Methods for the Treatment of Cattle Manure—A Review
Abstract
:1. Introduction
2. Characteristics of Cattle Manure
2.1. Moisture Content
2.2. Ash Content
2.3. Volatiles
2.4. Energy Content
3. Current Waste Management/Disposal Practices
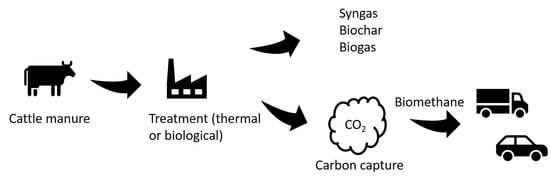
4. Advanced Options for the Treatment/Conversion of Cattle Manure
4.1. Biological Treatment
4.1.1. Composting and Vermicompost
4.1.2. Anaerobic Digestion
- (1).
- Hydrolysis: In this step, extracellular enzymes transform complex, undissolved material (carbohydrates, proteins and fats) into their respective monomers (sugars, amino acids, lipids), which are taken by the microorganisms for further degradation;
- (2).
- Acidogenesis: In this step, the dissolved compounds present in cells of fermentative bacteria convert simple monomers into volatile fatty acids (VFAs), alcohols, lactic acid, CO2, H2, NH3 and H2S, as well as new cell material;
- (3).
- Acetogenesis (intermediary acid production): In this step, digestion products (higher volatile fatty acids) are transformed into acetate, H2 and CO2, as well as new cell material;
- (4).
- Methanogenesis: In this stage, acetate, hydrogen plus carbonate, formate or methanol are converted into methane, CO2 and new cell material.
4.2. Thermochemical Conversion
4.2.1. Pyrolysis
4.2.2. Gasification
4.2.3. Hydrothermal Carbonisation
4.2.4. Hydrothermal Liquefaction
4.2.5. Fuel Production from Cattle Manure
4.2.6. Enzymatic Fermentation into Ethanol
4.3. Thermochemical Versus Biological Treatment
5. Environment and Sustainability
5.1. Contribution to the Energy and Climate Targets
5.2. Sustainability Matters
5.3. Future Prospects
6. Conclusions
Funding
Conflicts of Interest
References
- DEFRA. Farming Statistics Provisional Crop Areas, Yields and Livestock Populations. At June 2016—United Kingdom; F.R.A. Department for Environment: London, UK, 2016.
- USDA. Cattle Inventory; U.S.D.O. National Agricultural Statistics Service (NASS): Washington, DC, USA, 2019.
- FAOSTAT. Live Animals—Cattle; FAOSTAT Nations: Rome, Italy, 2018. [Google Scholar]
- Carlin, N.T.; Annamalai, K.; Harman, W.L.; Sweeten, J.M. The economics of reburning with cattle manure-based biomass in existing coal-fired power plants for NOx and CO2 emissions control. Biomass Bioenergy 2009, 33, 1139–1157. [Google Scholar]
- Smith, K.A.; Williams, A.G. Production and management of cattle manure in the UK and implications for land application practice. Soil Manag. 2016, 32, 73–82. [Google Scholar] [CrossRef]
- Chambers, B.; Nicholson, N.; Smith, K.; Pain, B.; Cumby, T.; Scotford, I. Making Better Use of Livestock Manures on Arable Land, 2nd ed.; Ministry of Agriculture, Fisheries and Food: London, UK, 2001.
- DEFRA. Emissions of Air Pollutants in the UK, 1970 to 2017; F.R.A. Department for Environment: London, UK, 2019.
