | υ (OH) | Stretching in hydroxyl groups |
| 2926 | υ (C–H) | Stretching in alkyl groups |
| 2870 | υ (C–H) | Stretching in alkyl groups |
| 1745 | υ (C=O) | Stretching in aldehydes, ketones groups and esters |
| 1642 | υ (C=O) | Stretching in cyclic amide |
| 1640 | υ (C=C) υ (C=O) | Stretching in olefins carbonyl groups of carbon material highly conjugated in graphite layer |
| 1552 | υ (C=O) | Assigned to carbonyl groups conjugated in aromatic |
| 1540 | υ (C=C) | Assigned to skeletal stretch in condensed aromatic system |
| 1520 | υ (C=C) | Aromatic skeletal stretching bands |
| 1462 | υ (C–H) | Stretching in aromatic ring |
| 1460 | υ (C=O) | Stretching in cyclic amide |
| 1444 | υ (C=C), δ (CH) | Stretching in aromatic skeletal and ester |
| 1150–1200 | υ (C–O–C), υ (C–C), | Stretching vibration in pyranose ring skeletal or stretching in aromatic ring |
| 1137 | υ (C–O) | stretching in phenolic ring, carboxylic moiety |
| 1069 | υ (C–O–C) | Asymmetrical stretch vibrations |
| 870, 690 | γ (C–H) | Aromatic C–H out-of-plane bending vibrations |
| 610 | υ (O–H) | Stretching in OH groups |
| 450 | υ (C–C) | Stretching in C–C vibrations |
| Elemental Spectrum | Binding Energy (eV) | Assignation |
|---|---|---|
| C1s | 284.1–284.4 | C–C; C–H; graphite type |
| 284.8–285.2 | C–OH; amorphous carbon, hydroxyl groups, phenolic, alcohol or ether aromatic carbon | |
| 285.5–286.1 | C–O–C; carbonyl groups | |
| 286.3–287.6 | O–C=O; carboxyl and ester groups | |
| 289.5–290.0 | π–π* transitions in the aromatic carbon | |
| O1s | 530.0–531.6 | C=O |
| 532.7–533.3 | C–OH or C–O–C | |
| 534.8–535.7 | chemisorbed oxygen | |
| N1s | 398.0–398.3 | N-6 or pyridine-like structures |
| 400.0–400.3 | N-5, i.e., pyrrolic | |
| 401.1–401.6 | graphitic nitrogen | |
| 402.3–404.0 | N-oxides |
| Model | Equation | |
|---|---|---|
| Langmuir–Freundlich isotherm/Sips isotherm | [140] | |
| Redlich–Peterson isotherm | [141] | |
| Tóth isotherm | [142] | |
| Dubinin-Astakhov (DA) equation | [143] | |
| Contaminant | Biomass Precursor | Synthesis Conditions | Biochar Characteristics | Adsorption Parameters | Main Interactions Controlling Adsorption | Ref. |
|---|---|---|---|---|---|---|
| Antibiotics | ||||||
| Sulfamethoxazole | Pine sawdust | Treatment in FeCl2, KOH and KNO3 solutions 90 °C | SBET = 126 m2·g−1 O = 14.2% pHpzc = 9.46 | Co = 0.5–9.0 mg·L−1 W = 20 mg·L−1 T = 25 °C pH = 4.5–8.5 qmax = 19.1 mg·g−1 | π–π electron donor acceptor (EDA) Hydrophobic | [160] |
| Sulfamethoxazole | Giant reed (Arundo donax L.) | Carbonization 300–600 °C | SBET ≤ 88 m2·g−1 O = 9.0–21.0% | Co = 0–80 mg·L−1 Room temperature pH = 1.0–12.0 qmax = 3.5 mg·g−1 | π–π EDA Hydrophobic Pore filling | [161] |
