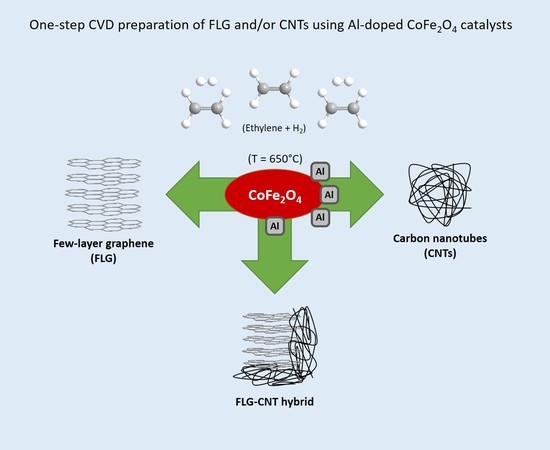
Preparation of Few-Layer Graphene/Carbon Nanotube Hybrids Using Oxide Spinel Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. c-CVD Synthesis
2.3. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ji, H.; Cheng, H.-M.; Ruoff, R.S. Mass production and industrial applications of graphene materials. Natl. Sci. Rev. 2018, 5, 90–101. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Matte, H.S.S.R.; Subrahmanyam, K.S. Synthesis and Selected Properties of Graphene and Graphene Mimics. Acc. Chem. Res. 2013, 46, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, L.; Zhang, C.; Casillas, G.; Sun, Z.; Yan, Z.; Ruan, G.; Peng, Z.; Raji, A.-R.O.; Kittrell, C.; et al. A seamless three-dimensional carbon nanotube graphene hybrid material. Nat. Commun. 2012, 3, 1225. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.M.; Novais, H.C.; Bacsa, R.; Serp, P.; Bachiller-Baeza, B.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Freire, C. Polyoxotungstate@Carbon Nanocomposites As Oxygen Reduction Reaction (ORR) Electrocatalysts. Langmuir 2018, 34, 6376–6387. [Google Scholar] [CrossRef]
- Dong, X.; Ma, Y.; Zhu, G.; Huang, Y.; Wang, J.; Chan-Park, M.B.; Wang, L.; Huang, W.; Chen, P. Synthesis of graphene-carbon nanotube hybrid foam and its use as a novel three-dimensional electrode for electrochemical sensing. J. Mater. Chem. 2012, 22, 17044–17048. [Google Scholar] [CrossRef]
- Xia, K.; Zhan, H.; Gu, Y. Graphene and Carbon Nanotube Hybrid Structure: A Review. Procedia IUTAM 2017, 21, 94–101. [Google Scholar] [CrossRef]
- Dasgupta, A.; Rajukumar, L.P.; Rotella, C.; Lei, Y.; Terrones, M. Covalent three-dimensional networks of graphene and carbon nanotubes: Synthesis and environmental applications. Nano Today 2017, 12, 116–135. [Google Scholar] [CrossRef]
- Badhulika, S.; Terse-Thakoor, T.; Chaves Villarreal, C.; Mulchandani, A. Graphene hybrids: Synthesis strategies and applications in sensors and sensitized solar cells. Front. Chem. 2015, 3, 38. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, L.; Liu, T. Strategies for the Hybridization of CNTs with Graphene. In Graphene-Carbon Nanotube Hybrids for Energy and Environmental Applications; Springer: Singapore, 2017; pp. 21–51. [Google Scholar]
- Eswaraiah, V.; Jyothirmayee Aravind, S.S.; Balasubramaniam, K.; Ramaprabhu, S. Graphene-Functionalized Carbon Nanotubes for Conducting Polymer Nanocomposites and Their Improved Strain Sensing Properties. Macromol. Chem. Phys. 2013, 214, 2439–2444. [Google Scholar] [CrossRef]
- Raji, A.-R.O.; Villegas Salvatierra, R.; Kim, N.D.; Fan, X.; Li, Y.; Silva, G.A.L.; Sha, J.; Tour, J.M. Lithium Batteries with Nearly Maximum Metal Storage. ACS Nano 2017, 11, 6362–6369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maarouf, A.A.; Kasry, A.; Chandra, B.; Martyna, G.J. A graphene–carbon nanotube hybrid material for photovoltaic applications. Carbon 2016, 102, 74–80. [Google Scholar] [CrossRef]
- Machado, B.F.; Marchionni, A.; Bacsa, R.R.; Bellini, M.; Beausoleil, J.; Oberhauser, W.; Vizza, F.; Serp, P. Synergistic effect between few layer graphene and carbon nanotube supports for palladium catalyzing electrochemical oxidation of alcohols. J. Energy Chem. 2013, 22, 296–304. [Google Scholar] [CrossRef]
- Wang, D.; Fang, G.; Zheng, Q.; Geng, G.; Ma, J. Construction of hierarchical porous graphene–carbon nanotubes hybrid with high surface area for high performance supercapacitor applications. J. Solid State Electrochem. 2017, 21, 563–571. [Google Scholar] [CrossRef]
- Axet, M.R.; Bacsa, R.R.; Machado, B.F.; Serp, P. Adsorption on and Reactivity of Carbon Nanotubes and Graphene. In Handbook of Carbon Nano Materials; World Scientific Publishing: Singapore, 2014; pp. 39–183. [Google Scholar]
- Darmawan, C.C.; Ye, L.; Samani, M.K.; Fu, Y.; Liu, J. Graphene-CNT hybrid material as potential thermal solution in electronics applications. In Proceedings of the 2017 IMAPS Nordic Conference on Microelectronics Packaging (NordPac), Gothenburg, Sweden, 18–20 June 2017; pp. 190–193. [Google Scholar]
