1. Introduction
As countries around the world transform their national energy strategies to meet stringent carbon reduction targets, carbon capture and storage (CCS) is being viewed as an essential tool for reducing carbon emissions from industrial processes. Notable projects include Quest managed by Shell in Canada which managed to capture 4 million tonnes of carbon dioxide by May 2019 [
1] and the Northern Lights project in Norway which aims to develop a full-scale CCS value chain in Norway by 2024 [
2]. CCS could be a suitable “add-on” technology to energy processes that currently utilise hydrocarbons as the feedstock. For example, steam methane reforming which produces hydrogen from natural gas could supply the carbon dioxide byproduct to CCS to make the overall process carbon-neutral (this may a viable solution for countries with existing supplies of natural gas such as in the United Kingdom (UK)). Without CCS, such processes will not be acceptable in future.
There are specific measurement capabilities that need to be developed to support CCS. For example, flow metering is important for calculating the amount of carbon dioxide that has been sent into storage sites and this requires traceable flow metering methods and an understanding of how gas quality affects accuracy of the measurements. Setting and meeting operational conditions for CCS processes require accurate determination of physical property measurements. Leak detection or monitoring is also an important measurement to ensure carbon dioxide does not escape following storage. This paper focuses on measurement challenges for gas quality, particularly the measurement of low-level impurities in the carbon dioxide, as well as the overall composition (i.e., level of high concentration components).
Various literature is available which outlines the issues of carbon dioxide purity in CCS processes (as reviewed in
Section 3). The presence of large amounts of impurities during the compression stage could lead to large inefficiencies or cause corrosion of storage/pipeline materials [
3]. An additional concern is the possible leakage of hazardous impurities to the atmosphere during storage; this risk can be minimised by simply ensuring that harmful impurities are not present in the carbon dioxide before the storage step.
The relevant document that covers carbon dioxide gas quality is the International Organisation for Standardisation Technical Report ISO/TR 27921 which states that CCS operators must develop their own purity specification tailored to their process; however, there are currently no standards that provide guidance on how to perform this.
The aim of this paper is to review available literature on the required purity of carbon dioxide for the different stages of carbon capture and storage for energy processes (combustion and steam methane reforming) to develop and provide a carbon dioxide purity specification. This specification has been provided to facilitate the development of a gas metrology infrastructure to support laboratories in performing quality assurance measurements.
2. Purity across the CCS Process
2.1. Production
2.1.1. Pre-Combustion
Pre-combustion is the process where carbon dioxide is removed before the actual conversion of fuel to energy. This may involve reforming (gas) or gasifying (coal) the fuel to syngas, and utilising water gas shift to produce more hydrogen from water (which also converts carbon monoxide to carbon dioxide). The carbon dioxide can be removed leaving a hydrogen-rich fuel. In this case, the likely impurities that may be present in the carbon dioxide are nitrogen, oxygen, hydrogen, methane, carbon monoxide, and sulphur-based compounds such as hydrogen sulphide. There is not expected to be significant levels of impurities that would originate from air, such as sulphur dioxide or nitrogen oxides, as the carbon dioxide is removed before the combustion step [
4]. The level of carbon dioxide in the final streams would entirely depend on the raw materials and process, but is expected to be between 5–20 cmol·mol
−1 for syngas and closer to 15–50 cmol·mol
−1 following water gas shift [
5,
6,
7].
2.1.2. Post-Combustion
Post-combustion is the process where the fuel is directly combusted using air without an initial reforming or gasification step. The trace elements that may be present in carbon dioxide for this process are nitrogen, oxygen, water, sulphur oxides, nitrous oxides, particulates, hydrochloric acid, hydrofluoric acid, mercury, other metals, and other trace contaminants. Combustion of coal is likely to provide more sulphur oxides and nitrous oxides, but lower oxygen compared to combustion of natural gas [
8].
2.1.3. Oxy-Fuel
An oxy-fuel process utilises pure oxygen instead of air to perform combustion. By removing inert nitrogen from the oxidising gas, the process can reduce fuel consumption (however, there may be additional costs from the air separation unit). The possible contaminants could be argon, oxygen, nitrogen, sulphur dioxide, sulphur trioxide, nitrogen monoxide, nitrogen dioxide, carbon monoxide, and other trace contaminants from the air [
9].
