Selection of Mixed Amines in the CO2 Capture Process
Abstract
:1. Introduction
| Amine | Chemical Structure | Advantage | Drawback | References |
|---|---|---|---|---|
| Primary amine | H2N-CH2-CH2-OH (MEA) |
|
| [12] |
| H2N-CH2-CH2-O-CH2-CH2-OH (DGA) | ||||
| Secondary amine | HN-(CH2-CH2-OH)2 (DEA) |
|
| [9] |
| HN-(CH2-C(OH)-CH3)2 (DIPA) | ||||
| Tertiary amine | N-(CH2-CH2-OH)3 (TEA) |
|
| [13,14] |
| CH3-N-(CH2-CH2-OH)2 (MDEA) | ||||
| Steric hindrance | HN-CH-(CH3)2-CH2-OH (AMP) |
|
| [15,16] |
| Piperazine | C4H10N2 (PZ) |
|
| [8] |
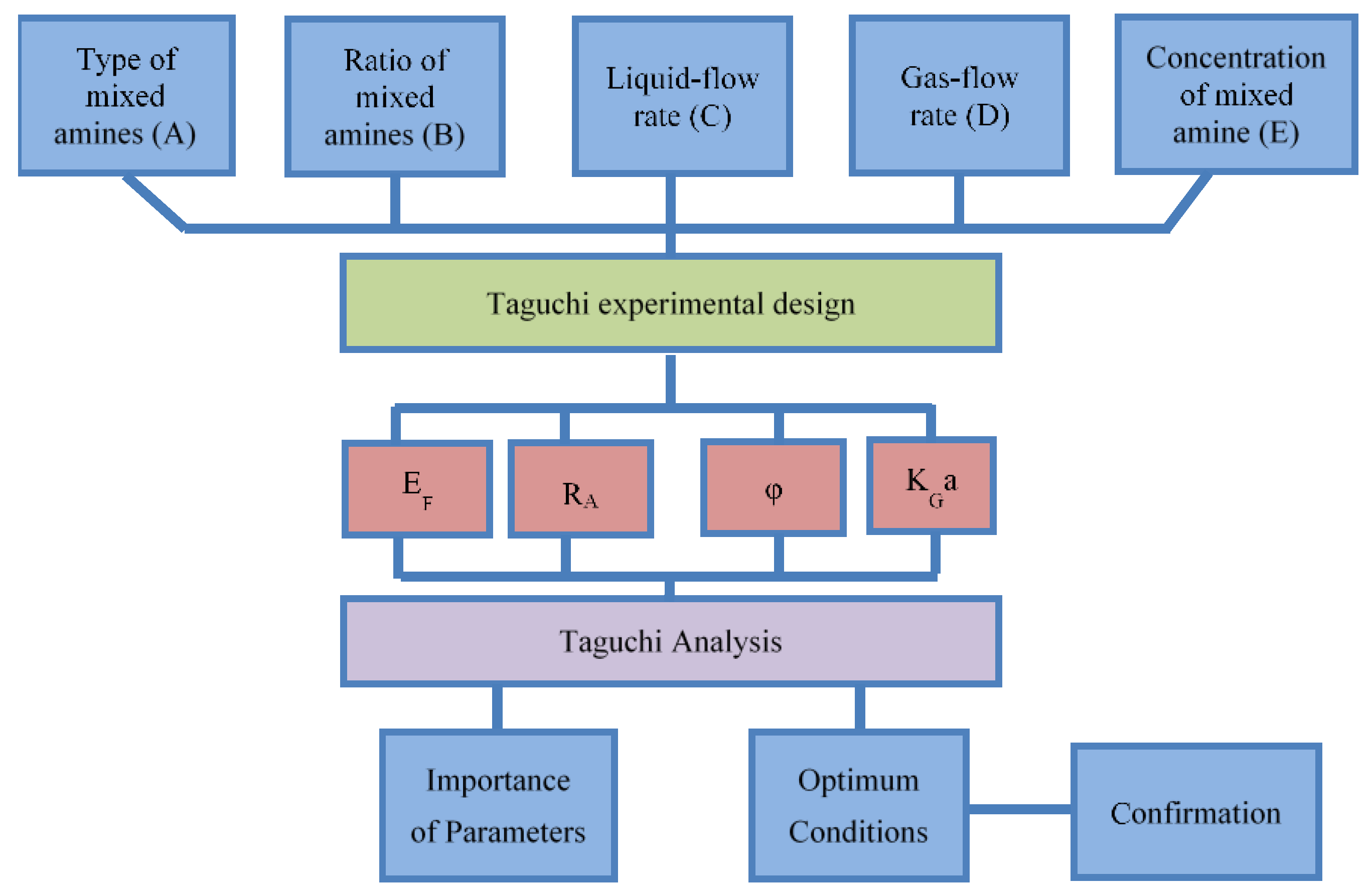
2. Experimental Design and Procedure
2.1. Absorption Experiment Design
2.2. Calculation of Experimental Data
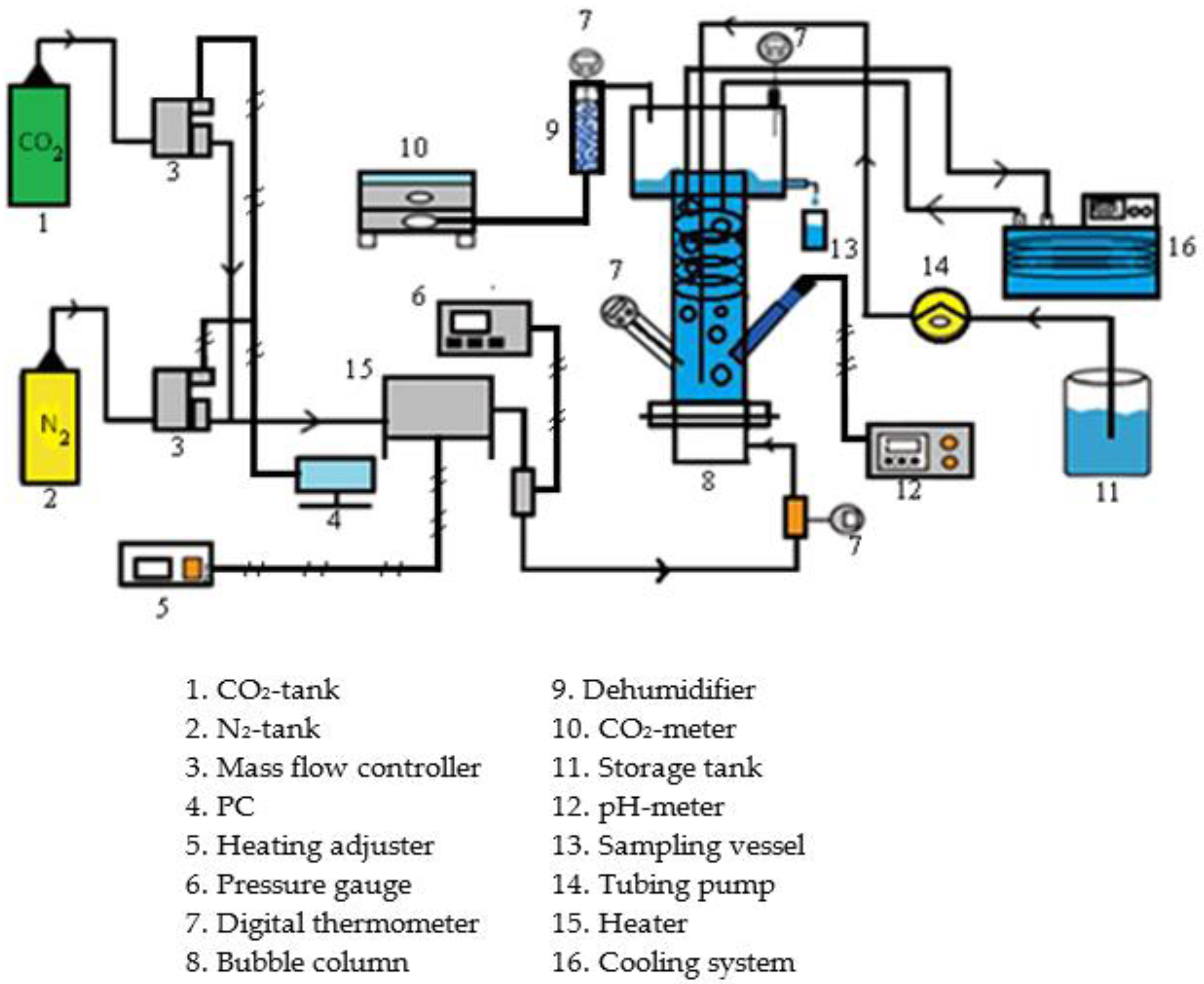
2.3. Experimental Devices and Procedures
3. Results and Discussion
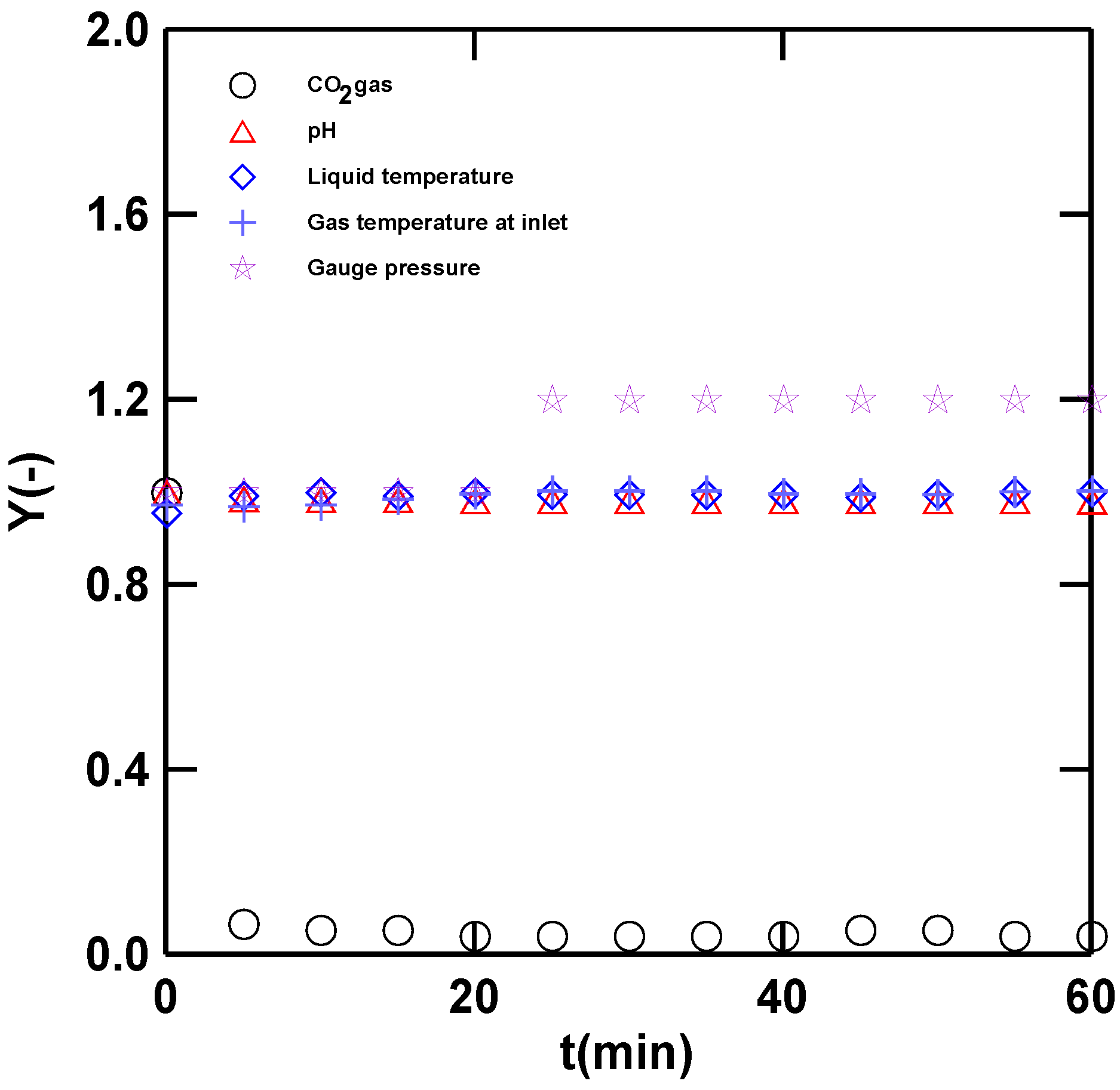
3.1. Operation and Data Calculation of the Steady-State Condition
3.2. Taguchi Analysis
3.3. Confirmation of the Optimum Condition
3.4. Comparisons of Mixed Amines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, M.T.; Allinson, G.W.; Wiley, D.E. Comparison of MEA capture cost for low CO2 emissions sources in Australia. Int. J. Greenh. Gas Con. 2011, 5, 49–60. [Google Scholar] [CrossRef]
- Han, K.; Ahn, C.K.; Lee, M.S. Performance of an ammonia-based CO2 Capture pilot facility in iron and steel industry. Int. J. Greenh. Gas Con. 2014, 27, 239–246. [Google Scholar] [CrossRef]
- Li, T.; Keener, T.C. A review: Desorption of CO2 from rich solutions in chemical absorption processes. Int. J. Greenh. Gas Con. 2016, 51, 290–304. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol. Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef] [Green Version]