- Zhou, D.-M.; Hao, X.Z.; Wang, Y.J.; Dong, Y.H.; Cang, L. Copper and Zn uptake by radish and pakchoi as affected by application of livestock and poultry manures. Chemosphere 2005, 59, 167–175. [Google Scholar]
- Technology Focus. An alternative manure treatment technology. Filtr. Sep. 2014, 51, 44–45. [Google Scholar]
- De Mendonça Costa, M.S.S.; Cestonaro, T.; de Mendonça Costa, L.A.; Rozatti, M.A.T.; Carneiro, L.J.; Pereira, D.C.; Lorin, H.E.F. Improving the nutrient content of sheep bedding compost by adding cattle manure. J. Clean. Prod. 2015, 86, 9–14. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [PubMed]
- Hepperly, P.; Lotter, D.; Ulsh, C.Z.; Seidel, R.; Reider, C. Compost, manure and synthetic fertilizer influences crop yields, soil properties, nitrate leaching and crop nutrient content. Compost Sci. Util. 2009, 17, 117–126. [Google Scholar] [CrossRef]
- Schlegel, A.J.; Assefa, Y.; Bond, H.D.; Wetter, S M.; Stone, L.R. Corn response to long–term applications of cattle manure, swine effluent, and inorganic nitrogen fertilizer. Agron. J. 2015, 107, 1701–1710. [Google Scholar] [CrossRef]
- Leclerc, A.; Laurent, A. Framework for estimating toxic releases from the application of manure on agricultural soil: National release inventories for heavy metals in 2000–2014. Sci. Total Environ. 2017, 590–591, 452–460. [Google Scholar] [CrossRef]
- Brown, P.; Broomfield, M.; Cardenas, L.; Choudrie, S.; Kilroy, E.; Jones, L.; MacCarthy, J.; Passant, N.; Thistlethwaite, G.; Thomson, A.; et al. UK Greenhouse Gas Inventory, 1990 to 2016; Annual Report for Submission under the Framework Convention on Climate Change; E.I.S. Department for Business: London, UK, 2018.
- Brown, P.; Broomfield, M.; Cardenas, L.; Choudrie, S.; Kilroy, E.; Jones, L.; Passant, N.; Thomson, A.; Wakeling, D. UK Greenhouse Gas Inventory, 1990 to 2015; Annual Report for submission under the Framework Convention on Climate Change; Department for Business, Energy and Industrial Strategy: London, UK, 2017.
- Myhre, G.; Shindell, D.; Bréon, F.M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, J.-F.; Lamarque, D.; Lee, B.; Mendoza, T. Anthropogenic and natural radiative forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M.M.B., Allen, S.K., Boschunget, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- USDA. Dairy 2007: Facility Characteristics and Cow Comfort on U.S. Dairy Operations; USDA: Washington, DC, USA, 2010.
- Shen, X.; Huang, G.; Yang, Z.; Han, L. Compositional characteristics and energy potential of Chinese animal manure by type and as a whole. Appl. Energy 2015, 160, 108–119. [Google Scholar]
- Phyllis2. Database for Biomass and Waste; Energy Research Centre of The Netherlands: Petten, The Netherlands, 2012. [Google Scholar]
- Wang, L.; Shahbazi, A.; Hanna, M.A. Characterization of corn stover, distiller grains and cattle manure for thermochemical conversion. Biomass Bioenergy 2011, 35, 171–178. [Google Scholar] [CrossRef]
- Sweeten, J.M.; Annamalai, K.; Thien, B.; McDonald, L.A. Co-firing of coal and cattle feedlot biomass (FB) fuels. Part I. Feedlot biomass (cattle manure) fuel quality and characteristics. Fuel 2003, 82, 1167–1182. [Google Scholar]