- Li, W.; Dichiara, A.; Bai, J. Carbon nanotube–graphene nanoplatelet hybrids as high-performance multifunctional reinforcements in epoxy composites. Compos. Sci. Technol. 2013, 74, 221–227. [Google Scholar] [CrossRef]
- Patel, S.C.; Alam, O.; Zhang, D.; Grover, K.; Qin, Y.-X.; Sitharaman, B. Layer-by-layer, ultrasonic spray assembled 2D and 3D chemically crosslinked carbon nanotubes and graphene. J. Mater. Res. 2017, 32, 370–382. [Google Scholar] [CrossRef]
- Rao, R.; Chen, G.; Arava, L.M.R.; Kalaga, K.; Ishigami, M.; Heinz, T.F.; Ajayan, P.M.; Harutyunyan, A.R. Graphene as an atomically thin interface for growth of vertically aligned carbon nanotubes. Sci. Rep. 2013, 3, 1891. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Li, B.; Wei, A.; Cao, X.; Chan-Park, M.B.; Zhang, H.; Li, L.-J.; Huang, W.; Chen, P. One-step growth of graphene–carbon nanotube hybrid materials by chemical vapor deposition. Carbon 2011, 49, 2944–2949. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Gao, C.; Kim, N.D.; Fan, X.; Wang, G.; Peng, Z.; Hauge, R.H.; Tour, J.M. Growing Carbon Nanotubes from Both Sides of Graphene. ACS Appl. Mater. Interfaces 2016, 8, 7356–7362. [Google Scholar] [CrossRef]
- Kumar, K.; Kim, Y.-S.; Li, X.; Ding, J.; Fisher, F.T.; Yang, E.-H. Chemical Vapor Deposition of Carbon Nanotubes on Monolayer Graphene Substrates: Reduced Etching via Suppressed Catalytic Hydrogenation Using C2H4. Chem. Mater. 2013, 25, 3874–3879. [Google Scholar] [CrossRef]
- Bacsa, R.; Serp, P.; Lecante, P.; Pavlenko, E.; Cameán, I.; Ramos, A.; Garcia, A.B.; Bacsa, W.S. Interlayer interaction and disorder in few layer graphene powders prepared by fluidized bed chemical vapor deposition. In Proceedings of the Nanotech 2014, Washington, DC, USA, 15–18 June 2014; pp. 68–71. [Google Scholar]
- Bacsa, R.R.; Cameán, I.; Ramos, A.; Garcia, A.B.; Tishkova, V.; Bacsa, W.S.; Gallagher, J.R.; Miller, J.T.; Navas, H.; Jourdain, V.; et al. Few layer graphene synthesis on transition metal ferrite catalysts. Carbon 2015, 89, 350–360. [Google Scholar] [CrossRef] [Green Version]
- Corrias, M.; Kihn, Y.; Kalck, P.; Serp, P. CVD from ethylene on cobalt ferrite catalysts: The effect of the support. Carbon 2005, 43, 2820–2823. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serp, P.; Machado, B.F. Nanostructured Carbon Materials for Catalysis; Royal Society of Chemistry: Croydon, UK, 2015; p. 570. [Google Scholar]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C. Science of Fullerenes and Carbon Nanotubes; Academic Press: Cambridge, MA, USA, 1996; p. 965. [Google Scholar]




| Sample | FWHMD (cm−1) | FWHMG * (cm−1) | FWHMG’ (cm−1) | ID/IG | IG/IG’ |
|---|---|---|---|---|---|
| CNTs | 53.9 | 67.7 | 81.4 | 1.18 | 1.71 |
| FLG-CNT hybrid (mixed catalysts) | 53.9 | 64.5 | 75.1 | 1.48 | 2.03 |
| FLG-CNT hybrid (single catalyst) | 47.4 | 52.7 | 62.2 | 1.36 | 1.19 |
| FLG | 42.3 | 32.8 | 56.0 | 0.92 | 0.94 |
| Sample | Yield (gC gcat−1) | Ash Content (wt.%) | SBET (m2 g−1) | d002 | |
|---|---|---|---|---|---|
| FWHM | Center | ||||
| CNTs | 74 | 3.9 | 179 | 1.66 | 25.65 |
| FLG-CNT hybrid (mixed catalysts) | 25 | 2.8 | 161 | 1.71 | 26.02 |
| FLG-CNT hybrid (single catalyst) | 5.6 | 4.0 | 57 | 0.85 | 26.15 |
| CNT@FLG | 6.2 | - | - | - | - |
| FLG | 5.1 | <1.0 | 40 | 0.68 | 26.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, B.F.; Bacsa, R.R.; Rivera-Cárcamo, C.; Serp, P. Preparation of Few-Layer Graphene/Carbon Nanotube Hybrids Using Oxide Spinel Catalysts. C 2019, 5, 28. https://0-doi-org.brum.beds.ac.uk/10.3390/c5020028
Machado BF, Bacsa RR, Rivera-Cárcamo C, Serp P. Preparation of Few-Layer Graphene/Carbon Nanotube Hybrids Using Oxide Spinel Catalysts. C. 2019; 5(2):28. https://0-doi-org.brum.beds.ac.uk/10.3390/c5020028
Chicago/Turabian StyleMachado, Bruno F., Revathi R. Bacsa, Camila Rivera-Cárcamo, and Philippe Serp. 2019. "Preparation of Few-Layer Graphene/Carbon Nanotube Hybrids Using Oxide Spinel Catalysts" C 5, no. 2: 28. https://0-doi-org.brum.beds.ac.uk/10.3390/c5020028