Insights into the Electrochemical Behavior of Mercury on Graphene/SiC Electrodes
Abstract
:1. Introduction
2. Materials and Methods
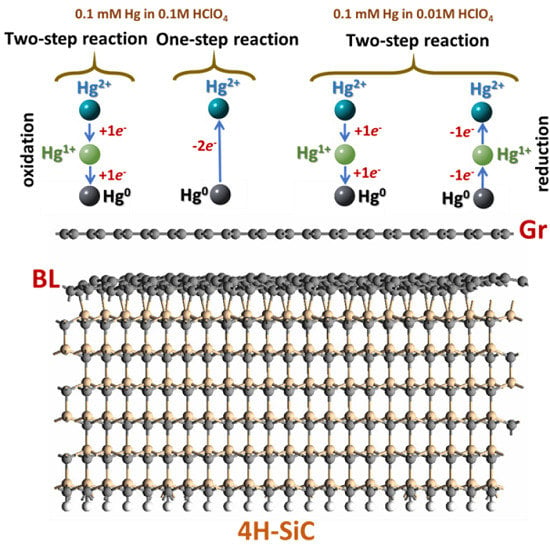
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cincinelli, A.; Martellini, T. Indoor Air Quality and Health. Int. J. Environ. Res. Public Health 2017, 14, 1286. [Google Scholar] [CrossRef] [PubMed]
- Montero-Montoya, R.; López-Vargas, R.; Arellano-Aguilar, O. Volatile Organic Compounds in Air: Sources, Distribution, Exposure and Associated Illnesses in Children. Ann. Glob. Health 2018, 84, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Tago, D.; Andersson, H.; Treich, N. Pesticides and Health: A Review of Evidence on Health Effects, Valuation of Risks, and Benefit-Cost Analysis. Prefer. Meas. Health 2014, 24, 203–295. [Google Scholar]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 231. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [PubMed]
- Gbogbo, F.; Arthur-Yartel, A.; Bondzie, J.A.; Dorleku, W.-P.; Dadzie, S.; Kwansa-Bentum, B.; Ewool, J.; Billah, M.K.; Lamptey, A.M. Risk of heavy metal ingestion from the consumption of two commercially valuable species of fish from the fresh and coastal waters of Ghana. PLoS ONE 2018, 13, e0194682. [Google Scholar] [CrossRef]
- Manzoor, J.; Sharma, M.; Wani, K.A. Heavy metals in vegetables and their impact on the nutrient quality of vegetables: A review. J. Plant Nutr. 2018, 41, 1744–1763. [Google Scholar] [CrossRef]
- Chen, S.-S.; Lin, Y.-W.; Kao, Y.-M.; Shih, Y.-C. Trace elements and heavy metals in poultry and livestock meat in Taiwan. Food Addit. Contam. Part B 2013, 6, 231–236. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef]
- Wojcik, D.P.; Godfrey, M.E.; Christie, D.; Haley, B.E. Mercury toxicity presenting as chronic fatigue, memory impairment and depression: Diagnosis, treatment, susceptibility, and outcomes in a New Zealand general practice setting (1994–2006). Neuroendocrinol. Lett. 2006, 27, 415–423. [Google Scholar] [PubMed]
- Björkman, L.; Lundekvam, B.F.; Lægreid, T.; Bertelsen, B.I.; Morild, I.; Lilleng, P.; Lind, B.; Palm, B.; Vahter, M. Mercury in human brain, blood, muscle and toenails in relation to exposure: An autopsy study. Environ. Health 2007, 6, 30. [Google Scholar]
- Hodgson, S.; Nieuwenhuijsen, M.J.; Elliott, P.; Jarup, L. Kidney disease mortality and environmental exposure to mercury. Am. J. Epidemiol. 2007, 165, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G. Mercury and Acrodynia. J. Orthomol. Med. 1995, 10, 145–146. [Google Scholar]
- Murata, K.; Weihe, P.; Budtz-Jørgensen, E.; Jørgensen, P.J.; Grandjean, P. Delayed brainstem auditory evoked potential latencies in 14-year-old children exposed to methylmercury. J. Pediatr. 2004, 144, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, C.M.; Chew, E.H.; Hashemy, S.I.; Lu, J.; Holmgren, A. Inhibition of the human thioredoxin system: A molecular mechanism of mercury toxicity. J. Biol. Chem. 2008, 283, 11913–11923. [Google Scholar] [CrossRef]
- GB 5749, Standards for Drinking Water Quality; National Standard of the People’s Republic of China: Beijing, China, 2006.