- Maglinao, A.L., Jr.; Capareda, S.C.; Nam, H. Fluidized bed gasification of high tonnage sorghum, cotton gin trash and beef cattle manure: Evaluation of synthesis gas production. Energy Conv. Manag. 2015, 105, 578–587. [Google Scholar] [CrossRef]
- Nam, H.; Maglinao, A.L., Jr.; Capareda, S.C.; Rodriguez-Alejandro, D.A. Enriched-air fluidized bed gasification using bench and pilot scale reactors of dairy manure with sand bedding based on response surface methods. Energy 2016, 95, 187–199. [Google Scholar] [CrossRef]
- Brugger, D.; Windisch, W.M. Environmental responsibilities of livestock feeding using trace mineral supplements. Anim. Nutr. 2015, 1, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, F.A.; Chambers, B.J.; Williams, J.R.; Unwin, R.J. Heavy metal contents of livestock feeds and animal manures in England and Wales. Bioresour. Technol. 1999, 70, 23–31. [Google Scholar] [CrossRef]
- WRAP. Specification for Composted Materials—BSI_PASS 100; The Waste & Resources Action Programme (WRAP): Banbury, UK, 2011. [Google Scholar]
- Yang, X.; Li, Q.; Tang, Z.; Zhang, W.; Yu, G.; Shen, Q.; Zhao, F.J. Heavy metal concentrations and arsenic speciation in animal manure composts in China. Waste Manag. 2017, 64, 333–339. [Google Scholar] [CrossRef]
- Misselbrook, T.H.; Smith, K.A.; Johnson, R.A.; Paina, B.F. Slurry application techniques to reduce ammonia emissions: Results of some UK Field-scale experiments. Biosyst. Eng. 2002, 81, 313–321. [Google Scholar] [CrossRef]
- Green, M.J.; Leach, K.A.; Breen, J.E.; Ohnstad, I.; Tuer, S.; Archer, S.C.; Bradley, A.J. Recycled manure solids as bedding for dairy cattle: A scoping study. Cattle Pract. 2014, 22, 207–214. [Google Scholar]
- Bradley, A. Risks, Benefits and Optimal Management of Recycled Manure Solids for use as Bedding for Dairy Cattle; AHDB Dairy: Kenilworth, UK, 2015. [Google Scholar]
- Cao, H.; Xin, Y.; Yuan, Q. Prediction of biochar yield from cattle manure pyrolysis via least squares support vector machine intelligent approach. Bioresour. Technol. 2016, 202, 158–164. [Google Scholar] [CrossRef]
- EPA. Wastewater Technology Fact Sheet—Anaerobic Lagoons; U.S.E.P. Agency: Washington, DC, USA, 2012.
- BIOWiSH. BIOWiSH Technologies. Available online: https://int.biowishtechnologies.com/ (accessed on 9 February 2019).
- LWR. Livestock Water Recycling. Available online: http://www.livestockwaterrecycling.com/ (accessed on 9 February 2019).
- Allance. Allance Fertilizer Machinery. Available online: http://www.fertilizer-machine.net/ (accessed on 9 March 2019).
- DariTech. Beddingmaster. Available online: http://www.daritech.com/products.html (accessed on 9 March 2019).
- McLanahan. Sand-Manure Separators (SMS). Available online: http://mclanahan.com/products/sand-manure-separators-sms/ (accessed on 20 March 2019).
- GEA. Manure Separators. Available online: http://www.gea.com/en/productgroups/farm-equipment/manure-separators/index.jsp (accessed on 20 March 2019).
- HoST. Microferm® and Macroferm®: Manure Digestion. Available online: http://www.host.nl/en/biogas-plants/manure-digestion-microferm/?gclid=EAIaIQobChMI-ajpm77y1AIVFBbTCh1nOAluEAAYBCAAEgIJm_D_BwE (accessed on 20 March 2019).
- DairyEnergy. Micro Anaerobic Biogas Systems Designed To Run On Only Dairy Slurry. Dairy Energy; 2017. Available online: https://www.dairyenergy.co.uk/ (accessed on 20 March 2019).