| Sulfamethoxazole and sulfapyridine | Pine wood | Carbonization 400–500 °C | SBET = 101–434 m2·g−1 O = 6.6–16.1% | pH = 1.7–11.0 Room temperature qmax SMX = 22.8 mg·g−1 qmax SP= 22.4 mg·g−1 | π–π EDA | [162] |
| Sulfamethoxazole, sulfathiazole and sulfamethazine | Bamboo | Carbonization at 380 °C and H3PO4 treatment | SBET < 1 m2·g−1 O = 18.9–39.5% | W = 100 mg·L−1 T = 21–30 °C pH = 1.0–10.0 qmax SMX = 88.1 mg·g−1 qmax STZ = 237 mg·g−1 qmax SMZ = 65.7 mg·g−1 | Neutral species: H-bonds π–π EDA Lewis acid-base Positive species: π–π EDA Negative species: Proton exchange π–π EDA | [163] |
| Tetracycline | Rice husk | Carbonization at 500 °C and H2SO4 or KOH treatments | SBET = 34–118 m2·g−1 O = 15.4–24.4% | Co = 50–1000 mg·L−1 W = 5 g·L−1 T = 30 °C Natural pH qmax = 58.8 mg·g−1 | Surface areaπ–π EDA O-groups | [164] |
| Tetracycline | Rice husk | Methanol treatment of the biochar | SBET = 66 m2·g−1 O = 23.4% | Co = 100 mg·L−1 W = 1 g·L−1 T = 30 °C Natural pH qmax = 95 mg·g−1 | π–π EDA O-groups | [165] |
| Tetracycline | Rice straw and Swine manure | Carbonization at 700 °C and treatment with H3PO4 | SBET = 227–372 m2·g−1 O = 4.7–7.2% pHpzc = 9.46 | Co = 30–200 mg·L−1 W = 166–33 mg·L−1 T = 25 °C pH = 5.0–9.0 qmax = 167.5 mg·g−1 | H-bonding π–π EDA | [166] |
| Tetracycline | Municipal sewage sludge | Carbonization at 400–800 °C and nitric or glacial acetic acid treatment | SBET = 24–202 m2·g−1 | Co = 50–800 mg·L−1 W = 1 g·L−1 T = 25–45 °C pH = 3.0–11.0 qmax = 287 mg·g−1 | π–π stacking pore filling | [167] |
| Sterorids | ||||||
| 17β-estradiol | Rice straw | Carbonization at 400–600 °C | SBET = 5.1–7.7 m2·g−1 | W = 200 mg·L−1 T = 28 °C pH = 3.0–12.0 qmax = 64.9 mg·g−1 | π–π interaction, electrostatic repulsion, film diffusion and multilayer adsorption | [168] |
| 17β-estradiol | Bagasse | Carbonization at 400–800 °C | SBET =167–339 m2·g−1 O = 10.1–19.2% | Co = 0.6–3.0 mg·L−1 W = 200 mg·L−1 T = 25 °C pH = 3.0–9.0 qmax = 50.2 mg·g−1 | H-bonds π–π interaction | [169] |
| Anti-inflammatory | ||||||
| Ibuprofen | Pine wood | Carbonization at 425 °C | SBET ~1 m2·g−1 O = 19.0% pHpzc = 2.0 | Co = 25–100 mg·L−1 T = 5–25 °C pH = 2.0–10.0 qmax = 22.7 mg·g−1 | O-groups | [170] |
| Ibuprofen | Rice straw | Carbonization at 400–600 °C | SBET = 71.3–63.0 m2·g−1 O = 30–49% pHs = 9.1–9.3 | Co = 10–100 mg·L−1 W = 50 mg·L−1 Room temperature qmax = 170 mg·g−1 | π–π interaction Electrostatic attraction | [171] |
| Diclofenac | Pig manure | Carbonization at 400 °C | SBET = 43.5 m2·g−1 pHIEP = 2.15 | Co = 0.1–10 mg·L−1 W = 2 g·L−1 T = 25 °C pH = 6.5 qmax = 12.5 mg·g−1 | electrostatic interactions, H-bonding, hydrophobic effects, π–π EDA | [172] |