2.1.4. Steam Methane Reforming
Steam methane reforming is a process similar to combustion but where methane from natural gas is reacted with water instead of air. The resulting gas then goes through a water gas shift reaction to produce a stream of hydrogen and carbon dioxide. The carbon dioxide can be separated and captured whilst the pure hydrogen can be used in chemical processes or as a clean fuel (for example for powering fuel cell hydrogen vehicles).
The expected carbon dioxide compositions from these processes (following purification) are shown in
Table 1.
2.2. Capture or Separation and Transport
The capture step refers to the stage of the process where pure carbon dioxide is extracted from the waste stream of an industrial process, whilst a separation step would remove impurities from the stream to leave pure carbon dioxide. The carbon dioxide could be present in flue gas from power plants, steam methane reforming, or chemical processes. The gas will usually go through a purification step, such as amine scrubbing, to produce a stream of pure carbon dioxide which can be liquefied before transporting for storage or utilisation [
13]. According to the literature, amine scrubbing usually captures 85–90% of the carbon dioxide from flue gas [
14], but it should be noted that this would entirely depend on the set-up and type of scrubbers used. The purity of the carbon dioxide following amine scrubbing can be better than 99.95% depending on the performance and lifetime of the scrubber [
15].
Table 2 provides an overview of different technologies that are commonly used for the capture or separation step including typical compositions some details.
Before transporting through pipelines, the carbon dioxide is usually dehydrated to ensure levels of water are below 50 µmol·mol
−1. According to an article by the World Resources Institute, there are three main types of pipelines that are categorised on the basis of different purity levels of carbon dioxide that can be present; the main impurities of concern are hydrogen sulphide, sulphur, methane, hydrocarbons, carbon monoxide, nitrogen, oxygen, and water [
16].
As shown in
Table 2, amine scrubbing appears to be one of the more suitable conventional methods for obtaining high-purity carbon dioxide from flue gas.
2.3. Storage
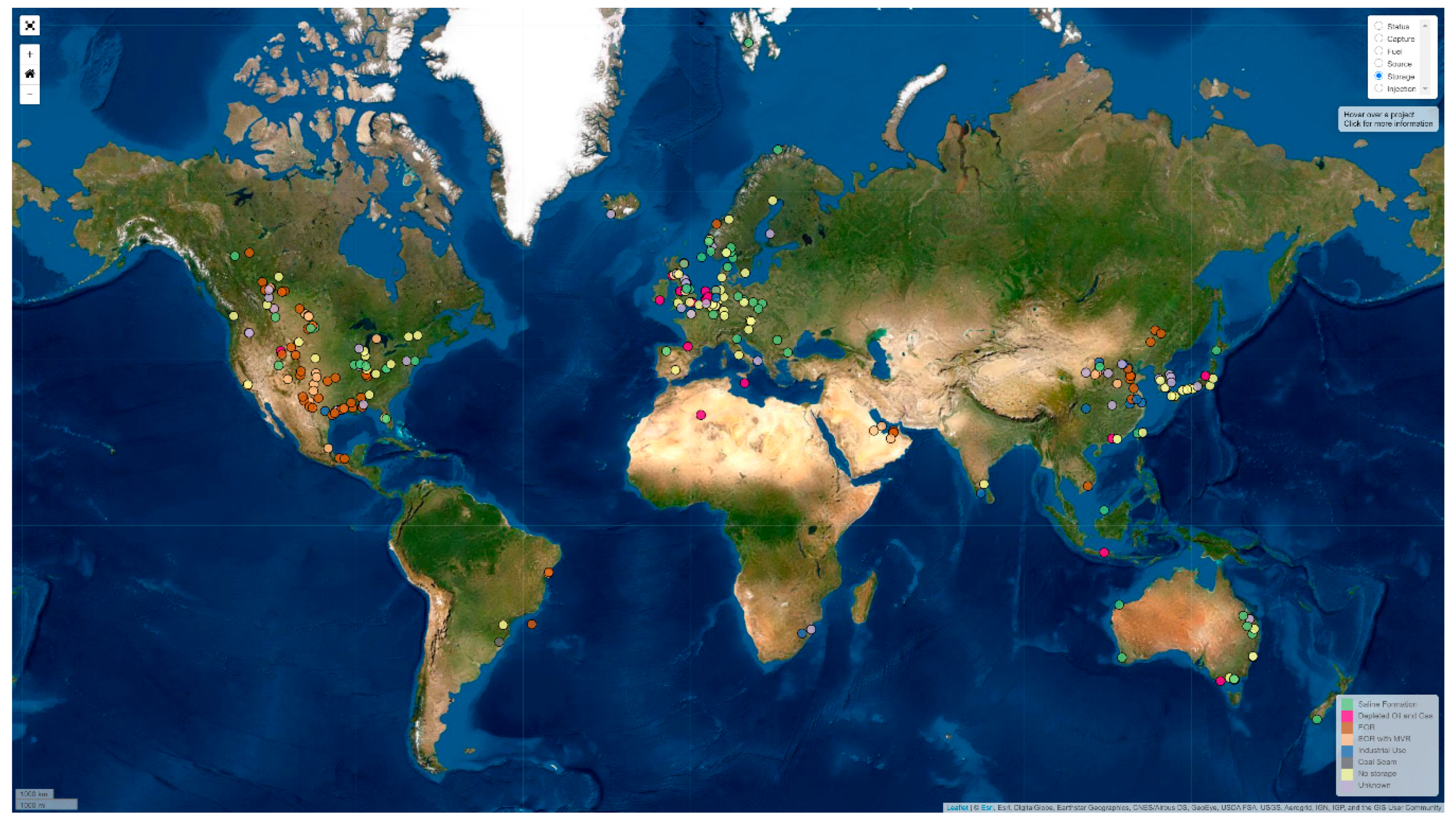
Worldwide, there are several projects that are using CCS to store carbon dioxide using different types of storage sites. There are many databases showing the CCS sites that are currently in operation including through the Scottish Carbon Capture and Storage research group (SCCS), as shown in
Figure 1 [