- Lazcano, C.; Gómez-Brandón, M.; Domínguez, J. Comparison of the effectiveness of composting and vermicomposting for the biological stabilization of cattle manure. Chemosphere 2008, 72, 1013–1019. [Google Scholar] [CrossRef]
- Eghball, B.; Power, J.F.; Gilley, J.E.; Doran, J.W. Nutrient, carbon, and mass loss during composting of beef cattle feedlot manure. J. Environ. Qual. 1997, 26, 189–193. [Google Scholar] [CrossRef]
- Domínguez, J.; Edwards, C.A.; Subler, S. A comparison of composting and vermicomposting. Biocycle 1997, 4, 57–59. [Google Scholar]
- Swati, A.; Hait, S. Fate and bioavailability of heavy metals during vermicomposting of various organic wastes—A review. Process Saf. Environ. Prot. 2017, 109, 30–45. [Google Scholar] [CrossRef]
- Pellegrini, L.A.; De Guido, G.; Langé, S. Biogas to liquefied biomethane via cryogenic upgrading technologies. Renew. Energy 2018, 124, 75–83. [Google Scholar] [CrossRef]
- Xavier, C.A.; Moset, V.; Wahid, R.; Møller, H.B. The efficiency of shredded and briquetted wheat straw in anaerobic co-digestion with dairy cattle manure. Biosyst. Eng. 2015, 139, 16–24. [Google Scholar] [CrossRef]
- Henze, M.; van Loosdrecht, M.C.; Ekama, G.A.; Brdjanovic, D. Biological Wastewater Treatment—Principles, Modelling and Design; IWA Publishing: London, UK, 2008. [Google Scholar]
- Luque, R.; Campelo, J.; Clark, J. Handbook of Biofuels Production—Processes and Technologies; Woodhead Publishing: Cambridge, UK, 2011. [Google Scholar]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Lehtomäki, A.; Huttunen, S.; Rintala, J.A. Laboratory investigations on co-digestion of energy crops and crop residues with cow manure for methane production: Effect of crop to manure ratio. Resour. Conserv. Recycl. 2007, 51, 591–609. [Google Scholar] [CrossRef]
- Kalamaras, S.D.; Kotsopoulos, T.A. Anaerobic co-digestion of cattle manure and alternative crops for the substitution of maize in South Europe. Bioresour. Technol. 2014, 172, 68–75. [Google Scholar] [CrossRef]
- Ledda, C.; Schievano, A.; Scaglia, B.; Rossoni, M.; Fernández, F.G.A.; Adani, F. Integration of microalgae production with anaerobic digestion of dairy cattle manure: An overall mass and energy balance of the process. J. Clean. Prod. 2016, 112, 103–112. [Google Scholar] [CrossRef]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.W.; Choi, S.K.; Choi, Y.S.; Kim, S.J. Production of biocrude-oil from swine manure by fast pyrolysis and analysis of its characteristics. Renew. Energy 2015, 79, 14–19. [Google Scholar] [CrossRef]
- Yuan, X.; He, T.; Cao, H.; Yuan, Q. Cattle manure pyrolysis process: Kinetic and thermodynamic analysis with isoconversional methods. Renew. Energy 2017, 107, 489–496. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Zhu, X.; Venderbosch, R. A correlation between stoichiometrical ratio of fuel and its higher heating value. Fuel 2005, 84, 1007–1010. [Google Scholar] [CrossRef]
- Mathieu, P.; Dubuisson, R. Performance analysis of a biomass gasifier. Energy Conv. Manag. 2002, 43, 1291–1299. [Google Scholar] [CrossRef]
- Gordillo, G.; Annamalai, K.; Carlin, N. Adiabatic fixed-bed gasification of coal, dairy biomass, and feedlot biomass using an air–steam mixture as an oxidizing agent. Renew. Energy 2009, 34, 2789–2797. [Google Scholar] [CrossRef]
- Lv, P.M.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air–steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef]
- Gil, J.; Corella, J.; Aznar, M.P.; Caballero, M.A. Biomass gasification in atmospheric and bubbling fluidized bed: Effect of the type of gasifying agent on the product distribution. Biomass Bioenergy 1999, 17, 389–403. [Google Scholar] [CrossRef]
- Gordillo, G.; Annamalai, K. Adiabatic fixed bed gasification of dairy biomass with air and steam. Fuel 2010, 89, 384–391. [Google Scholar] [CrossRef]