- Abdulkadir, A.; Rayer, A.V.; Quang, D.V.; Hadri, N.E.; Dindi, A.; Feron, P.H.M.; Abu-Zahra, M.R.M. Heat of absorption and specific heat of carbon dioxide in aqueous solutions of monoethanolamine, 3-piperdienmethonal and their blends. Energy Procedia 2014, 63, 2070–2081. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, P.D.; Kenig, E.Y. Absorption of CO2 into aqueous blends of alkanolamines prepared from renewable resources. Chem. Eng. Sci. 2007, 62, 7344–7350. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.F.; Qin, S.J.; Zheng, Z.S. A kinetics study on the absorption of carbon dioxide into mixed aqueous solution of methyldiethanolamine and piperazine. Ind. Eng. Chem. Res. 2001, 40, 3785–3791. [Google Scholar] [CrossRef]
- Van Wagener, D.H.; Rochelle, G.T. Stripper configurations for CO2 capture by aqueous monoethanolamine and piperazine. Energy Procedia 2011, 4, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Adeosun, A.; Abu-Zahra, M.R.M. Evaluation of amine-blend solvent systems for CO2 post-combustion capture applications. Energy Procedia 2013, 37, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Ali Khan, A.; Halder, G.; Saha, A.K. Kinetic effect and absorption performance of piperizine activator into aqueous solutions of 2-amino-1-methyl-1-propanol through post-combustion CO2 capture. Korean J. Chem. Eng. Eng. 2019, 63, 1090–1101. [Google Scholar] [CrossRef]
- Mondal, M.K. Absorption of carbon dioxide into a mixed aqueous solution of diethanolamine and piperazine. Indian J. Chem. Technol. 2010, 17, 431–435. [Google Scholar]