17]. This section discusses the conventional types of CCS storage sites with a focus on the possible impact of impurities in the carbon dioxide.
2.3.1. Deep Saline Aquifers
Saline aquifers are underground rocks that are water-permeable and end up saturated with brine. Storage of carbon dioxide via saline aquifers is optimised by injecting the carbon dioxide as a supercritical fluid. Once injected into the aquifers, the carbon dioxide is trapped beneath a layer of gas impermeable rock (such as caprock). The Shell Quest CCS facility in Alberta, Canada is one such process that has used this type of storage [
1]. Any requirements for quality may arise from concerns around carbon dioxide leaks as some impurities present in the gas could be hazardous to humans and/or the environment. Additionally, the presence of noncondensable impurities such as nitrogen, hydrogen, and oxygen may lead to difficulties with the compression step and limit storage capacity (as these impurities may reduce overall density) [
18]. Some impurities, such as water and sulphur dioxide, may react together to form sulphuric acid, which could cause mineral dissolution [
18].
2.3.2. Unmineable Coal Seams
Studies have shown that porous coal is efficient at trapping carbon dioxide [
19]. Therefore, coal mines that are not accessible for mining may be suitable for storing carbon dioxide. This process provides the additional benefit that the carbon dioxide can displace methane from the mine which can be extracted and utilised as a source of energy (for example, this methane could be injected into the natural gas grid following clean-up) [
20]. The San Juan Basin Allison Unit Project in New Mexico is one example where unmineable coal seams were used to store carbon dioxide [
21].
2.3.3. Depleted Oil and Gas Reservoirs
Enhanced oil recovery (EOR) is the process of feeding carbon dioxide into depleted oil reservoirs to extract remaining oil. This provides two benefits as the process allows carbon dioxide to be securely stored underground whilst displacing the remains of oil that otherwise would have been unobtainable. It is estimated that an additional 470 billion barrels of oil could potentially be extracted by EOR from discovered oil sites worldwide, which would lead to the sequestration of 140 billion metric tons of carbon dioxide [
22]. This is already a widely used technique where carbon dioxide is readily available; however, there may be issues over the suitability of a given depleted reservoir for long-term carbon dioxide storage. One notable facility is the Century Plant in Texas which has a storage capacity of 8.4 million metric tons of carbon dioxide per annum as is being used for EOR. It is noted as one of the biggest CCS facilities worldwide currently [
23].