- Thanapal, S.S.; Annamalai, K.; Sweeten, J.M.; Gordillo, G. Fixed bed gasification of dairy biomass with enriched air mixture. Appl. Energy 2012, 97, 525–531. [Google Scholar] [CrossRef]
- Annamalai, K.; Priyadarsan, S.; Arumugam, S.; Sweeten, J.M. Energy conversion: Principles for coal, animal waste, and biomass fuels. In Encyclopedia of Energy Engineering and Technology, 2nd ed.; Capenhart, B.L., Ed.; Taylor & Francis: Abingdon, UK, 2008; pp. 476–497. [Google Scholar]
- Pecchi, M.; Baratieri, M. Coupling anaerobic digestion with gasification, pyrolysis or hydrothermal carbonization: A review. Renew. Sustain. Energy Rev. 2019, 105, 462–475. [Google Scholar] [CrossRef]
- Zhou, S.; Liang, H.; Han, L.; Huang, G.; Yang, Z. The influence of manure feedstock, slow pyrolysis, and hydrothermal temperature on manure thermochemical and combustion properties. Waste Manag. 2019, 88, 85–95. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, X.; Yuan, Q. Effects of process parameters on the distribution characteristics of inorganic nutrients from hydrothermal carbonization of cattle manure. J. Environ. Manag. 2018, 209, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, J.; Zhang, Y.; Liu, Z. Hydrothermal liquefaction of typical livestock manures in China: Biocrude oil production and migration of heavy metals. J. Anal. Appl. Pyrolysis 2018, 135, 133–140. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Juliana, P.M.; Torres-Mayanga, P.; Forster-Carneiro, T.; Meireles, M. Supercritical water gasification of biomass for hydrogen production: Variable of the process. Food Public Health 2015, 5, 92–101. [Google Scholar]
- Tushar, M.S.H.K.; Dutta, A.; Xu, C. Catalytic supercritical gasification of biocrude from hydrothermal liquefaction of cattle manure. Appl. Catal. B Environ. 2016, 189, 119–132. [Google Scholar] [CrossRef]
- Chen, S.; Wen, Z.; Liao, W.; Liu, C.; Kincaid, R.L.; Harrison, J.; ElliottM, D.C.; Brown, D.; Stevens, D.J. Studies into Using Manure in a Biorefinery Concept. In Twenty-Sixth Symposium on Biotechnology for Fuels and Chemicals; Davison, B.H., Ed.; Humana Press: New York, NY, USA, 2005. [Google Scholar]
- Vancov, T.; Schneider, R.C.S.; Palmer, J.; McIntosh, S.; Stuetz, R. Potential use of feedlot cattle manure for bioethanol production. Bioresour. Technol. 2015, 183, 120–128. [Google Scholar] [CrossRef]
- Lin, C.-L.; Wu, M.-H.; Weng, W.-C. Effect of the type of bed material in two-stage fluidized bed gasification reactors on hydrogen gas synthesis and heavy metal distribution. Int. J. Hydrog. Energy 2019, 44, 5633–5639. [Google Scholar] [CrossRef]
- European Commission. Fourth Progress Report on the Promotion and use of Energy From Renewable Sources for the United Kingdom 2016. Available online: https://ec.europa.eu/energy/en/topics/renewable-energy/progress-reports (accessed on 20 March 2019).
- European Commission. Renewable Energy Policy Database and Support Legal Sources on Renewable Energy—Legal Sources on Renewable Energy 2014. Available online: http://www.res-legal.eu/home/ (accessed on 20 March 2019).
- Alliance, U.S.C. United States Climate Alliance. Available online: https://www.usclimatealliance.org/ (accessed on 20 March 2019).
- Aguirre-Villegas, H.A.; Larson, R.A. Evaluating greenhouse gas emissions from dairy manure management practices using survey data and lifecycle tools. J. Clean. Prod. 2017, 143, 169–179. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- EBA. Biogas and Biomethane 2015. Available online: http://european-biogas.eu/publications-homepage/biogas-and-biomethane/ (accessed on 20 March 2019).
- Biogas. The Official Information Portal on Anaerobic Digestion. Available online: http://www.biogas-info.co.uk/about/biogas/ (accessed on 20 March 2019).
