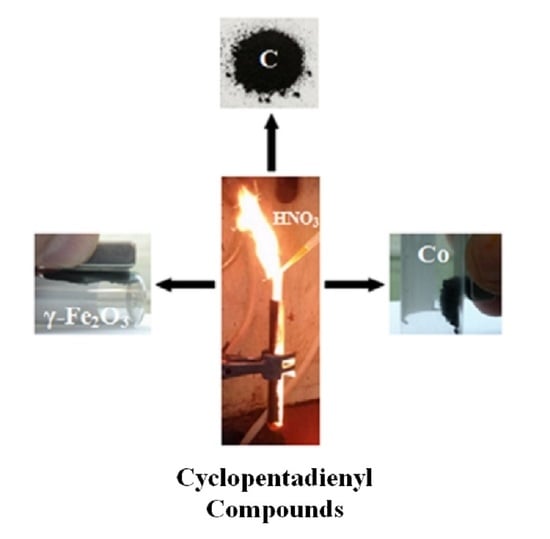
Hypergolic Materials Synthesis through Reaction of Fuming Nitric Acid with Certain Cyclopentadienyl Compounds
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Cyclopentadienyllithium-HNO3 hypergolic Pair
3.2. Ferrocene- and Cobaltocene-HNO3 Hypergolic Pairs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baikousi, M.; Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Avgeropoulos, A.; Gournis, D.; Karakassides, M.A. Direct production of carbon nanosheets by self-ignition of pyrophoric lithium dialkylamides in air. Mater. Lett. 2019, 254, 58–61. [Google Scholar] [CrossRef]
- Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Synthesis of highly crystalline graphite from spontaneous ignition of in situ derived acetylene and chlorine at ambient conditions. Molecules 2020, 25, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmpes, N.; Asimakopoulos, G.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Functional carbon materials derived through hypergolic reactions at ambient conditions. Nanomaterials 2020, 10, 566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmpes, N.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Gioti, C.; Karakassides, M.A.; Gournis, D. Hypergolics in carbon nanomaterials synthesis: New paradigms and perspectives. Molecules 2020, 25, 2207. [Google Scholar] [CrossRef] [PubMed]
- Chalmpes, N.; Tantis, I.; Bakandritsos, A.; Bourlinos, A.B.; Karakassides, M.A.; Gournis, D. Rapid carbon formation from spontaneous reaction of ferrocene and liquid bromine at ambient conditions. Nanomaterials 2020, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Trent, C.H.; Zucrow, M.J. The hypergolic reaction of dicyclopentadiene with white fuming nitric acid. J. Am. Rocket Soc. 1951, 21, 129–131. [Google Scholar] [CrossRef]
- Roh, J.-S. Structural study of the activated carbon fiber using laser Raman spectroscopy. Carbon Lett. 2008, 9, 127–130. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Rodil, S.E.; Robertson, J. Interpretation of infrared and Raman spectra of amorphous carbon nitrides. Phys. Rev. B 2003, 67, 155306. [Google Scholar] [CrossRef] [Green Version]
- D’Arsié, L.; Esconjauregui, S.; Weatherup, R.S.; Wu, X.; Arter, W.E.; Sugime, H.; Cepek, C.; Robertson, J. Stable, efficient p-type doping of graphene by nitric acid. RSC Adv. 2016, 6, 113185–113192. [Google Scholar] [CrossRef] [Green Version]
- Bakandritsos, A.; Pykal, M.; Błoński, P.; Jakubec, P.; Chronopoulos, D.D.; Poláková, K.; Georgakilas, V.; Čépe, K.; Tomanec, O.; Ranc, V.; et al. Cyanographene and graphene acid: Emerging derivatives enabling high-yield and selective functionalization of graphene. ACS Nano 2017, 11, 2982–2991. [Google Scholar] [CrossRef] [PubMed]
- Zoppellaro, G.; Bakandritsos, A.; Tuček, J.; Błoński, P.; Susi, T.; Lazar, P.; Bad’ura, Z.; Steklý, T.; Opletalová, A.; Otyepka, M.; et al. Microwave energy drives “on–off–on” spin-switch behavior in nitrogen-doped graphene. Adv. Mater. 2019, 31, 1902587. [Google Scholar] [CrossRef] [PubMed]
- Zygouri, P.; Tsoufis, T.; Kouloumpis, A.; Patila, M.; Potsi, G.; Sevastos, A.A.; Sideratou, Z.; Katsaros, F.; Charalambopoulou, G.; Stamatis, H.; et al. Synthesis, characterization and assessment of hydrophilic oxidized carbon nanodiscs in bio-related applications. RSC Adv. 2018, 8, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Amara, D.; Margel, S. Solventless thermal decomposition of ferrocene as a new approach for the synthesis of porous superparamagnetic and ferromagnetic composite microspheres of narrow size distribution. J. Mater. Chem. 2011, 21, 15764–15772. [Google Scholar] [CrossRef]
- Amara, D.; Margel, S. Synthesis and characterization of elemental iron and iron oxide nano/microcomposite particles by thermal decomposition of ferrocene. Nanotechnol. Rev. 2013, 2, 333. [Google Scholar] [CrossRef]
- Dormans, G.J.M.; Meekes, G.J.B.M.; Staring, E.G.J. OMCVD of cobalt and cobalt silicide. J. Cryst. Growth 1991, 114, 364–372. [Google Scholar] [CrossRef]
- Wei, X.-W.; Zhou, X.-M.; Wu, K.-L.; Chen, Y. 3-D flower-like NiCo alloy nano/microstructures grown by a surfactant-assisted solvothermal process. CrystEngComm 2011, 13, 1328–1332. [Google Scholar] [CrossRef]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalmpes, N.; Bourlinos, A.B.; Šedajová, V.; Kupka, V.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Hypergolic Materials Synthesis through Reaction of Fuming Nitric Acid with Certain Cyclopentadienyl Compounds. C 2020, 6, 61. https://0-doi-org.brum.beds.ac.uk/10.3390/c6040061
Chalmpes N, Bourlinos AB, Šedajová V, Kupka V, Moschovas D, Avgeropoulos A, Karakassides MA, Gournis D. Hypergolic Materials Synthesis through Reaction of Fuming Nitric Acid with Certain Cyclopentadienyl Compounds. C. 2020; 6(4):61. https://0-doi-org.brum.beds.ac.uk/10.3390/c6040061
Chicago/Turabian StyleChalmpes, Nikolaos, Athanasios B. Bourlinos, Veronika Šedajová, Vojtěch Kupka, Dimitrios Moschovas, Apostolos Avgeropoulos, Michael A. Karakassides, and Dimitrios Gournis. 2020. "Hypergolic Materials Synthesis through Reaction of Fuming Nitric Acid with Certain Cyclopentadienyl Compounds" C 6, no. 4: 61. https://0-doi-org.brum.beds.ac.uk/10.3390/c6040061