2.4. Utilisation
There are many uses for carbon dioxide in industry that includes preparation of food and chemical processing. The European Industrial Gases Association (EIGA) have developed a purity specification for carbon dioxide (
Table 3) that is widely used by the food and beverage industry, “EIGA document 70/17”; in order to meet these specifications, additional clean-up and analysis would be required following carbon dioxide capture [
24]. There are several uses for carbon dioxide in chemical processing to manufacture oxygenated organics, minerals, carbon fuels, and plastics. The biggest use of carbon dioxide in industry is in the production of urea at an average of 109.5 million tonnes per years. The remaining is used for the synthesis of other important feedstock chemicals such as salicylic acid, methanol, and polycarbonates. The medical industry is the second biggest user of carbon dioxide with an average use of 400 tonnes per year, in which it is used as an insufflation gas in laparoscopic procedures (keyhole surgery). The beverage industry is also a key user of carbon dioxide, in which it is used to carbonate certain drinks like beer and cider. It is also used in its supercritical form to decaffeinate coffee.
Purity specifications and gas analysis methods for carbon dioxide utilisation are important and are already fairly established in specific industries (such as beverage); in other cases, gas quality assurance would be controlled by the individual companies receiving carbon dioxide. As this paper focuses on carbon dioxide for storage, gas quality assurance for carbon dioxide utilisation is considered outside of its scope and, as such, is not discussed further.
3. Quality Requirements for CCS
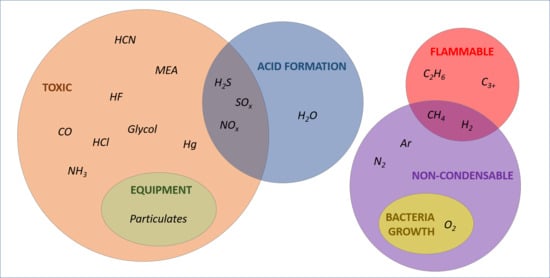
This section reviews previous and ongoing CCS projects where the possible impurities have been identified and threshold amount fractions for compounds have been specified (
Table 4). A focus of the review was to understand the amount fraction limits that have previously been used and the rationale behind these threshold limits in order to develop a draft purity specification.
3.1. Water (H2O)
Various project reports have listed water as an impurity that must be limited; it is a common byproduct of combustion and a possible impurity in hydrogen from the steam methane-reforming process [
4,
16,
25,
26,
28]. If water is present with hydrogen sulphide or sulphide dioxide, there is a risk of corrosion through the production of sulphuric acid [
4,
12,
25]. Similarly, the reaction of water with carbon dioxide could produce carbonic acid [
16]. Additionally, hydrates could form from the reaction of water with certain components which can lead to interruption to flow [
26,
32].
3.2. Hydrogen Sulphide (H2S), Sulphur Oxides (SOx), and Nitrogen Oxides (NOx)
Hydrogen sulphide and sulphur dioxide in the presence of water can form sulphuric acid, which would be corrosive to pipelines. Similarly, nitrous oxides in the presence of water can form nitric acid [
27]. Nitrogen oxide was noted to act as a catalyst for the oxidation of sulphide dioxide to sulphuric acid also leading the sulphur precipitates [
18]. Hydrogen sulphide alone can cause the gas to turn sour, which would affect production equipment [
25]. Additionally, if any of these gases are present in a carbon dioxide leak (from the storage site), the resulting release could be toxic [
12,
25]. In one report, a limit of 50 µmol·mol
−1 for hydrogen sulphide is recommended for health and safety reasons [
16]. It is also noted that hydrogen sulphide may cause severe pore blocking at geologic storage sites [
18].
3.3. Carbon Monoxide (CO)
Carbon monoxide could be present in carbon dioxide at low percentage volume levels. It appears that the main concern is around toxicity if a carbon dioxide leak was to occur [
29]. The CarbonNet Project [
30] set a carbon monoxide range of 900–5000 µmol·mol
−1; the lower limit and upper limit were set to allow a range of potential carbon dioxide sources to be received as part of the project.
3.4. Oxygen (O2), Methane (CH4), Nitrogen (N2), Argon (Ar), and Hydrogen (H2)
These gases are considered “light” and, if present in carbon dioxide at high levels, can increase compression power requirements and decrease carbon dioxide storage capacity by lowering the density [
25,
32]; therefore, a maximum level of 4–5 cmol·mol
−1 has been specified in several CCS projects [
4,
29,
30,
33]. Another consideration specifically for EOR is that oxygen could lead to both combustion of hydrocarbons in the oil field [
27,
29] and enhanced growth of bacteria [
16,
29] (for both cases, a limit of 10 µmol·mol
−1 was recommended). Oxygen was noted to have an effect on the dissolution of caprock; however, the levels required to cause this were significantly high and not likely due to its low solubility [
18]. As methane and hydrogen are flammable gases, one project recommended to keep the volume of these gases low in the carbon dioxide to avoid losing useful product with high energy content [
4].