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for transformation of biogas to biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Gasrec. Gasrec Ltd. Available online: https://www.gasrec.co.uk/ (accessed on 20 March 2019).


| Ash Composition (wt % of Ash) | Beef Cattle Manure [19] a | Dairy Cattle Manure [19] b | Cattle Manure [20] |
|---|---|---|---|
| P2O5 | 7.19 | 19.13 | 3.0 |
| K2O | 7.02 | 4.79 | 6.40 |
| Na2O | 2.29 | 1.44 | 2.0 |
| CaO | 7.95 | 8.90 | 13.90 |
| MgO | 5.42 | 6.24 | 3.70 |
| Fe2O3 | 1.89 | 2.03 | 1.70 |
| CuO | 0.04 | 0.04 | - |
| ZnO | 0.08 | 0.04 | - |
| SO3 | - | - | 2.80 |
| SiO2 | - | - | 53.50 |
| Alkali index (kg alkali/GJ) | 1.34 | 1.18 | - |
| Initial deformation temperature (°C) c | 1232 | ||
| Softening or spherical temperature (°C) c | 1271 |
| Property | Fresh Cattle Manure [21] | Feedlot Manure [22] | Beef Cattle Manure [19] a | Beef Cattle Manure [23] | Cattle/Cow Manure [20] b | Dairy Cattle Manure (Sand Bedding) [24] | Feedlot Manure (Low Ash) [4] | Feedlot Manure (High Ash) [4] |
|---|---|---|---|---|---|---|---|---|
| Moisture (wt %) | 70.7 | 40.2 ± 1.0 | 75.66 ± 7.8 | 13.08 ± 0.5 | 39.24 ± 35.3 | |||
| Ash (wt %) | ||||||||
| Wet basis | 10.9 | 21.5 ± 0.4 | ||||||
| Dry basis | 37.2 | 35.9 ± 0.1 | 22.64 ± 11.9 | 29.80 ± 2.8 | 27.72 ± 17.9 | 48.8 ± 3.1 | 13.58 | 45.23 |
| Heating value (MJ/kg) | ||||||||
| Wet basis | 3.9 | 8.01 ± 0.2 | ||||||
| Dry basis | 13.3 | 13.5 ± 0.5 | 15.21 | 15.93 ± 0.3 | 8.7 ± 1.6 | 18.65 | 11.24 | |
| Dry (af) | 21.2 | 20.08 ± 0.53 | 21.58 | 20.53 | ||||
| Volatiles (wt %) | ||||||||
| Wet basis | 15.2 | |||||||
| Dry basis | 52 | 50.2 ± 0.9 | 64.58 ± 8.1 | 59.05 ± 0.4 | 49.1 ± 2.9 | |||
| Dry (af) | 82.8 | 83.91 ± 8 | ||||||
| Fixed carbon (wt %) | ||||||||
| Dry basis | 11.15 ± 2.9 | 2.2 ± 0.8 | ||||||
| Dry (af) | 16.09 ± 8 | |||||||
| Elemental analysis (%, dry and ash-free basis) | ||||||||
| C | 49.38 | 49.66 | 48.66 | 50.43 | 45.54 ± 11.5 | 32.42 | 57.43 | 59.06 |
| H | 6.46 | 5.62 | 6.80 | 7.18 | 6.31 ± 0.14 | 4.43 | 6.82 | 7.03 |
| O | 39.79 | 39.09 | 41.24 | 39.29 | 38.25 ± 3.22 | 60.55 | 31.26 | 28.91 |
| N | 3.33 | 4.28 | 2.79 | 2.55 | 2.2 ± 1.23 | 2.15 | 3.88 | 4.22 |
| S | 1.05 | 1.36 | 0.76 | 0.57 | 0.58 ± 0.41 | 0.45 | 0.62 | 0.78 |
| Chemical formula | CH1.559O0.605 N0.058 S0.008 | CH1.684O0.639 N0.05 S0.006 | ||||||
| Limits in Compost | ||||||
|---|---|---|---|---|---|---|
| UK (BS EN 13650) [27] | USA [28] | China [28] | Dairy Cattle Manure [26] | Dairy Cattle Slurry [26] | Beef Cattle Manure [26] | |
| As | − | 41 | 15 | 0.57–4.83 | <0.1–4.48 | 0.39–1.53 |
| Cd | 1.5 | 39 | 3 | <0.1–0.53 | <0.1–1.74 | <0.1–0.24 |