- Vaidya, P.D.; Kenig, E.Y. CO2-alkanomine reaction kinetics: A review of recent work. Chem. Eng. Technol. 2007, 30, 1467–1474. [Google Scholar] [CrossRef]
- Rangwala, H.A.; Morrell, B.R.; Mather, A.E.; Otto, F.D. Absorption of CO2 into aqueous tertiary amine/mea solutions. Can. J. Chem. Eng. 1992, 70, 482–490. [Google Scholar] [CrossRef]
- Xiao, J.; Li, C.C.; Li, M.H. Kinetics of absorption of carbon dioxide into aqueous solutions of 2-amino-2-methyl-1-propanol+monoethanolamine. Chem. Eng. Sci. 2000, 55, 161–175. [Google Scholar] [CrossRef]
- Choi, W.J.; Seo, J.B.; Jang, S.Y.; Jung, J.H.; Oh, K.J. Removal characteristics of CO2 using aqueous MEA/AMP solutions in the absorption and regeneration process. J. Environ. Sci. 2009, 21, 907–913. [Google Scholar] [CrossRef]
- Neveux, T.; Moullec, Y.L.; Corriou, J.P.; Favre, E. Energy performance of CO2 capture processes: Interaction between process design and solvent. Chem. Eng. Trans. 2013, 35, 337–342. [Google Scholar] [CrossRef]
- Dash, S.K.; Samanta, A.N.; Bandyopadhyay, S.S. Simulation and parametric study of post combustion CO2 capture process using (AMP+PZ) blended solvent. Inter. J. Greenh. Gas. Con. 2014, 21, 130–139. [Google Scholar] [CrossRef]
- Diao, Y.F.; Zheng, X.Y.; He, B.S.; Chen, C.H.; Xu, X.C. Experimental study on capuring CO2 greenhouse gas by ammonia scrubbing. Energy Convers. Manag. 2004, 45, 2283–2296. [Google Scholar] [CrossRef]
- Oyenekan, B.A.; Rochelle, G.T. Alternative stripper configurations for CO2 capture by aqueous amines. AICHE J. 2007, 53, 3144–3154. [Google Scholar] [CrossRef]