| Antimicrobial | ||||||
| Triclosan | Activated sludge biosolid | Carbonization at 300–800 °C and HCl acid treatment | SBET = 21–141 m2·g−1 O = 4.2–16% pHs = 2.9–3.5 | Co = 0.2–0.3 mg·L−1 W = 0.2–1.0 g·L−1 T = 25 °C pH = 3.0–11.0 qmax = 0.87 mg·g−1 | Hydrophobicity, hydrogen bonding and π-stacking | [173] |
| Pesticides | ||||||
| Atrazine and imidacloprid | Five agricultural wastes | Carbonization at 600 °C and treatment with H3PO4 | SBET = 159–246 m2·g−1 O = 8.3–12.2% pHs = 6.9–10.1 | Co = 1.0–10.0 µg·L−1 W = 1.0 g·L−1 T = 27 °C qmax atraz= 5.2 mg·g−1 qmax imid= 4.5 mg·g−1 | Atrazine: H/C ratio Mesopore Imidacloprid: H/C ratio Polarity | [174] |
| Atrazine | Corn straw | Carbonization at 200 and 450 °C in presence and absence of NH4H2PO4 | SBET = 45–356 m2·g−1 O = 20.6–22.1% | Co = 0.5–50.0 mg·L−1 W = 125–500 mg·L−1 T = 10–30 °C pH = 2.0–9.0 qmax = 84.5 mg·g−1 | Microporosity π–π EDA | [175] |
| Atrazine | Pig manure | Carbonization at 350 or 700 °C and ash removal | SBET = 23.8–218.1 m2·g−1 O = 4.8–20.3% pHs = 6.2–9.5 | Co = 2.0–40.0 mg·L−1 W = 1.25 g·L−1 T = 20–24 °C pH = 6.5 | Hydrophobicity Pore filling π–π EDA | [176] |
| Atrazine | Several biomass wastes | Carbonization at 450 °C | SBET = 41.2–62.2 m2·g−1 O = 11.6–17.6% pHpzc = 8.3–9.2 | Co = 5.0–35.0 mg·L−1 W = 10 g·L−1 T = 10–40 °C pH = 4.5–11.0 qmax ~ 1.5 mg·g−1 | Pore filling | [177] |
| Plasticizers | ||||||
| Dibutyl phthalate (DBP) | Different plants and manure | Carbonization at 200 and 450 °C and ash removal | SCO2 = 162–402 m2·g−1 O = 10.2–16.7% | Co = 0.1–10.0 mg·L−1 T = 23 °C | π–π EDA | [178] |
| Dimethyl phthalate (DMP) Diethyl phthalate (DEP) Dibutyl phthalate (DBP) | Peanut shells | Carbonization at 300 or 700 °C and HNO3/H2SO4 acid treatment | SBET = 7.0–381.0 m2·g−1 O = 8.7–42.5% | Co = 1.0–100.0 mg·L−1 Room temperature pH = 7 qmax DMP = 110.5 mg·g−1 qmax DEP = 506.7 mg·g−1 qmax DBP = 216.1 mg·g−1 | Hydrophylic π–π EDA | [179] |
| Contaminant | Activating Agent and Synthesis Conditions | Biomass Precursor | Activated Carbon Characteristics | Adsorption Parameters | Main Interactions Controlling Adsorption | Ref. |
|---|---|---|---|---|---|---|
| Antibiotics | ||||||
| Tetracycline | NaOH r = 3:1 700 °C | Macadamia nut shells | SBET = 1524 m2·g−1 pHpzc = 8.74 Acid groups = 0.63 mmol·g−1 Basic groups = 1.0 mmol·g−1 | Co = 250–800 mg·L−1 T = 25 °C pH = 3.0–10.0 qmax = 455.3 mg·g−1 | At low pH, π–π dispersion. At medium-high pH, hydrogen bonding. | [104] |