| Cr | 100 | − | 150 | 0.77–21.40 | <0.2–12.9 | 0.79–2.05 |
| Cu | 200 | 1500 | − | 26.2–55.8 | <1.0–352 | 10.5–27.9 |
| Ni | 50 | 420 | − | 1.7–9.1 | 0.1–11.4 | 0.2–3.1 |
| Pb | 200 | 300 | 50 | <1.0–9.18 | <1.0–16.9 | <1.0–6.4 |
| Zn | 400 | 2800 | − | 99–238 | <5–727 | 41–274 |
| Technology | Company | Features |
|---|---|---|
| Bioremediation of dairy wastewater | BIOWiSH Technologies [34] | Biocatalyst can be added directly or mixed with water to the lagoon inflow. The process results in a significant reduction of biochemical oxygen demand (BOD), and total suspended solids (TSS) by almost 50% |
| Mechanical (membranes) and chemical treatments | Livestock Water Recycling [35] | The process extracts up to 75% of water from manure. By concentrating and segregating the nutrients, it results in clean, potable water, dry solids that are rich in both phosphorus and organic nitrogen and concentrated stable ammonium and potassium liquid. |
| Windrow Composting method | Allance Fertiliser Machinery [36] | Process up to 50,000 ton/year. Waste is dumped on ground to form piles of 1.5–2.5 m height. The process takes 4–6 weeks. In the first two weeks, turning is required every two to three days when the temperature is 55 °C or above. Finally, the compost is dried, ground to produce a finer material, and screened to remove larger particles (e.g., fractions >10 mm will be discarded). |
| Recycled manure solids (RMS) | DariTech [37] | There are composter options for up to 1400 cows. Dewatered flush manure is first fed into a separator to deliver solids with 35% (dry matter) of manure solids. Then the solids are composted using bacteria within and then air is supplied. |
| Separators and bedding systems | Sand-Manure Separators (SMS), McLanahan [38] | 80 to 90% of sand can be captured, which can be recycled as bedding. It also processes a nearly sand-free manure effluent. |
| Separators | GEA [39] | Process daily manure output of up to 300 cows per hour. The process allows to recover liquid and fibres contained in manure in order to produce compost or bedding |
| Anaerobic digestion | HoST bio-energy installations [40] | The process consists of a digester integrated with combined heat and power (CHP). There are two versions: one capable of producing 65–150 kWe and the other 200–400 kWe |
| Anaerobic digestion | Dairy Energy [41] | It requires 1500 m3 of liquid manure per annum and 200 of space. It has a capacity of 11–44 kW using a CHP system. |
| Raw Cattle Manure (Straw as Bedding) | Control | Composting | Vermicomposting | Composting + Vermi-Composting | |
|---|---|---|---|---|---|
| pH | 7.70–8.94 | 8.89–8.78 a | 8.86–8.07 a | 7.73–7.51 b | 7.85–7.14 b |
