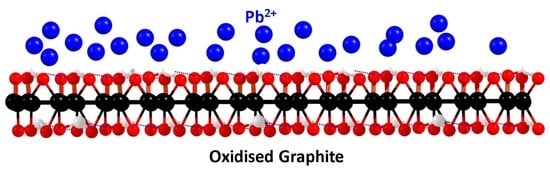
Removal of Lead by Oxidized Graphite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Oxidation of Graphite
2.3. Linear Range Determination of Square Wave Anodic Stripping Voltammetry (SWASV)
2.4. Optimum Dosage of Oxidized Graphite for the Adsorption of Lead Ions (Pb2+)
2.5. Adsorption of Pb2+ Ions
3. Results and Discussion
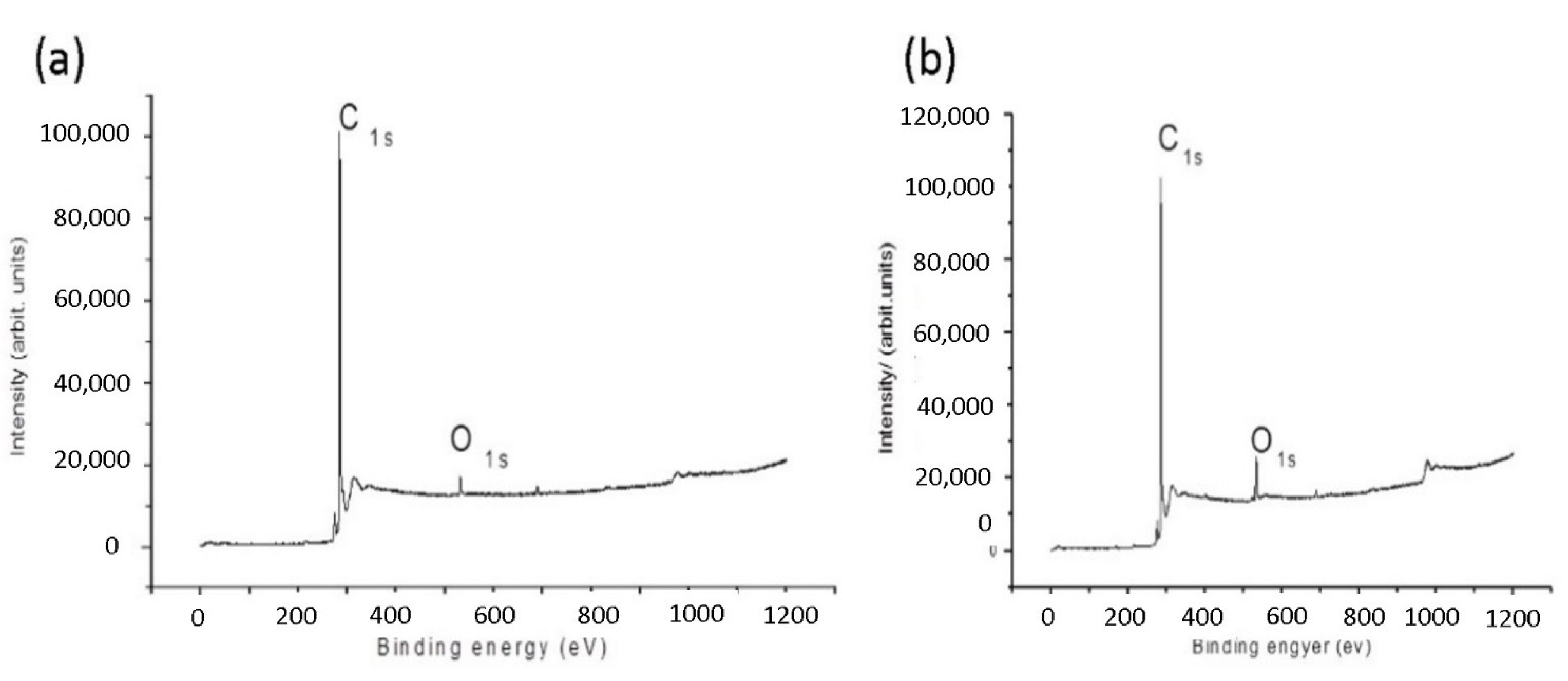
3.1. XPS Characterization
3.2. Effective Concentration of Pb2+ Ions
3.3. Effective Amount of Oxidized Graphite Powder on Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lester, J.N. Significance and behavior of heavy metals in waste water treatment processes I. Sewage treatment and effluent discharge. Sci. Total Environ. 1983, 30, 1–44. [Google Scholar] [CrossRef]
- Smith, W.H. Lead contamination of the roadside ecosystem. J. Air Pollut. Control Assoc. 1976, 26, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.; Green, D. Toward eliminating children’s lead exposure: A comparison of policies and their outcomes in three lead producing and using countries. Environ. Res. Lett. 2020, 15, 103008. [Google Scholar] [CrossRef]
- Khoder, M.I.; Hassan, S.K.; El-Abssawy, A.A. An evaluation of loading rate of dust, Pb, Cd, and Ni and metals mass concentration in the settled surface dust in domestic houses and factors affecting them. Indoor Built Environ. 2010, 19, 391–399. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Xiong, Z.; Wang, M.; Liu, Q. Environmental pollution effect analysis of lead compounds in China Based on Life Cycle. Int. J. Environ. Res. Public Health 2020, 17, 2184. [Google Scholar] [CrossRef] [Green Version]
- Rieuwerts, J.S.; Farago, M.E. Lead contamination in smelting and mining environments and variations in chemical forms and bioavailability. Chem. Speciat. Bioavailab. 1995, 7, 113–123. [Google Scholar] [CrossRef]