- Daneshvar, N.; Moattar, M.T.Z.; Abdi, M.A.; Aber, S. Carbon dioxide equilibrium absorption in muti-component systems of CO2+TIPA+MEA+H2O, at low CO2 partial pressures: Experimental solubility data, corrosion study and molding with artificial neural network. Sep. Purif. Technol. 2004, 37, 135–147. [Google Scholar] [CrossRef]
- Mangalapally, H.P.; Notz, R.; Hoch, S.; Asprion, N.; Sieder, G.; Garcia, H.; Hasse, H. Pilot plant Experimental studies of post combustion CO2 capture by reactive absorption with MEA and new solvents. Energy Procedia 2009, 1, 963–970. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, J.H.; Lee, I.Y.; Jang, K.R.; Shim, J.G. Performance evaluation of newly developed absorbents for CO2 capture. Energy Procedia 2011, 4, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.Q.; Liu, D.W.; Kirk, D.W.; Yang, J.; Bao, S. Aqueous blended amine MEA+DETA solutions for CO2 absorption. APEST 2012, 6, 459–464. [Google Scholar]
- Lin, P.H.; Wong, D.S.H. Carbon dioxide capture and regeneration with amine/alchol/water. Int. J. Greenh. Gas Con. 2014, 26, 69–75. [Google Scholar] [CrossRef]
- Bosch, H.; Versteeg, G.F.; van Swaaij, W.P.M. Gas-liquid mass transfer with parallel reversible reactions—III. Absorption of CO2 into solutions of blends of amines. Chem. Eng. Sci. 1989, 44, 2745–2750. [Google Scholar] [CrossRef] [Green Version]
- David, A.; James, G.; Critchfield, E.; Rochelle, G.T. CO2 absorption/desorption in mixtures of methyldiethanolamine with monoethanolamine. Chem. Eng. Sci. 1991, 46, 2829–2845. [Google Scholar] [CrossRef]
- Versteeg, G.F.; Dijck, L.A.J.; Swaaij, W.P.M. On the kinetics between CO2 and Alkaloamines both in aqueous and non-aqueous solutions. An overview. Chem. Eng. Commun. 1996, 114, 113. [Google Scholar] [CrossRef]