| EC (dS m−1) | 1.25 ± 0.08 | 1.32 ± 0.08 a | 2.13 ± 10 b | 0.78 ± 0.02 c | 0.72 ± 0.04 c |
| C to N ratio | 17.0 ± 0.74 | 15.7 ± 1.09 a | 17.5 ± 0.33 a | 11.1 ± 0.24 b | 11.3 ± 0.16 b |
| Total C (g kg−1 dw) | 399.2 ± 2.8 | 395.7 ± 3.2 a | 384.9 ± 2.7 a | 314.0 ± 5.4 b | 309.0 ± 8.6 b |
| Total N (g kg−1 dw) | 23.6 ± 0.9 | 25.6 ± 1.7 ab | 22.0 ± 0.3 a | 28.3 ± 0.2 b | 27.4 ± 0.8 b |
| DON (mg kg−1 dw) | 2190 ± 380 | 2260 ± 244 a | 2571 ± 896 a | 3726 ± 153 a | 2165 ± 198 a |
| NH4–N (mg kg-1 dw) | 610 ± 92 | 534 ± 128 a | 1235 ± 291 b | 276 ± 24 a | 191 ± 30 a |
| NO3–N (mg kg-1 dw) | 19 ± 15 | 0 ± 0 a | 721 ± 184 b | 917 ± 113 b | 829 ± 110 b |
| DOC (mg kg−1 dw) | 4406 ± 704 | 6819 ± 772 a | 9338 ± 2103 a | 5249 ± 302 a | 4825 ± 387 a |
| Available P (mg kg−1 dw) | 211 ± 6 | 175 ± 7 a | 342 ± 22 b | 111 ± 3 c | 109 ± 6 c |
| Stage I | Stage II | Stage III | |
|---|---|---|---|
| Conversion degree (α) | 0.05–0.35 | 0.35–0.55 | 0.55–0.85 |
| Apparent activation energy (E, kJ mol−1) | 149.62 ± 19.95 | 172.81 ± 3.25 | 262.16 ± 86.10 |
| Decomposition temperature (°C) | 105–300 | 300–330 | 330–800 |
| Beef Cattle Manure (T = 730 °C, ER = 0.35) [23] | Pine Wood (T = 780–830 °C, ER = 0.18–0.45) [64] | Dairy Biomass (T = 519–1015 °C, ER = 0.16–0.63, S/F = 0.4–0.8) [65] | Beef Cattle Manure (Equilibrium Conditions: T = 850 °C, ER = 0.3) [19] | |
|---|---|---|---|---|
| Synthesis Gas Composition (%vol) | ||||
| Hydrogen | 7.72 ± 0.26 | 5.0–16.3 | 13.48–25.45 | 19.12 |
| Carbon monoxide | 10.92 ± 0.23 | 9.9–22.4 | 4.77–11.73 | 19.21 |
| Carbon dioxide | 14.18 ± 0.43 | 9.0–19.4 | 11–25.2 | 8.25 |
| Methane | 4.38 ± 0.13 | 2.2–6.2 | 0.43–1.73 | 0.64 |
| Ethane | 0.43 ± 0.02 | 0.2–3.3 a | 0.20–0.69 | |
| Nitrogen | 56.67 ± 1.33 | 41.6–61.6 | 45.03 | |
| Water | 7.75 | |||
| LHV (MJ Nm−3) | 4.19 | 3.7–8.4 | ||
| HHV (MJ Nm−3) | 3.27–4.28 | 5.14 | ||
| Gas yield (Nm3 kg−1 biomass) | 1.87 | 1.25–2.45 | 2.16 | |
| Carbon conversion efficiency (%) | 90.45 | |||
| Cold Gasification efficiency (%), ηG | 49.19 | |||
| Energy conversion efficiency (%) | 24–69 | 72.36 | ||
| Biochar Proximate Analysis (db) (%wt) | ||||
| Volatile matter | 16.2 | |||
| Ash | 81.18 | |||
| Fixed carbon | 2.62 | |||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Font-Palma, C. Methods for the Treatment of Cattle Manure—A Review. C 2019, 5, 27. https://0-doi-org.brum.beds.ac.uk/10.3390/c5020027
Font-Palma C. Methods for the Treatment of Cattle Manure—A Review. C. 2019; 5(2):27. https://0-doi-org.brum.beds.ac.uk/10.3390/c5020027
Chicago/Turabian StyleFont-Palma, Carolina. 2019. "Methods for the Treatment of Cattle Manure—A Review" C 5, no. 2: 27. https://0-doi-org.brum.beds.ac.uk/10.3390/c5020027