- Meyer, P.A.; McGeehin, M.A.; Falk, H. A global approach to childhood lead poisoning prevention. Int. J. Hyg. Environ. Health 2003, 206, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Kordas, K.; Ravenscroft, J.; Cao, Y.; McLean, E.V. Lead Exposure in low and middle-income countries: Perspectives and lessons on patterns, injustices, economics, and politics. Int. J. Environ. Res. Public Health 2018, 15, 2351. [Google Scholar] [CrossRef] [Green Version]
- Rădulescu, A.; Lundgren, S. A pharmacokinetic model of lead absorption and calcium competitive dynamics. Sci. Rep. 2019, 9, 14225. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalination 2016, 7, 387–419. [Google Scholar]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Bankole, M.T.; Abdulkareem, A.S.; Mohammed, I.A.; Ochigbo, S.S.; Tijani, J.O.; Abubakre, O.K.; Roos, W.D. Selected heavy metals removal from electroplating wastewater by purified and polyhydroxylbutyrate functionalized carbon nanotubes adsorbents. Sci. Rep. 2019, 9, 4475. [Google Scholar] [CrossRef] [Green Version]
- Dean, J.G.; Bosqui, F.L.; Lanouette, K.H. Removing heavy metals from waste water. Environ. Sci. Technol. 1972, 6, 518–522. [Google Scholar] [CrossRef]
- Li, A.Y.; Deng, H.; Jiang, Y.H.; Ye, C.H.; Yu, B.G.; Zhou, X.L.; Ma, A.Y. Superefficient removal of heavy metals from wastewater by Mg-Loaded biochars: Adsorption characteristics and removal mechanisms. Langmuir 2020, 36, 9160–9174. [Google Scholar] [CrossRef]
- Mnasri-Ghnimi, S.; Frini-Srasra, N. Removal of heavy metals from aqueous solutions by adsorption using single and mixed pillared clays. Appl. Clay Sci. 2019, 179, 105151. [Google Scholar] [CrossRef]
- Arora, R. Adsorption of Heavy Metals—A Review. Mater. Today Proc. 2019, 18, 4745–4750. [Google Scholar] [CrossRef]
- Netzer, A.; Hughes, D.E. Adsorption of copper, lead and cobalt by activated carbon. Water Res. 1984, 18, 927–933. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Kyzas, G.Z.; Triantafyllidis, K.S.; Matis, K.A. Activated carbons for the removal of heavy metal ions: A systematic review of recent literature focused on lead and arsenic ions. Open Chem. 2015. [Google Scholar] [CrossRef]
- Ouni, L.; Ramazani, A.; Taghavi Fardood, S. An overview of carbon nanotubes role in heavy metals removal from wastewater. Front. Chem. Sci. Eng. 2019, 13, 274–295. [Google Scholar] [CrossRef]
