Expendable Graphite as an Efficient Flame-Retardant for Novel Partial Bio-Based Rigid Polyurethane Foams
Abstract
:1. Introduction
2. Experimental
2.1. Materials
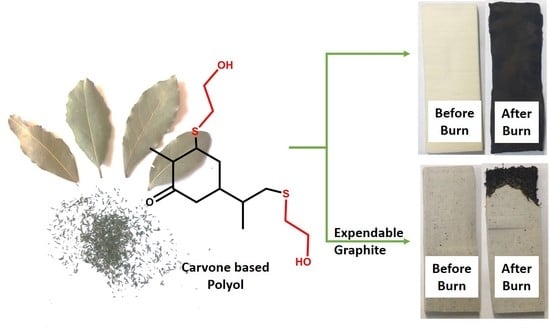
2.2. Synthesis of Carvone-Based Polyol
2.3. Polyol Characterizations
2.4. Synthesis of Flame-Retardant Rigid Polyurethane
2.5. Characterizations of Polyurethane Foams
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Yan, M.; Cochran, E.W.; Kessler, M.R. Biorenewable Polymers Based on Acrylated Epoxidized Soybean Oil and Methacrylated Vanillin. Mater. Today 2015, 5, 18–22. [Google Scholar] [CrossRef]
- Zhang, C.; Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Highly Flame Retardant and Bio-Based Rigid Polyurethane Foams Derived from Orange Peel Oil. Polym. Eng. Sci. 2018, 58, 2078–2087. [Google Scholar] [CrossRef]
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Biobased Polyurethanes Prepared from Different Vegetable Oils. ACS Appl. Mater. Interfaces 2015, 7, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Javni, I.; Zhang, W.; Petrović, Z.S. Effect of Different Isocyanates on the Properties of Soy-Based Polyurethanes. J. Appl. Polym. Sci. 2003, 88, 2912–2916. [Google Scholar] [CrossRef]
- Ramanujam, S.; Zequine, C.; Bhoyate, S.; Neria, B.; Kahol, P.; Gupta, R. Novel Biobased Polyol Using Corn Oil for Highly Flame-Retardant Polyurethane Foams. C—J. Carbon Res. 2019, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Castor-Oil Derived Nonhalogenated Reactive Flame-Retardant-Based Polyurethane Foams with Significant Reduced Heat Release Rate. J. Appl. Polym. Sci. 2018, 47276. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Sustainable Flame-Retardant Polyurethanes Using Renewable Resources. Ind. Crops Prod. 2018, 123, 480. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Radojcic, D.; Kahol, P.K.; Chen, J.; Mishra, S.R.; Gupta, R.K. Highly Flame-Retardant Bio-Based Polyurethanes Using Novel Reactive Polyols. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Chen, J.; Mishra, S.R.; Gupta, R.K. Highly Flame-Retardant Polyurethane Foam Based on Reactive Phosphorus Polyol and Limonene-Based Polyol. J. Appl. Polym. Sci. 2018, 135, 16–19. [Google Scholar] [CrossRef]
- Ding, H.; Huang, K.; Li, S.; Xu, L.; Xia, J.; Li, M. Synthesis of a Novel Phosphorus and Nitrogen-Containing Bio-Based Polyol and Its Application in Flame Retardant Polyurethane Foam. J. Anal. Appl. Pyrolysis 2017, 128, 102–113. [Google Scholar] [CrossRef]
- Montero de Espinosa, L.; Meier, M.A.R. Plant Oils: The Perfect Renewable Resource for Polymer Science?! Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, C.C.C.R.; da Fonseca, M.M.R. Carvone: Why and How Should One Bother to Produce This Terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Babrauskas, V.; Lucas, D.; Eisenberg, D.; Singla, V.; Dedeo, M.; Blum, A. Flame Retardants in Building Insulation: A Case for Re-Evaluating Building Codes. Build. Res. Inf. 2012, 40, 738–755. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A Review of Recent Progress in Phosphorus-Based Flame Retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Wilkie, C.A. The Utility of Nanocomposites in Fire Retardancy. Materials (Basel) 2010, 3, 4580–4606. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ding, X.; Su, Y. Properties of Rigid Polyurethane Foams Produced by the Addition of Phosphorus Compounds. Am. J. Mater. Res. 2014, 1, 14–19. [Google Scholar]
- Chen, Y.; Jia, Z.; Luo, Y.; Jia, D.; Li, B. Environmentally Friendly Flame-Retardant and Its Application in Rigid Polyurethane Foam. Int. J. Polym. Sci. 2014, 2014. [Google Scholar] [CrossRef]