- Villanueva, C.M.; Kogevinas, M.; Cordier, S.; Templeton, M.R.; Vermeulen, R.; Nuckols, J.R.; Nieuwenhuijsen, M.J.; Levallois, P. Assessing Exposure and Health Consequences of Chemicals in Drinking Water: Current State of Knowledge and Research Needs. Environ. Health Perspect. 2014, 122, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Kallithrakas, N.; Foteinis, S. Recent Advances in the Analysis of Mercury in Water—Review. Curr. Anal. Chem. 2016, 12, 122–136. [Google Scholar] [CrossRef]
- Suvarapu, L.N.; Baek, S.-O. Recent Developments in the Speciation and Determination of Mercury Using Various Analytical Techniques. J. Anal. Methods Chem. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Leopold, K.; Foulkes, M.; Worsfold, P. Methods for the determination and speciation of mercury in natural waters—A review. Anal. Chim. Acta 2010, 663, 127–138. [Google Scholar] [CrossRef]
- Li, P.; Liu, B.; Zhang, D.; Sun, Y.; Liu, J. Graphene field-effect transistors with tunable sensitivity for high performance Hg (II) sensing. Appl. Phys. Lett. 2016, 109, 153101. [Google Scholar] [CrossRef]
- An, J.H.; Park, S.J.; Kwon, O.S.; Bae, J.; Jang, J. High-Performance Flexible Graphene Aptasensor for Mercury Detection in Mussels. ACS Nano 2013, 7, 10563–10571. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lu, G.; Chang, J.; Mao, S.; Yu, K.; Cui, S.; Chen, J. Hg(II) Ion Detection Using Thermally Reduced Graphene Oxide Decorated with Functionalized Gold Nanoparticles. Anal. Chem. 2012, 84, 4057–4062. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Gan, Y.; Liang, T.; Hu, Q.; Wang, Q.; Ren, T.; Sun, Q.; Wan, H.; Wang, P. Graphene FET array biosensor based on ssDNA aptamer for ultrasensitive Hg2+ detection in environmental pollutants. Front. Chem. 2018, 6, 333. [Google Scholar] [CrossRef] [PubMed]
- Martín-Yerga, D.; Costa-García, A. Recent advances in the electrochemical detection of mercury. Curr. Opin. Electrochem. 2017, 3, 91–96. [Google Scholar] [CrossRef]
- Suherman, A.L.; Tanner, E.E.L.; Compton, R.G. Recent developments in inorganic Hg2+ detection by voltammetry. Trends Anal. Chem. 2017, 94, 161–172. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S.; Morozov, S.; Novoselov, K. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhao, X.; He, Y.; Zhu, H. Graphene-Based Sensors; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 157–174. [Google Scholar]
- Devi, N.R.; Sasidharan, M.; Sundramoorthy, A.K. Gold Nanoparticles-Thiol-Functionalized Reduced Graphene Oxide Coated Electrochemical Sensor System for Selective Detection of Mercury Ion. J. Electrochem. Soc. 2018, 165, B3046–B3053. [Google Scholar] [CrossRef]
- Wang, S.; Zhai, S.; Li, Y.; Zhang, C.; Han, Z.; Peng, R.; Wang, X.; Lin, Z.; Lin, L.; Liu, Y.; et al. Simultaneously detection of Pb2+ and Hg2+ using electrochemically reduced graphene oxide. Int. J. Electrochem. Sci. 2018, 13, 785–796. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Taher, M.A.; Heydari, A.; Ghanei-Motlagh, R.; Gupta, V.K. A novel voltammetric sensor for sensitive detection of mercury(II) ions using glassy carbon electrode modified with graphene-based ion imprinted polymer. Mater. Sci. Eng. C 2016, 63, 367–375. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.; Wang, S.; Li, J.; Tang, J.; Wang, S.; Wang, Y. Sensitive and selective detection of Hg2+ based on an electrochemical platform of PDDA functionalized rGO and glutaraldehyde cross-linked chitosan composite film. RSC Adv. 2016, 6, 69815–69821. [Google Scholar] [CrossRef]