| Tetracycline and ciprofloxacin | H3PO4 r = 2:1 450 °C | Lignin | SBET = 933 m2·g−1 pHpzc = 2.60 Acid groups = 3.9 mmol·g−1 Basic groups = 0.18 mmol·g−1 | Co = 180–600 mg·L−1 W = 1.0 g·L−1 T = 20–40 °CpH = 5.5 qmax TC = 475.5 mg·g−1 qmax CP = 418.6 mg·g−1 | H bonding | [193] |
| Tetracycline | H3PO4 450 °C | Apricot stones | SBET = 308 m2·g−1 pHpzc = 2.13 | Co = 100–200 mg·L−1 T = 30–55 °C pH = 1.5–8.5 qmax = 308.3 mg·g−1 | Low pH, electrostatic attraction. Medium pH, π–π EDA. High pH, electrostatic repulsion | [194] |
| Tetracycline | H3PO4–Fe(NO3)3 450 °C | Iris tectorum | SBET = 1371 m2·g−1 Acid groups = 3.8 mmol·g−1 | Co = 350–800 mg·L−1 W = 600 mg·L−1 T = 22 °C pH = 2.0–11.0 qmax = 769.2 mg·g−1 | Electrostatic attraction | [195] |
| Tetracycline | ZnCl2 r = 6:1 600 °C | Tomato industrial waste | SBET = 1093 m2·g−1 pHpzc = 6.17 Acid groups = 1.17 meq·g−1 Basic groups = 1.04 meq·g−1 | Co = 200–400 mg·L−1 W = 200–1000 mg·L−1 T = 15–35 °C pH = 5.7 qmax = 500.0 mg·g−1 | -- | [196] |
| Tetracycline | ZnCl2 r = 6:1 600 °C | Grape industrial processing pulps | SBET = 1455 m2·g−1 pHpzc = 5.86 Acid groups = 1.24 meq·g−1 Basic groups = 0.88 meq·g−1 | Co = 200–400 mg·L−1 W = 200–1000 mg·L−1 T = 15–35 °C pH = 5.7 qmax = 625.0 mg·g−1 | -- | [197] |
| Tetracycline | ZnCl2 r = 0.5:1 700 °C | Oak charcoals | SBET = 224 m2·g−1 | Co = 25–100 mg·L−1 W = 0.25–3.5 g·L−1 T = 20 °C pH = 3.0–11.0 qmax = 282.1 mg·g−1 | Low-medium pH, π–π dispersion and hydrophobic High pH electrostatic repulsion | [198] |
| Tetracycline | NaOH800 °C | Pinus taeda | SBET = 960 m2·g−1 pHpzc = 6.83 | Co = 20–100 mg·L−1 W = 100 mg·L−1 T = 20 °C pH = 3.0–9.0 qmax = 274.8 mg·g−1 | Low-medium pH, π–π dispersion and hydrophobic High pH electrostatic repulsion | [105] |
| Tetracycline, oxytetracycline and chlortetracycline | H3PO4 r ~ 1:1 900 °C | Hazelnut shell | SBET = 1425 m2·g−1 pHpzc = 4.2 | Co = 0.1–1.0 g·L−1 W = 4 g·L−1 T = 20–50 °C pH = 2.0–10.0 qmax TC = 302.9 mg·g−1 qmax OTC= 321.5 mg·g−1 qmax ClT = 313.5 mg·g−1 | Low pH, repulsive forces (+/+) Medium pH, hydrogen bonding, EDA, and π–π dispersion High pH, repulsive forces (-/-) | [199] |
| Sulfamethoxazole | H3PO4 K2CO3 r = 1:1 800 °C | Bleached pulp | SBET = 814–965 m2·g−1 pHpzc = 2.3–4.9 | Co = 0.035–0.30 g·L−1 T = 25 °C pH = 7.3 qmax = 13.0 mg·g−1 | Repulsive forces | [200] |
| Amoxicillin | ZnCl2 r = 2:1 900 °C | Macauba palm waste | SBET = 907 m2·g−1 pHIEP = 5.9 | Co = 10–1000 mg·L−1 W = 10 g·L−1 T = 25 °C pH = 3.0–9.0 qmax = 38.0 mg·g−1 | At low pH electrostatic attraction | [201] |
| Steroids | ||||||
| Ethinylestradiol | ZnCl2 r = 2:1900 °C | Macauba palm waste | SBET = 907 m2·g−1 pHIEP = 5.9 | Co = 10–1000 mg·L−1 W = 10 g·L−1 T = 25 °C pH = 3.0–9.0 qmax = 38.0 mg·g−1 | π–π interaction | [201] |