- Xing, W.; Yuan, H.; Zhang, P.; Yang, H.; Song, L.; Hu, Y. Functionalized Lignin for Halogen-Free Flame Retardant Rigid Polyurethane Foam: Preparation, Thermal Stability, Fire Performance and Mechanical Properties. J. Polym. Res. 2013, 20, 234. [Google Scholar] [CrossRef]
- Konig, A.; Malek, A.; Fehrenbacher, U.; Brunklaus, G.; Wilhelm, M.; Hirth, T. Silane-Functionalized Flame-Retardant Aluminum Trihydroxide in Flexible Polyurethane Foam. J. Cell. Plast. 2010, 46, 395. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, P.; Liu, Y. Flame Retardant Property of Novel Intumescent Flame Retardant Rigid Polyurethane Foams. Polym. Eng. Sci. 2013, 53, 2478–2485. [Google Scholar] [CrossRef]
- Camino, G.; Duquesne, S.; Delobel, R.; Eling, B.; Lindsay, C.; Roels, T. Mechanism of Expandable Graphite Fire Retardant Action in Polyurethanes. ACS Symp. Ser. 2001, 797, 90–109. [Google Scholar] [CrossRef]
- Bhoyate, S.; Mensah-Darkwa, K.; Kahol, P.K.; Gupta, R.K. Recent Development on Nanocomposites of Graphene for Supercapacitor Applications. Curr. Graphene Sci. 2017, 1, 26. [Google Scholar] [CrossRef]
- Bhoyate, S.; Mensah-Darkwa, K.; Kahol, P.K.; Gupta, R.K. Advancement in Light Energy Harvesting by Using Tailored Graphene in DSSC and BHJ Solar Cells. Curr. Graphene Sci. 2019, 3, 1. [Google Scholar] [CrossRef]
- Gupta, R.; Ionescu, M.; Wan, X.; Radojcic, D.; Bilic, N. New Polyols with Isocynauric Structure by Thiol-Ene “Click” Chemistry Reactions. J. Cell. Plast. 2017, 53, 639–662. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in Renewable Polymers from Natural Terpenes, Terpenoids, and Rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef] [PubMed]
- Elbers, N.; Ranaweera, C.K.; Ionescu, M.; Wan, X.; Kahol, P.K.; Gupta, R.K. Synthesis of Novel Biobased Polyol via Thiol-Ene Chemistry for Rigid Polyurethane Foams. J. Renew. Mater. 2017, 5, 74–83. [Google Scholar] [CrossRef]
- Desroches, M.; Caillol, S.; Lapinte, V.; Auvergne, R.M.; Boutevin, B. Synthesis of Biobased Polyols by Thiol–Ene Coupling from Vegetable Oils. Macromolecules 2011, 44, 2489. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Liang, H.; Yang, Z.; Yuan, T.; Luo, Y.; Li, P.; Yang, Z.; Zhang, C. A Solvent-Free and Scalable Method To Prepare Soybean-Oil-Based Polyols by Thiol–Ene Photo-Click Reaction and Biobased Polyurethanes Therefrom. ACS Sustain. Chem. Eng. 2017, 5, 7365–7373. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Yang, R. Effects of Dimethyl Methylphosphonate, Aluminum Hydroxide, Ammonium Polyphosphate, and Expandable Graphite on the Flame Retardancy and Thermal Properties of Polyisocyanurate–Polyurethane Foams. Ind. Eng. Chem. Res. 2015, 54, 5876–5884. [Google Scholar] [CrossRef]
- Wang, W.J.; He, K.; Dong, Q.X.; Fan, Y.; Zhu, N.; Xia, Y.B.; Li, H.F.; Wang, J.; Yuan, Z.; Wang, E.P.; et al. Influence of Aluminum Hydroxide and Expandable Graphite on the Flammability of Polyisocyanurate-Polyurethane Foams. Appl. Mech. Mater. 2013, 368, 741. [Google Scholar] [CrossRef]
- Gharehbaghi, A.; Bashirzadeh, R.; Ahmadi, Z. Polyurethane Flexible Foam Fire Resisting by Melamine and Expandable Graphite: Industrial Approach. J. Cell. Plast. 2011, 47, 549. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The Influence of Crude Glycerol and Castor Oil-Based Polyol on the Structure and Performance of Rigid Polyurethane-Polyisocyanurate Foams. Ind. Crops Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Hu, X.-M.; Wang, D.-M. Enhanced Fire Behavior of Rigid Polyurethane Foam by Intumescent Flame Retardants. J. Appl. Polym. Sci. 2013, 129, 238–246. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Dong, Q.; Xie, M.; Liu, P.; Ding, Y.; Zhang, S.; Yang, M.; Zheng, G. Core-Shell Expandable Graphite @ Aluminum Hydroxide as a Flame-Retardant for Rigid Polyurethane Foams. Polym. Degrad. Stab. 2017, 146, 267–276. [Google Scholar] [CrossRef]