The presence of high-level impurities can also affect measurements such as flow metering, density, speed of sound, viscosity, and thermal conductivity. Changes in density can reduce the amount of carbon dioxide being transported, whilst an increase in the maximum two-phase pressure could lead to increased risk of ductile fracture. This would also affect the phase behaviour of carbon dioxide [
32]. As discussed later in
Section 4.5, further work is required to accurately model parameters such as these to traceable gas compositions.
3.5. Ammonia (NH3) and Amines
Ammonia and amines are potential impurities that can be present in carbon dioxide from the scrubbing stage. From the literature, the detrimental effects to the process only appear to be noted as toxicity from a carbon dioxide leak [
29]; however, it is possible that these impurities could react with sulphuric acid or nitric acid to form particulates (ammonium sulphate or ammonium nitrate, respectively).
3.6. Ethane (C2H6) and Hydrocarbons (C3+)
Similarly to methane, other hydrocarbons in the carbon dioxide stream need to be kept to not only lower amount fractions to avoid explosive atmospheres, but also to reduce loss of a valuable energy product. One project noted that high levels of hydrocarbons in carbon dioxide could lead to asphyxiation, although carbon dioxide itself would also act as an asphyxiant [
29].
3.7. Particulates
A purity specification set by the National Energy Technology Laboratory (NETL) in the United States of America (USA) includes particulates as one of the criteria; however, there appears to be no recommended maximum mass concentration or rationale [
29]. Particulate matter could increase wear of machinery and pipelines [
32], while it is also likely to induce pore blocking at the CCS site.
3.8. Other Impurities
Additional impurities that have been provided in the NETL purity specification include hydrogen cyanide, mercury, hydrochloric acid, and hydrofluoric acid as toxic components that could be hazardous in a carbon dioxide leak, as well as glycol due to its damage to seals and other equipment. The specifications also include selexol, although the effects are unknown. The authors of the report suggest further work is required to set maximum allowed levels for these impurities [
29].
Table 5 provides a summary of the purity requirements identified in this section. Where different reports provide alternative maximum limits, a rationale for the selected limit is provided and the relevant references indicated. Where maximum impurity levels are selected on the basis of long-term exposure limits (for health and safety considerations), the work exposure limits provided by the UK’s Health and Safety Executive (HSE) have been used to ensure they comply with UK recommended best practice [
31]. The quality requirements for carbon dioxide utilisation are not included in the table as there are various uses of carbon dioxide, some of which may not adhere to national or international regulations but a company’s own internal practice. Not enough information on selexol is currently known to provide maximum limits.
4. Recommendations
4.1. Assessing Effects of Carbon Dioxide Quality
Further research is required to produce accurate and traceable data that can be used to assign maximum amount fraction threshold values with a sufficient degree of confidence. For example, rigorous testing of pipeline and underground storage materials is required to understand how the presence of impurities may affect, for example, corrosion. Some impurities such as nitrogen, oxygen, and argon should be limited as the presence of these components at a high level could reduce storage capacity. However, it is clear that the conditions of storage, such as temperature and pressure, also determine how much effect the presence of noncondensable gases such as nitrogen, oxygen, and hydrogen would have on storage capacity [
18]. A more detailed study may be required to select an optimum condition compromising between efficiency of the compression stage and purity levels that can be achieved with conventional purification methods (ultimately looking at the economics of both). This study would require further investigation into the expected purity of carbon dioxide from amine scrubbers to understand levels of impurities on the basis of specific operation conditions. Until these studies are carried out,
Table 5 probably provides the best available guidance on a suitable carbon dioxide purity specification for CCS processes.