- Nyairo, W.N.; Eker, Y.R.; Kowenje, C.; Akin, I.; Bingol, H.; Tor, A.; Ongeri, D.M. Efficient adsorption of lead (II) and copper (II) from aqueous phase using oxidized multiwalled carbon nanotubes/polypyrrole composite. Sep. Sci. Technol. 2018, 53, 1498–1510. [Google Scholar] [CrossRef]
- Abiman, P.; Wildgoose, G.G.; Crossley, A.; Compton, R.G. Quantitative studies of metal Ion adsorption on a chemically modified carbon surface: Adsorption of Cd(II) and Hg(II) on glutathione modified carbon. Electroanalysis 2009, 21, 897–903. [Google Scholar] [CrossRef]
- McKenzie, R.M. The adsorption of lead and other heavy metals on oxides of manganese and iron. Soil Res. 1980, 18, 61–73. [Google Scholar] [CrossRef]
- Rashed, M.N. Lead removal from contaminated water using mineral adsorbents. Environmentalist 2001, 21, 187–195. [Google Scholar] [CrossRef]
- Shi, B.; Zuo, W.; Zhang, J.; Tong, H.; Zhao, J. Removal of Lead(II) Ions from aqueous solution using jatropha curcas L. Seed Husk Ash as a Biosorbent. J. Environ. Qual. 2016, 45, 984–992. [Google Scholar] [CrossRef]
- Mathew, B.B.; Jaishankar, M.; Biju, V.G.; Krishnamurthy Nideghatta, B. Role of bioadsorbents in reducing toxic metals. J. Toxicol. 2016, 2016, 4369604. [Google Scholar] [CrossRef] [Green Version]
- Reddad, Z.; Gerente, C.; Andres, Y.; Le Cloirec, P. Adsorption of several metal ions onto a low-cost biosorbent: Kinetic and Equilibrium Studies. Environ. Sci. Technol. 2002, 36, 2067–2073. [Google Scholar] [CrossRef]
- Xie, R.; Jin, Y.; Chen, Y.; Jiang, W. The importance of surface functional groups in the adsorption of copper onto walnut shell derived activated carbon. Water Sci. Technol. 2017, 76, 3022–3034. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Yang, X.; Xu, G.; Yu, H. Removal of lead from aqueous solutions by ferric activated sludge-based adsorbent derived from biological sludge. Arab. J. Chem. 2019, 12, 4142–4149. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, I.M.; Ahmed, S.A.; Shehata, N.; Sheneshen, E.S.; Fathy, M.; Shehata, A. A novel approach for the removal of lead (II) ion from wastewater using Kaolinite/Smectite natural composite adsorbent. Appl. Water Sci. 2018, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Bala, T.; Prasad, B.L.V.; Sastry, M.; Kahaly, M.U.; Waghmare, U.V. Interaction of different metal ions with carboxylic acid group: A quantitative study. J. Phys. Chem. A 2007, 111, 6183–6190. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M. Adsorption of lead ions from aqueous solution by okra wastes. Int. J. Phys. Sci. 2007, 2, 178–184. [Google Scholar]
- Zavvar Mousavi, H.; Seyedi, S. Kinetic and equilibrium studies on the removal of Pb(II) from aqueous solution using nettle ash. J. Chil. Chem. Soc. 2009, 55, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Alghamdi, A.A.; Al-Odayni, A.-B.; Saeed, W.S.; Al-Kahtani, A.; Alharthi, F.A.; Aouak, T. Efficient Adsorption of Lead (II) from Aqueous Phase Solutions Using Polypyrrole-Based Activated Carbon. Materials (Basel) 2019, 12, 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
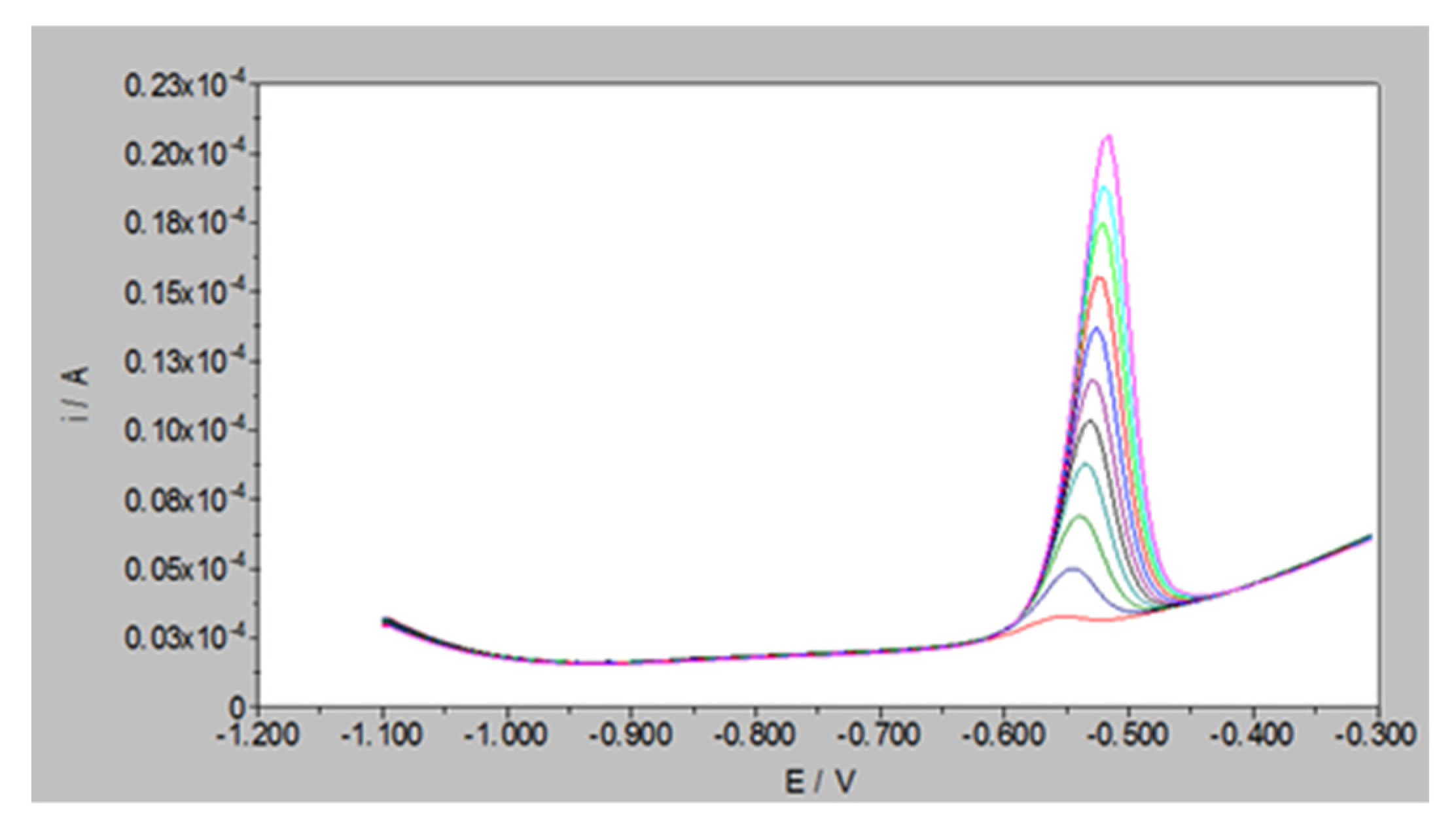


| Initial Concentration of Pb2+ Ion (Ci µM) | Average Adsorbed Moles of Pb2+ Ions in mol mg−1 (×10−10) |
|---|---|
| 50 | 44.863 |
| 100 | 73.333 |
| 200 | 80.806 |
| 300 | 82.313 |
| 400 | 85.816 |
| 500 | 84.400 |
| Dosage of Oxidised Graphite (mg) | Average Adsorbed Moles of Pb2+ Ions in mol mg−1 (×10−10) |
|---|---|
| 100 | 73.333 |
| 200 | 81.613 |
| 300 | 80.883 |
| 400 | 81.410 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvanantharajah, N.; Iyngaran, P.; Abiman, P.; Kuganathan, N. Removal of Lead by Oxidized Graphite. C 2021, 7, 23. https://0-doi-org.brum.beds.ac.uk/10.3390/c7010023
Selvanantharajah N, Iyngaran P, Abiman P, Kuganathan N. Removal of Lead by Oxidized Graphite. C. 2021; 7(1):23. https://0-doi-org.brum.beds.ac.uk/10.3390/c7010023
Chicago/Turabian StyleSelvanantharajah, Namasivayam, Poobalasuntharam Iyngaran, Poobalasingam Abiman, and Navaratnarajah Kuganathan. 2021. "Removal of Lead by Oxidized Graphite" C 7, no. 1: 23. https://0-doi-org.brum.beds.ac.uk/10.3390/c7010023