- Xie, Y.-L.; Zhao, S.-Q.; Ye, H.-L.; Yuan, J.; Song, P.; Hu, S.-Q. Graphene/CeO2 hybrid materials for the simultaneous electrochemical detection of cadmium(II), lead(II), copper(II), and mercury(II). J. Electroanal. Chem. 2015, 757, 235–242. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Y.; Zhai, J.; Bond, A.M.; Zhang, J. Direct Electrodeposition of Graphene-Gold Nanocomposite Films for Ultrasensitive Voltammetric Determination of Mercury(II). Electroanalysis 2014, 26, 121–128. [Google Scholar] [CrossRef]
- Wang, X.-M.; Wu, S.-G.; Liu, H.; Zhou, L.; Zhao, Q.-P. Graphene-gold nanoparticle composite film modified electrode for determination of trace mercury in environmental water. Chin. J. Chem. Phys. 2013, 26, 590–596. [Google Scholar] [CrossRef]
- Wei, Y.; Gao, C.; Meng, F.-L.; Li, H.-H.; Wang, L.; Liu, J.-H.; Huang, X.-J. SnO2/reduced graphene oxide nanocomposite for the simultaneous electrochemical detection of cadmium(II), lead(II), copper(II), and mercury(II): An interesting favorable mutual interference. J. Phys. Chem. C 2012, 116, 1034–1041. [Google Scholar] [CrossRef]
- Yao, Y. Facile Synthesis of Au Nanoparticles@Reduced Graphene Oxide Nanocomposition-Modified Electrode for Simultaneous Determination of Copper and Mercury Ions. Int. J. Electrochem. Sci. 2019, 14, 3844–3855. [Google Scholar] [CrossRef]
- Palisoc, S.T.; Bentulan, J.M.O.; Natividad, M.T. Determination of trace heavy metals in canned food using Graphene/AuNPs/[Ru(NH3)6]3+/Nafion modified glassy carbon electrodes. J. Food Meas. Charact. 2019, 13, 169–176. [Google Scholar] [CrossRef]
- Mejri, A.; Mars, A.; Elfil, H.; Hamzaoui, A.H. Graphene nanosheets modified with curcumin-decorated manganese dioxide for ultrasensitive potentiometric sensing of mercury(II), fluoride and cyanide. Microchim. Acta 2018, 185, 529. [Google Scholar] [CrossRef]
- Wen, G.-L.; Zhao, W.; Chen, X.; Liu, J.-Q.; Wang, Y.; Zhang, Y.; Huang, Z.-J.; Wu, Y.-C. N-doped reduced graphene oxide/MnO2 nanocomposite for electrochemical detection of Hg2+ by square wave stripping voltammetry. Electrochim. Acta 2018, 291, 95–102. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Z.; Hu, Y.; Zhan, H.; Zhu, J.; Ge, X. Fabrication of high-intensity electron transfer electrochemiluminescence interface for Hg2+ detection by using reduced graphene oxide-Au nanoparticles nanocomposites and CdS quantum dots. J. Electroanal. Chem. 2018, 823, 397–406. [Google Scholar] [CrossRef]
- Shtepliuk, I.; Yakimova, R. Interaction of epitaxial graphene with heavy metals: Towards novel sensing platform. Nanotechnology 2019, 30, 294002. [Google Scholar] [CrossRef] [PubMed]
- Shtepliuk, I.; Khranovskyy, V.; Yakimova, R. Combining graphene with silicon carbide: Synthesis and properties—A review. Semicond. Sci. Technol. 2016, 31, 113004. [Google Scholar] [CrossRef]
- Pearce, R.; Iakimov, T.; Andersson, M.; Hultman, L.; Spetz, A.L.; Yakimova, R. Epitaxially grown graphene based gas sensors for ultra sensitive NO2 detection. Sens. Actuators B Chem. 2011, 155, 451–455. [Google Scholar] [CrossRef]
- Ogawa, Y.; Hu, B.; Orofeo, C.M.; Ikeda, K.-I.; Tsuji, M.; Mizuno, S.; Hibino, H.; Ago, H. Domain Structure and Boundary in Single-Layer Graphene Grown on Cu(111) and Cu(100) Films. J. Phys. Chem. Lett. 2012, 3, 219–226. [Google Scholar] [CrossRef]