4.2. Primary Reference Materials
When certifying the composition and purity of carbon dioxide used in the CCS process, the measurement needs to be traceable to the International System of Units (SI). This ensures that the measurements are accurate (providing confidence when transporting and storing the carbon dioxide) and internationally comparable, ensuring a common global assessment that can be easily compared to
Table 5 of this report. The National Physical Laboratory (NPL) is the UK’s National Metrology Institute and has the role of providing traceability for measurements of gas composition performed in UK industry. New purity requirements for CCS will require NPL to provide traceable primary reference materials containing the impurities listed in
Table 5 in a carbon dioxide gas matrix. This may be challenging due to the unique properties of carbon dioxide compared to other gases frequently used as the matrix gas in primary reference materials; as shown in
Figure 2, carbon dioxide liquefies at around 50 bar (at room temperature). Some laboratories would perhaps wish to use calibration standards in nitrogen (or alternative gas) matrices as opposed to carbon dioxide; however, testing would be required to ensure that changing the gas matrix would not affect accuracy of the measurement.
4.3. Sampling
Carbon dioxide can be sampled as a gas or a liquid depending on conditions (such as pressure and temperature) and it can also easily change phase once it has been sampled, including becoming a supercritical fluid. One study looked into the effects that water, nitrogen, oxygen, and argon had on the thermophysical properties and phase behaviour of carbon dioxide in a CCS system. The results indicated that an impurity at a level of 10% by mole in carbon dioxide would lower the overall mixture density [
34]. The potential change in phase when sampling liquid carbon dioxide from a CCS site would also raise the question of whether impurities in gas would behave the same as in liquid (i.e., if low-pressure carbon dioxide is sampled from a liquid storage site, would the impurities be present at the same level as in the original pressurised liquid?). If this did pose a problem, analytical techniques that are capable of monitoring purity whilst the carbon dioxide is in the liquid phase may need to be developed. This is a similar problem faced by liquid natural gas providers where Raman spectroscopy has been identified as a suitable technique for determining composition whilst still in the liquid phase [
35]. Additionally, there is the possibility that certain impurities segregate and collect at certain locations within the storage site (such as hydrogen sulphide, which is known to absorb to surfaces); this must be studied to understand whether sampling location has an effect on composition of the carbon dioxide sample.
4.4. Gas Analysis Methods
Due to the large number of impurities that may need to be analysed, it is likely that gas analysers capable of measuring several impurities in a single method would be used (such as gas chromatography with mass spectrometer) as opposed to combining several techniques that focus on a single impurity (such as cavity ringdown spectroscopy). These new analytical methods will be important for providing initial support to UK industries that require traceable measurements to verify the purity of carbon dioxide and to validate commercially available online purity analysers. There are several methods available for performing the analysis in accordance with the purity specifications of
Table 5; some options are shown in
Table 6 although this should not be considered a complete list.
As these methods have not been developed and tested specifically for impurity measurement in carbon dioxide, this work would need to be carried out to ensure methods are available for CCS operators to perform quality assurance measurements. There are several manufacturers who already offer gas analysers suitable for online monitoring the quality of carbon dioxide [
36,
37].
4.5. Impact of Gas Quality on Other Measurements
As mentioned in a paper by the National Engineering Laboratory (UK) [
38], the presence of impurities will also have an adverse effect on flow metering, which is necessary for regulatory measurement under the European Union (EU) Emissions Trading System (ETS). Flow metering is required for custody transfer and fiscal purposes and for monitoring the various processes across the CCS network, including controlling the volume of carbon dioxide being injected into the geological storage formation. Flow meters are generally designed to operate in one specific phase, either gas or liquid. However, even trace levels of contaminants will invalidate the phase diagram and equations of state for pure carbon dioxide. This is important because, under the ETS, the mass of annually transferred carbon dioxide is required to be determined within a maximum uncertainty of less than ±1.5%. Without knowing the exact phase envelope and physical properties of the carbon dioxide stream, it will be extremely difficult to control the CCS processes and achieve the required measurement uncertainty. An accurate model for equations of state is also required to correctly determine other physical properties such as density, speed of sound, and carbon dioxide phase, which are required to operate the CCS process safe and efficiently [
32].
5. Conclusions
CCS is a fairly new approach that is being implemented as a method for reducing carbon dioxide from energy processes, and the number of CCS operators globally is fairly low. As such, guidance and standards for carbon dioxide quality assurance are only starting to be developed; ISO/TR 27921 which provides guidance on this topic was published in 2020 and advises that each operator develops their own purity specifications on the basis of a risk assessment of their process.
In order to ensure operators can carry out gas quality assurance, they require traceable primary reference materials, suitable sampling methods, and validated purity analysis methods, which are not available. This paper provides a draft purity specification for carbon dioxide in CCS processes according to knowledge gained from previous CCS projects to support gas analysis laboratories, calibration gas producers, National Metrology Institutes, and standardisation committees to develop fit-for-purpose guidance, standards, and metrology infrastructure required for this importance measurement.