| β-blockers | ||||||
| Atenolol, acebutolol | CO2 700 °C | Palm kernel shell | SBET = 711 m2·g−1 pHpzc = 11.5 | Co = 100–250 mg·L−1 W = 1 g·L−1 T = 25 °C pH = 3.0–10.0 qmax ATE= 183.4 mg·g−1 qmax ACE= 225.4 mg·g−1 | Hydrogen bonding | [202] |
| Anti-inflammatory | ||||||
| Ibuprofen, naproxen, ketoprofen, diclofenac | H3PO4 | Olive-waste cakes | SBET = 793 m2·g−1 | T = 20–40 °C pH = 2.0–8.6 qmax IBU = 12.6 mg·g−1 qmax NAP= 39.5 mg·g−1 qmax KET= 24.7 mg·g−1 qmax DIC= 56.2 mg·g−1 | Low-medium pH hydrogen bonding and/or Van der Waals interaction. High pH, electrostatic repulsion | [203] |
| Ibuprofen | Steam 800 °C | Aegle marmelos shell | SBET = 308 m2·g−1 pHpzc = 7.2 | Co = 0.033–3.33 g·L−1 W = 1.0–45.0 mg·L−1 T = 15–45 °C pH = 2.0–6.0 qmax = 12.6 mg·g−1 | Electrostatic interactions | [204] |
| Ibuprofen | K2CO3 r = 1:1, 700 °C and steam 750 °C | Cork waste | SBET = 1060 m2·g−1 | T = 20–40 °C pH = 2.0–11–0 qmax = 416.7 mg·g−1 | Electrostatic interactions | [205] |
| Analgesic | ||||||
| Paracetamol | H3PO4 r = 3:1 500 °C | Olive stones | SBET = 990 m2·g−1 | Co =0.3–10.0 mg·L−1 T = 25 °C Natural pH qmax = 98.4 mg·g−1 | -- | [206] |
| Paracetamol | H3PO4 r = 1:1 600 °C | Spent tea leaves | SBET = 1208 m2·g−1 pHpzc = 2.02 | Co = 10–100 mg·L−1 W = 0.1–1.0 g·L−1 T = 25–60 °C pH = 3.0–11.0 qmax = 59.2 mg·g−1 | High pH, electrostatic repulsion | [207] |
| Antipyrine | FeCl3 r = 2:1 800 °C | Tara gum | SBET = 1680 m2·g−1 | Co = 10–100 mg·L−1 W = 200–400 mg·L−1 T = 20–60 °C Natural pH qmax = 275.0 mg·g−1 | Pore filling O-functionalities | [55] |
| Antiepileptic | ||||||
| Carbamazepine | H3PO4 K2CO3 r = 1:1 800 °C | Bleached pulp | SBET = 814–965 m2·g−1 pHpzc = 2.3–4.9 | Co = 0.035–0.30 g·L−1 T = 25 °C pH = 7.3 qmax = 92.0 mg·g−1 | -- | [200] |
| Carbamazepine | CO2 700 °C | Palm kernel shell | SBET = 711 m2·g−1 pHpzc = 11.5 | Co = 100–250 mg·L−1 W = 1 g·L−1 T = 25 °C pH = 3.0–10.0 qmax = 170.1 mg·g−1 | Hydrophobic and π–π intreactions | [202] |
| Carbamazepine | KOH r= 1:1 700 °C | Pomelo peels | SBET = 904 m2·g−1 pHpzc = 4.46 | Co = 10–100 mg·L−1 T = 25 °C pH = 2.0–12.0 qmax = 286.5 mg·g−1 | hydrophobic, π–π interactions and hydrogen bonding | [208] |
| Carbamazepine | H3PO4 435 °C | Peach stones | SBET = 1216 m2·g−1 pHpzc = 3.1 | Co = 100 mg·L−1 T = 30 °C pH naturalqmax = 335.0 mg·g−1 | π–π interactions | [209] |
| Stimulant | ||||||
| Caffeine | ZnCl2 (MW) * r = 1:1, 5.3 min, 1300 W | Eragrostis plana Nees leaves | SBET = 1250 m2·g−1 pHpzc = 3.65 Acid groups = 1.04 mmol·g−1 Basic groups = 0.12 mmol·g−1 | Co = 0.1–2.0 g·L−1 W = 0.5–10.0 g·L−1 T = 25 °C pH = 7.0 qmax = 235.5 mg·g−1 | π–π interactions | [210] |