- Derks, P.W.J.; Kleigeld, T.; van Aken, C.; Hogendoorn, J.A.; Versteeg, G.F. Kinetics of absorption of carbon dioxide in aqueous piperazine solutions. Chem. Eng. Sci. 2006, 61, 6837–6854. [Google Scholar] [CrossRef] [Green Version]
- Aroonwilas, A.; Veawab, A. Integration of CO2 capture unit using blended MEA-AMP solution into coal-fired power plants. Energy Procedia 2009, 1, 4315–4321. [Google Scholar] [CrossRef] [Green Version]
- Badea, A.A.; Dinca, C.F. CO2 capture from post-combustion gas by employing MEA absorption process–experimental investigation for pilot studies. UPB Sci. Bull. Ser. D 2012, 74, 21–32. [Google Scholar]
- Fu, K.; Chen, G.; Sema, T.; Zhang, X.; Liang, Z. Experimental study on mass transfer and prediction using artificial neural network for CO2 absorption into aqueous DETA. Chem. Eng. Sci. 2013, 100, 195–202. [Google Scholar] [CrossRef]
- Bishnoi, S.; Rochelle, G.T. Absorption of carbon dioxide into aqueous piperazine: Reaction kinetics, mass transfer and solubility. Chem. Eng. Sci. 2000, 55, 5531–5543. [Google Scholar] [CrossRef]
- Sakwattanapong, R.; Aroonwilas, A.; Veawab, A. Reaction rate of CO2 in aqueous MEA-AMP solution: Experiment and modeling. Energy Procedia 2009, 1, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, H.; Mello, L.; Salvagnini, W.; de Paulo, J.L. Absorption of carbon dioxide into aqueous solutions of alkanolamines in wetted wall column with film promoter. Chem. Eng. Trans. 2011, 25, 51–56. [Google Scholar] [CrossRef]
- Gómez-Díaz, D.; López, A.B.; La García, M.D.R.; Pacheco, R.R.; Gómez-Díaz, D. Carbon Dioxide Absorption in Triethanolamine Aqueous Solutions: Hydrodynamics and Mass Transfer. Chem. Eng. Technol. 2014, 37, 419–426. [Google Scholar] [CrossRef]