Author Contributions
A.M. led writing of the paper and performed majority of the literature review and metrology requirements. R.J.C.B. provided steer and contributed to the text particularly summarising the literature review and metrology requirements. R.W., D.H., S.B., P.J.B., D.R.W. and T.B. contributed to the literature review of carbon dioxide purity requirements. T.G., R.A.R. and A.J.F. contributed to the metrology requirements section. All authors have read and agreed to the published version of the manuscript
Funding
This research and the article processing charge was funded by the Department for Business, Energy, and Industrial Strategy.
Conflicts of Interest
The authors declared no conflict of interest.
References
- Shell Carbon Capture and Storage Projects. Available online: https://www.shell.com/sustainability/environment/climate-change/carbon-capture-and-storage-projects.html (accessed on 1 June 2020).
- Northern Lights about the Project. Available online: https://northernlightsccs.com/en/about (accessed on 1 June 2020).
- Wetenhall, B.; Aghajani, H.; Chalmers, H.; Benson, S.D.; Ferrari, M.C.; Li, J.; Race, J.M.; Singh, P.; Davison, J. Impact of CO2 impurity on CO2 compression, liquefaction and transportation. Energy Procedia 2014, 63, 2764–2778. [Google Scholar] [CrossRef] [Green Version]
- DYNAMIS. Dynamis CO2 Quality Recommendations; DYNAMIS: Oslo, Norway, 2007. [Google Scholar]
- Abdelaziz, O.; Gadalla, M.; Ashour, F. Simulation of biomethanol production from green syngas through sustainable process design. In Proceedings of the 4th International Conference on Simulation and Modeling Methodologies, Technologies and Applications (SIMULTECH), Vienna, Austria, 28–30 August 2014. [Google Scholar]
- Raibhole, V.; Sapali, S. Simulation and Parametric Analysis of Cryogenic Oxygen Plant for Biomass Gasification. Mech. Eng. Res. 2012, 2, 97. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-H.; Chen, C.-Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- De Visser, E.; Hendriks, C.; Barrio, M.; Mølnvik, M.J.; de Koeijer, G.; Liljemark, S.; Le Gallo, Y. Dynamis CO2 quality recommendations. Int. J. Greenh. Gas Control 2008, 2, 478–484. [Google Scholar] [CrossRef]
- Global CCS Institute. CO2 Capture Techologies—Oxy Combustion with CO2 Capture; Global CCS Institute: Melbourne, Australia, 2012. [Google Scholar]
- Kather, A. CO2 Quality and Other Relevant Issues. In Proceedings of the 2nd Working Group Meeting on CO2 Quality and Other Relevant Issues, Cottbus, Germany, 7 September 2009. [Google Scholar]
- White, V.; Torrente-Murciano, L.; Sturgeon, D.; Chadwick, D. Purification of oxyfuel-derived CO2. Energy Procedia 2009, 1, 399–406. [Google Scholar] [CrossRef]
- Bert Metz, O.D.; de Coninck, H.; Loos, M.; Meyer, L. Carbon Dioxide Capture and Storage; IPCC: Cambridge, UK, 2005. [Google Scholar]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.S. The Outlook for Power Plant CO2 Capture. In International Seminar on Nuclear War and Planetary Emergencies—42nd Session; World Scientific: Singapore, 2009; pp. 157–173. [Google Scholar]
- Walspurger, S.; Dijk, H.A.J. EDGAR CO2 Purity: Type and Quantities of Impurities Related to CO2 Point Source and Capture Technology: A Literature Study; ECN: Petten, The Netherlands, 2012. [Google Scholar]
- World Resources Institute. Guidelines for Carbon Dioxide Capture, Transport, and Storage; World Resources Institute: Washington, DC, USA, 2008. [Google Scholar]
- SCCS Global CCS Map. Available online: https://www.sccs.org.uk/expertise/global-ccs-map (accessed on 1 June 2020).