- Jeon, C.; Hwang, H.-N.; Lee, W.-G.; Jung, Y.G.; Kim, K.S.; Park, C.-Y.; Hwang, C.-C. Rotated domains in chemical vapor deposition-grown monolayer graphene on Cu(111): An angle-resolved photoemission study. Nanoscale 2013, 5, 8210. [Google Scholar] [CrossRef] [PubMed]
- Virojanadara, C.; Syväjärvi, M.; Yakimova, R.; Johansson, L.I.; Zakharov, A.A.; Balasubramanian, T. Homogeneous large-area graphene layer growth on6H-SiC(0001). Phys. Rev. B 2008, 78, 245403. [Google Scholar] [CrossRef]
- Yazdi, G.R.; Iakimov, T.; Yakimova, R. Epitaxial Graphene on SiC: A Review of Growth and Characterization. Crystals 2016, 6, 53. [Google Scholar] [CrossRef]
- Shtepliuk, I.; Vagin, M.; Ivanov, I.G.; Iakimov, T.; Yazdi, G.R.; Yakimova, R. Lead (Pb) interfacing with epitaxial graphene. Phys. Chem. Chem. Phys. 2018, 20, 17105–17116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shtepliuk, I.; Santangelo, M.F.; Vagin, M.; Ivanov, I.G.; Khranovskyy, V.; Iakimov, T.; Eriksson, J.; Yakimova, R. Understanding Graphene Response to Neutral and Charged Lead Species: Theory and Experiment. Materials 2018, 11, 2059. [Google Scholar] [CrossRef]
- Yakimova, R.; Iakimov, T.; Syväjärvi, M. Process for Growth of Graphene. U.S. Patent US9150417B2, 6 October 2015. [Google Scholar]
- Ivanov, I.G.; Hassan, J.U.; Iakimov, T.; Zakharov, A.A.; Yakimova, R.; Janzén, E. Layer-number determination in graphene on SiC by reflectance mapping. Carbon 2014, 77, 492–500. [Google Scholar] [CrossRef] [Green Version]
- Vagin, M.Y.; Sekretaryova, A.N.; Ivanov, I.G.; Håkansson, A.; Iakimov, T.; Syväjärvi, M.; Yakimova, R.; Lundström, I.; Eriksson, M. Monitoring of epitaxial graphene anodization. Electrochim. Acta 2017, 238, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Nomura, M.; Wakita, H.; Ohtaki, H. An extended x-ray absorption fine structure study of aqueous rare earth perchlorate solutions in liquid and glassy states. J. Chem. Phys. 1988, 89, 5153–5159. [Google Scholar] [CrossRef]
- Sémon, L.; Boehme, C.; Billard, I.; Hennig, C.; Lützenkirchen, K.; Reich, T.; Rossini, I.; Wipff, G.; Roßberg, A.; Roßberg, A. Do Perchlorate and Triflate Anions Bind to the Uranyl Cation in an Acidic Aqueous Medium? A Combined EXAFS and Quantum Mechanical Investigation. ChemPhysChem 2001, 2, 591–598. [Google Scholar] [CrossRef]
- Binnemans, K. Applications of tetravalent cerium compounds. In Handbook on the Physics and Chemistry of Rare Earths, 1st ed.; Gschneidner, K.A., Jr., Bünzli, J.-C.G., Pecharsky, V.K., Eds.; Elsevier Science Publisher B.V.: Amsterdam, The Netherlands, 2006; Volume 3, pp. 306–307. ISBN 9780080466729. [Google Scholar]
- Scharifker, B.; Hills, G. Theoretical and experimental studies of multiple nucleation. Electrochim. Acta 1983, 28, 879–889. [Google Scholar] [CrossRef]
- Frisch, M.J. Gaussian 16, Revision B. 01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, G.; Sundermann, A. Correlation consistent valence basis sets for use with the Stuttgart–Dresden–Bonn relativistic effective core potentials: The atoms Ga–Kr and In–Xe. J. Chem. Phys. 2001, 114, 3408–3420. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Martins, M.E.; Salvarezza, R.C.; Arvia, A.J. The electrodeposition of mercury from aqueous Hg22+ ion-containing acid solutions on smooth and columnar structured platinum electrodes. Electrochim. Acta 1998, 32, 438–450. [Google Scholar] [CrossRef]