- Shi, L.; Li, Z.-M.; Xie, B.-H.; Wang, J.-H.; Tian, C.-R.; Yang, M.-B. Flame Retardancy of Different-Sized Expandable Graphite Particles for High-Density Rigid Polyurethane Foams. Polym. Int. 2006, 55, 862–871. [Google Scholar] [CrossRef]
- Tan, S.Q.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid Polyurethane Foams from a Soybean Oil-Based Polyol. Polymer (Guildf) 2011, 52, 2840. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Hu, L.; Zhou, Y. Synthesis of Rigid Polyurethane Foams with Castor Oil-Based Flame Retardant Polyols. Ind. Crops Prod. 2014, 52, 380–388. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Hu, Y.; Zhang, F.; Shang, Q.; Lei, W.; Zhou, Y.; Cai, Z. Castor Oil-Based Polyfunctional Acrylate Monomers: Synthesis and Utilization in UV-Curable Materials. Prog. Org. Coat. 2018, 121, 236–246. [Google Scholar] [CrossRef]
- Ranaweera, C.K.; Ionescu, M.; Bilic, N.; Wan, X.; Kahol, P.K.; Gupta, R.K. Biobased Polyols Using Thiol-Ene Chemistry for Rigid Polyurethane Foams with Enhanced Flame-Retardant Properties. J. Renew. Mater. 2017, 5, 1. [Google Scholar] [CrossRef]
- Ye, L.; Meng, X.-Y.; Liu, X.-M.; Tang, J.-H.; Li, Z.-M. Flame-Retardant and Mechanical Properties of High-Density Rigid Polyurethane Foams Filled with Decabrominated Dipheny Ethane and Expandable Graphite. J. Appl. Polym. Sci. 2009, 111, 2372–2380. [Google Scholar] [CrossRef]














| Compound | Jeffol | EG 0 | EG 0.5 | EG 1.5 | EG 3 | EG 5 | EG 8 | EG 10 |
|---|---|---|---|---|---|---|---|---|
| C-2-ME-Polyol | 0 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Jeffol 522 | 20 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| A-1 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 |
| Water | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| T-12 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| B8404 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| MDI | 37.14 | 33.39 | 33.39 | 33.39 | 33.39 | 33.39 | 33.39 | 33.39 |
| EG | 0 | 0 | 0.5 | 1.5 | 3 | 5 | 8 | 10 |
| Wt.% of EG in the foam | 0 | 0 | 0.91 | 2.67 | 5.19 | 8.37 | 12.75 | 15.44 |
| N2 | N2 | N2 | Air | Air | Air | |
|---|---|---|---|---|---|---|
| Foam | Td5% | Td10% | Tdmax | Td5% | Td10% | Tdmax |
| 0 EG | 231.2 | 250.0 | 700 | 231.6 | 246.3 | 700 |
| 0.5 EG | 233.0 | 251.2 | 700 | 226.9 | 245.5 | 700 |
| 1.5 EG | 228.0 | 249.7 | 700 | 233.5 | 249.7 | 700 |
| 3.0 EG | 227.8 | 246.4 | 700 | 224.0 | 240.9 | 700 |
| 5.0 EG | 229.6 | 245.8 | 700 | 225.7 | 242.2 | 700 |
| 8.0 EG | 231.4 | 247.9 | 700 | 225.9 | 243.1 | 700 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. de Souza, F.; Choi, J.; Bhoyate, S.; Kahol, P.K.; Gupta, R.K. Expendable Graphite as an Efficient Flame-Retardant for Novel Partial Bio-Based Rigid Polyurethane Foams. C 2020, 6, 27. https://0-doi-org.brum.beds.ac.uk/10.3390/c6020027
M. de Souza F, Choi J, Bhoyate S, Kahol PK, Gupta RK. Expendable Graphite as an Efficient Flame-Retardant for Novel Partial Bio-Based Rigid Polyurethane Foams. C. 2020; 6(2):27. https://0-doi-org.brum.beds.ac.uk/10.3390/c6020027
Chicago/Turabian StyleM. de Souza, Felipe, Jonghyun Choi, Sanket Bhoyate, Pawan K. Kahol, and Ram K. Gupta. 2020. "Expendable Graphite as an Efficient Flame-Retardant for Novel Partial Bio-Based Rigid Polyurethane Foams" C 6, no. 2: 27. https://0-doi-org.brum.beds.ac.uk/10.3390/c6020027