- IEAGHG. Effects of Impurities on Geological Storage of CO2; IEAGHG: Cheltenham, UK, 2011. [Google Scholar]
- Das, S.; Dutta, P. Preliminary Understanding of CO2 Sequestration and Enhanced Methane Recovery in Raniganj Coalfield of India by Reservoir Simulation. Energy Procedia 2017, 114, 4643–4657. [Google Scholar] [CrossRef]
- Mazzotti, M.; Pini, R.; Storti, G.; Burlini, L. Carbon dioxide (CO2) sequestration in unmineable coal seams and use for enhanced coalbed methane recovery (ECBM). In Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology; Maroto-Valer, M.M., Ed.; Woodhead Publishing: Cambridge, UK, 2010; Volume 2, pp. 127–165. [Google Scholar]
- Corum, M.D.; Jones, K.B.; Warwick, P.D. CO2 Sequestration Potential of Unmineable Coal—State of Knowledge. Energy Procedia 2013, 37, 5134–5140. [Google Scholar] [CrossRef] [Green Version]
- Godec, M.; Kuuskraa, V.; Van Leeuwen, T.; Stephen Melzer, L.; Wildgust, N. CO2 storage in depleted oil fields: The worldwide potential for carbon dioxide enhanced oil recovery. Energy Procedia 2011, 4, 2162–2169. [Google Scholar] [CrossRef] [Green Version]
- Sonnichsen, N. Largest Global Carbon Sequestration Projects in Operation 2019; Statistica: New York, NY, USA, 2020. [Google Scholar]
- EIGA. Carbon Dioxide Food and Beverages Grade, source Qualification, Quality Standards and Verification; EIGA: Brussels, Belgium, 2017. [Google Scholar]
- SNC Lavalin. Impact of Impurities on CO2 Capture, Transport and Storage; SNC Lavalin: Montreal, QC, Canada, 2004. [Google Scholar]
- Anheden, M.; Andersson, A.; Bernstone, C.; Eriksson, S.; Yan, J.; Liljemark, S.; Wall, C. CO2 quality requirement for a system with CO2 capture, transport and storage. In Greenhouse Gas Control Technologies 7; Elsevier Science Ltd.: Oxford, UK, 2005; pp. 2559–2564. [Google Scholar]
- White, V.; Allam, R.; Miller, E. Purification of Oxyfuel-Derived CO2 for Sequestration or EOR; IEAGHG: Cheltenham, UK, 2007. [Google Scholar]
- Veritas, D.N. Design and Operation of CO2 Pipelines. In GEnergy Procedia 4; Elsevier Science Ltd.: Oxford, UK, 2011; pp. 3032–3039. [Google Scholar]
- NETL. CO2 Impurity Design Parameters; NETL: Pittsburgh, PA, USA, 2012.
- Harkin, T.; Filby, I.; Sick, H.; Manderson, D.; Ashton, R. Development of a CO2 Specification for a CCS Hub Network. Energy Procedia 2017, 114, 6708–6720. [Google Scholar] [CrossRef]
- Health and Safety Executive. EH40/2005 Workplace Exposure Limits, 3rd ed.; Health and Safety Executive: Buxton, UK, 2018.
- ISO/TR 27921:2020. Carbon Dioxide Capture, Transportation, and Geological Storage—Cross Cutting Issues—CO2 Stream Composition; International Organisation of Standardisation: Geneva, Switzerland, 2020. [Google Scholar]
- Wetenhall, B.; Race, J.M.; Downie, M.J. The Effect of CO2 Purity on the Development of Pipeline Networks for Carbon Capture and Storage Schemes. Int. J. Greenh. Gas Control 2014, 30, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Chapoy, A.; Nazeri, M.; Kapateh, M.; Burgass, R.; Coquelet, C.; Tohidi, B. Effect of impurities on thermophysical properties and phase behaviour of a CO2-rich system in CCS. Int. J. Greenh. Gas Control 2013, 19, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Nieuwenkamp, G. Final Publishable JRP Summary for ENG60 LNG II Metrological Support for LNG Custody Transfer and Transport Fuel Applications; Euramet: Braunschweig, Germany, 2017. [Google Scholar]
- Chromatotec. CO2 quality control—Gas chromatography solutions. In Gasworld Magazine; Gasworld: Truro, UK, 2018; p. 54. [Google Scholar]
- V&F Analyse- und Messtechnik GmbH, How Pure Is Your CO2? In Gasworld Magazine; Gasworld: Truro, UK, 2018.
- Hunter, N.G.L. Measurement Challenges for Carbon Capture and Storage. Meas. Control 2011, 44, 81–85. [Google Scholar]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).