- Al-Hindi, M.; Azizi, F. The effect of water type on the absorption and desorption of carbon dioxide in bubble columns. Chem. Eng. Comm. 2020, 207, 339–349. [Google Scholar] [CrossRef]
- Karamian, S.; Mowla, D. Esmaeilzadeh, The effect of various nanofluids on absorption intensification of CO2/SO2 in a single-bubble column. Processes 2019, 7, 393. [Google Scholar] [CrossRef] [Green Version]
- Beiki, H.; Ansaryan, O. Nanofluids application to promote CO2 absorption inside a bubble column: ANFIS and experimental study. Energy 2020, unpublished. [Google Scholar]
- Chakma, A.; Lemonier, J.P.; Chornet, E.; Overend, R.P. Absorption of CO2by aqueous triethanolamine (TEA) solutions in a high shear jet absorber. Gas Sep. Purif. 1989, 3, 65–70. [Google Scholar] [CrossRef]
- Fraley, S.; Oom, M.; Terrien, B.; Zalewski, J. Design of Experiments via Taguchi Methods: Orthogonal Arrays. 2012. Available online: https://controls.engin.umich.edu/wiki/index.php (accessed on 13 January 2021).
- Chen, P.C.; Luo, Y.X.; Cai, P.W. CO2 Capture Using Monoethanolamine in a Bubble-ColumnScrubber. Chem. Eng. Technol. 2015, 38, 274–282. [Google Scholar] [CrossRef]
- Chen, P.C.; Lin, S.Z. Optimization in the Absorption and Desorption of CO2 Using Sodium Glycinate Solution. Appl. Sci. 2018, 8, 2041. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.C.; Shi, W.; Du, R.; Chen, V. Scrubbing of CO2 Greenhouse gases, accompanied by precipitation in a continuous bubble-column Scrubber. Ind. Eng. Chem. Res. 2008, 47, 6336–6343. [Google Scholar] [CrossRef]