| Caffeine | H3PO4 500 °C | Pineapple leaves | SBET = 1031 m2·g−1 pHpzc = 2.80 Acid groups = 0.74 mmol·g−1 Basic groups = 0.59 mmol·g−1 | Co = 100–500 mg·L−1 W = 1.0 g·L−1 T = 25–55 °C pH = 2.0–9.0 qmax = 155.5 mg·g−1 | π–π interactions and hydrogen bonds | [211] |
| Pesticide | ||||||
| Atrazine | KOH r = 3:1 600 °C Different treatments | Hemp stem | SBET = 2067–2213 m2·g−1 pHpzc = 3.9–9.2 O = 6.0–21.0% | Co = 30 mg·L−1 W = 50–1000 mg·L−1 T = 25 °C pH = 5.0–9.0 qmax = 466.0 mg·g−1 | π–π and hydrophobic interactions | [212] |
| Atrazine | H3PO4 350 °C | Banana peel | pHpzc = 8.2 | Co = 1–150 mg·L−1 W = 15 g·L−1 T = 25–60 °C pH = 2.0–9.0 qmax = 14.4 mg·g−1 | -- | [213] |
| Glyphosate | KOH r = 0.25:1 650 °C | Waste newspaper | SBET = 535 m2·g−1 pHpzc = 12.0 | Co = 5–100 mg·L−1 W = 1 g·L−1 T = 28 °C pH = 2.0–9.0 qmax = 48.4 mg·g−1 | Electrostatic interactions | [214] |
| Carbofuran | KOH + CO2 r = 2.75:1 850 °C | Palm oil fronds | SBET = 1237 m2·g−1 | Co = 25–250 mg·L−1 W = 1.5 g·L−1 T = 30 °C pH = 2.0–12.0 qmax = 164.0 mg·g−1 | Electrostatic attraction | [215] |
| Diuron | H3PO4 | Grape seeds | SBET = 1139 m2·g−1 | Co ~ 5–33 mg·L−1 T = 15–45 °C Natural pH qmax = 129.1 mg·g−1 | -- | [216] |
| Plasticizers | ||||||
| Dibutyl phthalate Diethyl phthalate | H3PO4 r = 8:1 600 °C | Albizzia julibrissin pods | IN ** =1139 mg·g−1 pHpzc = 2.9 | Co = 150–400 mg·L−1 T = 10–40 °C pH = 2.0–10.0 qmax DBP = 1305 mg·g−1 qmax DEP = 457 mg·g−1 | -- | [217] |
| Dibutyl phthalate | ZnCl2 | Ginkgo leaves | SBET = 697 m2·g−1 | Co = 5–15 mg·L−1 W = 0.1–1.0 g·L−1 T = 25–35 °C pH = 1.0–13.0 qmax = 129.9 mg·g−1 | -- | [218] |
| Dibutyl phthalate | -- | Nutshell | SBET = 1224 m2·g−1 | Co = 3.0–6.0 mg·L−1 W = 40–140 mg·L−1 T = 25–55 °C pH = 3.0–9.0 qmax = 104.7 mg·g−1 | -- | [219] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. A Review on the Synthesis and Characterization of Biomass-Derived Carbons for Adsorption of Emerging Contaminants from Water. C 2018, 4, 63. https://0-doi-org.brum.beds.ac.uk/10.3390/c4040063
Bedia J, Peñas-Garzón M, Gómez-Avilés A, Rodriguez JJ, Belver C. A Review on the Synthesis and Characterization of Biomass-Derived Carbons for Adsorption of Emerging Contaminants from Water. C. 2018; 4(4):63. https://0-doi-org.brum.beds.ac.uk/10.3390/c4040063
Chicago/Turabian StyleBedia, Jorge, Manuel Peñas-Garzón, Almudena Gómez-Avilés, Juan J. Rodriguez, and Carolina Belver. 2018. "A Review on the Synthesis and Characterization of Biomass-Derived Carbons for Adsorption of Emerging Contaminants from Water" C 4, no. 4: 63. https://0-doi-org.brum.beds.ac.uk/10.3390/c4040063