- Orlik, M.; Galus, Z. Electrochemistry of Mercury. In Encyclopedia of Electrochemistry; Wiley: Hoboken, NJ, USA, 2007; Volume 7, pp. 958–991. [Google Scholar]
- Yasri, N.G.; Sundramoorthy, A.K.; Chang, W.-J.; Gunasekaran, S. Highly Selective Mercury Detection at Partially Oxidized Graphene/Poly(3,4-Ethylenedioxythiophene):Poly(Styrenesulfonate) Nanocomposite Film-Modified Electrode. Front. Mater. 2014, 1, 33. [Google Scholar] [CrossRef]
- Radhi, M.M.; Tan, W.T.; Ab Rahman, M.Z.B.; Kassim, A.B. Electrochemical Redox of Hg2+ Mediated by Activated Carbon Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2010, 5, 615–629. [Google Scholar]
- Brownson, D.A.C.; Banks, C.E. The Handbook of Graphene Electrochemistry; Springer: London, UK, 2014; Chapter 2; pp. 23–77. ISBN 978-1-4471-6427-2. [Google Scholar]
- Zanello, P. Inorganic Electrochemistry: Theory, Practice and Application; The Royal Society of Chemistry: Cambridge, UK, 2003; pp. 117–118. ISBN 0-85404-661-5. [Google Scholar]
- Klingler, R.J.; Kochi, J.K. Electron-transfer kinetics from cyclic voltammetry. Quantitative description of electrochemical reversibility. J. Phys. Chem. 1981, 85, 1731–1741. [Google Scholar] [CrossRef]
- Shtepliuk, I.; Vagin, M.; Iakimov, T.; Yakimova, R. Fundamentals of Environmental Monitoring of Heavy Metals Using Graphene. Chem. Eng. Trans. 2019, 73, 7–12. [Google Scholar]
- Vinokur, N.; Miller, B.; Avyigal, Y.; Kalish, R. Cathodic and Anodic Deposition of Mercury and Silver at Boron-Doped Diamond Electrodes. J. Electrochem. Soc. 1999, 146, 125. [Google Scholar] [CrossRef]
- Serruya, A.; Mostany, J.; Scharifker, B.R. The kinetics of mercury nucleation from Hg22+ and Hg2+ solutions on vitreous carbon electrodes. J. Electroanal. Chem. 1999, 464, 39–47. [Google Scholar] [CrossRef]
- Sharp, M.; Petersson, M.; Edström, K. Preliminary determinations of electron transfer kinetics involving ferrocene covalently attached to a platinum surface. J. Electroanal. Chem. Interfacial Electrochem. 1979, 95, 123–130. [Google Scholar] [CrossRef]
- Shtepliuk, I.; Caffrey, N.M.; Iakimov, T.; Khranovskyy, V.; Abrikosov, I.A.; Yakimova, R. On the interaction of toxic heavy metals (Cd, Hg, Pb) with graphene quantum dots and infinite graphene. Sci. Rep. 2017, 7, 3934. [Google Scholar] [CrossRef] [PubMed]
- Shtepliuk, I.; Yakimova, R. Interband transitions in closed-shell vacancy containing graphene quantum dots complexed with heavy metals. Phys. Chem. Chem. Phys. 2018, 20, 21528–21543. [Google Scholar] [CrossRef] [Green Version]








| Parameter | Anodic Process Hg0 − 2e−→Hg2+ | Cathodic Process Hg2+ + 2e−→Hg0 |
|---|---|---|
| Current density, mA/cm2 | 35.75 | −4.88 |
| Potential, V | 0.244 | 0.043 |
| Surface charge density, mC/cm2 | 90 | 16 |
| Electron transfer rate constant, 10−2 × cm∙s−1 | 6.4 | 0.89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shtepliuk, I.; Vagin, M.; Yakimova, R. Insights into the Electrochemical Behavior of Mercury on Graphene/SiC Electrodes. C 2019, 5, 51. https://0-doi-org.brum.beds.ac.uk/10.3390/c5030051
Shtepliuk I, Vagin M, Yakimova R. Insights into the Electrochemical Behavior of Mercury on Graphene/SiC Electrodes. C. 2019; 5(3):51. https://0-doi-org.brum.beds.ac.uk/10.3390/c5030051
Chicago/Turabian StyleShtepliuk, Ivan, Mikhail Vagin, and Rositsa Yakimova. 2019. "Insights into the Electrochemical Behavior of Mercury on Graphene/SiC Electrodes" C 5, no. 3: 51. https://0-doi-org.brum.beds.ac.uk/10.3390/c5030051