- Jou, F.Y.; Mather, A.E.; Otto, F.D. The solubility of CO2 in a 30 mass percent monoethanolamine solution. Can. J. Chem. Eng. 1995, 73, 140–147. [Google Scholar] [CrossRef]
- Chen, P.C.; Yu, S.C. CO2 Capture and Crystallization of Ammonia Bicarbonate in a Lab-Scale Scrubber. Crystals 2018, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.C.; Huang, C.H.; Su, T.; Chen, H.W.; Yang, M.W.; Tsao, J.M. Optimum conditions for thecapture of carbon dioxide with a bubble-column scrubber. Int. J. Greenh. Gas Con. 2015, 35, 47–55. [Google Scholar] [CrossRef]

| Factors | Level 1 | Level 2 | Level 3 | Level 4 |
|---|---|---|---|---|
| Type of mixed amines (A) | MEA + AMP | MEA + DIPA | MEA + TEA | MEA + PZ |
| Ratio of mixed amines [wt%] (B) | 5 | 10 | 15 | 20 |
| QL [mL/min] (C) | 150 | 200 | 250 | 300 |
| Qg [L/min] (D) | 3 | 6 | 9 | 12 |
| Concentration of mixed amine [M] (E) | 1 | 1.5 | 2 | 2.5 |
| NO | A | B | C | D | E |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 2 | 2 | 2 | 2 |
| 3 | 1 | 3 | 3 | 3 | 3 |
| 4 | 1 | 4 | 4 | 4 | 4 |
| 5 | 2 | 1 | 2 | 3 | 4 |
| 6 | 2 | 2 | 1 | 4 | 3 |
| 7 | 2 | 3 | 4 | 1 | 2 |
| 8 | 2 | 4 | 3 | 2 | 1 |
| 9 | 3 | 1 | 3 | 4 | 2 |
| 10 | 3 | 2 | 4 | 3 | 1 |
| 11 | 3 | 3 | 1 | 2 | 4 |
| 12 | 3 | 4 | 2 | 1 | 3 |
| 13 | 4 | 1 | 4 | 2 | 3 |
| 14 | 4 | 2 | 3 | 1 | 4 |
| 15 | 4 | 3 | 2 | 4 | 1 |
| 16 | 4 | 4 | 1 | 3 | 2 |
| NO. | EF (%) | RA × 104 (mol/s·L) | KGa (s−1) | (mole/mole·L) |
|---|---|---|---|---|
| NO.1 | 95.95 | 4.02 | 0.229 | 0.134 |
| NO.2 | 88.16 | 9.12 | 0.361 | 0.127 |
| NO.3 | 84.00 | 14.47 | 0.523 | 0.106 |
| NO.4 | 76.32 | 26.48 | 0.798 | 0.087 |
| NO.5 | 85.33 | 14.40 | 0.536 | 0.108 |
| NO.6 | 61.33 | 15.70 | 0.379 | 0.172 |
| NO.7 | 96.05 | 4.21 | 0.235 | 0.046 |
| NO.8 | 82.89 | 8.11 | 0.281 | 0.141 |
| NO.9 | 68.42 | 27.7 | 0.730 | 0.154 |
| NO.10 | 76.00 | 14.2 | 0.432 | 0.161 |
| NO.11 | 92.00 | 8.54 | 0.394 | 0.104 |
| NO.12 | 97.33 | 4.02 | 0.252 | 0.035 |
| NO.13 | 89.33 | 8.13 | 0.340 | 0.126 |
| NO.14 | 100 | 4.09 | 0.646 | 0.034 |
| NO.15 | 62.67 | 15.6 | 0.382 | 0.263 |
| NO.16 | 81.33 | 12.6 | 0.427 | 0.229 |
| EF (%) | RA × 104 (mol/s·L) | KGa (s−1) | System and Conditions | Reference |
|---|---|---|---|---|
| 36.54–86.84 | 2.30–8.56 | 0.051–0.189 | Sodium glycinate solution(pH-stat) pH = 9.5–11 Qg = 3–9 L/min T = 25–40 °C CL = 3–6 M yA1 = 15% | [42] |
| 17.5–97.5 | 3.68–56.8 | 0.0377–0.8881 | MEA solution(pH-stat) pH = 9–11 Qg = 4–9.5 L/min T = 25–45 °C yA1 = 15–65% | [41] |
| 10.9–100 | 3.21–10.99 | 0.0136–0.3302 | Ammonia solution(pH-stat) pH = 9.5 Qg = 4–9.5 L/min T = 25–60 °C yA1 = 15–60% | [45] |
| 21.3–90.6 | 1.03–11.48 | 0.015–0.246 | NaOH solution(pH stat) pH = 10–13 Qg = 3–12 L/min T = 25–55 °C yA1 = 15% | [46] |
| 61.33–100 | 4.02–27.70 | 0.229–0.789 | Mixed amine solutions Qg = 3–12 L/min Q = 150–300 mL/min T = 30 °C yA1 = 15% | This work |
| Outcome Data | Optimum Conditions | Importance of Parameters |
|---|---|---|
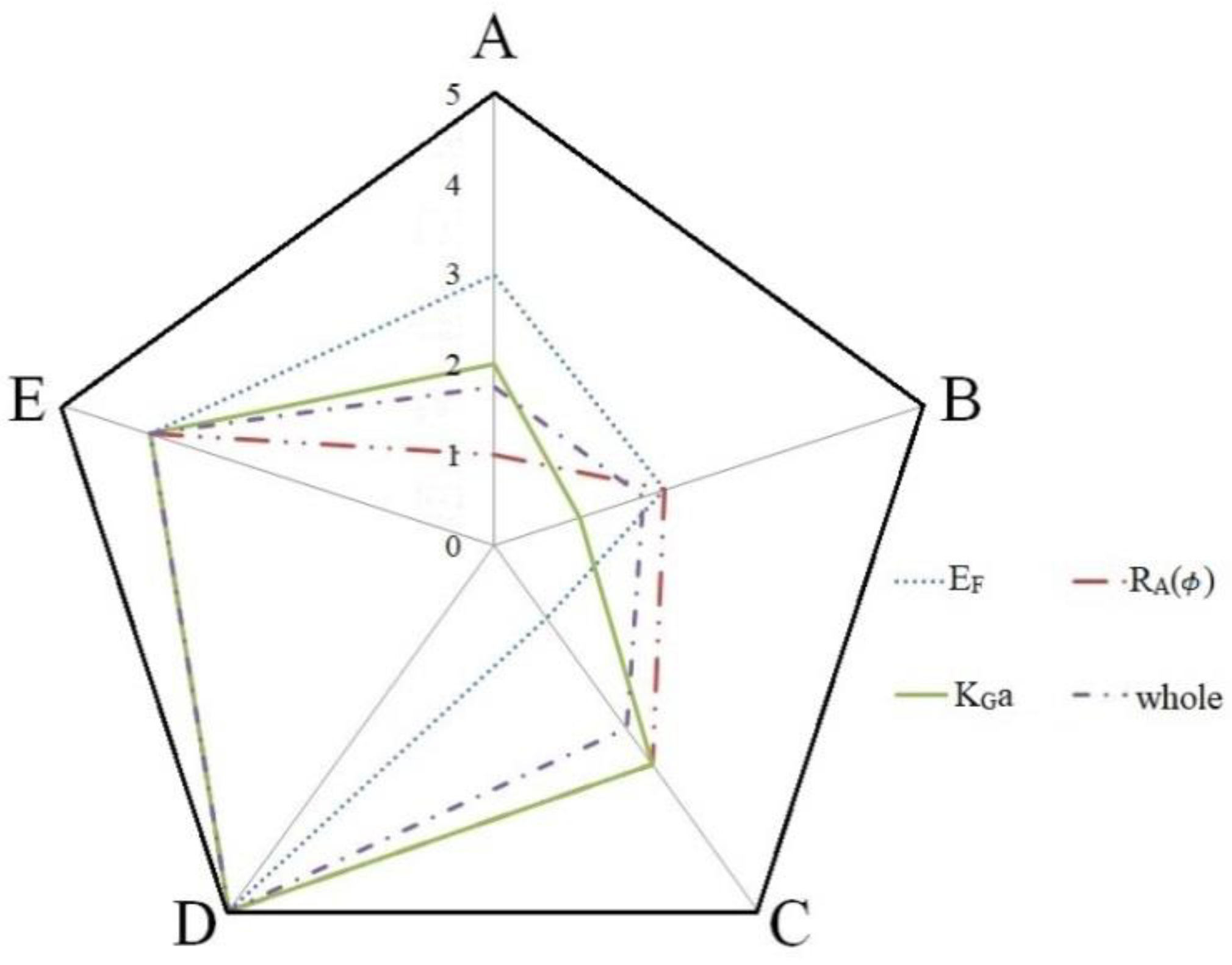
| EF | A1 B4 C4 D1 E4 | D > E > A > B > C |
| RA | A1 B3 C4 D4 E2 | D > E > C > B > A |
| KGa | A4 B2 C3 D4 E4 | D > E > C > A > B |
| A1 B1 C1 D4 E1 | D > E > C > B > A |
| NO. | EF (%) | RA × 104 (mole/s·L) | KGa (s−1) | (mole/mole·L) |
|---|---|---|---|---|
| 1–16 | 61.33–100 | 4.02–27.7 | 0.229–0.798 | 0.034–0.263 |
| Optimum value | 100 | 30.69 | 1.540 | 0.297 |
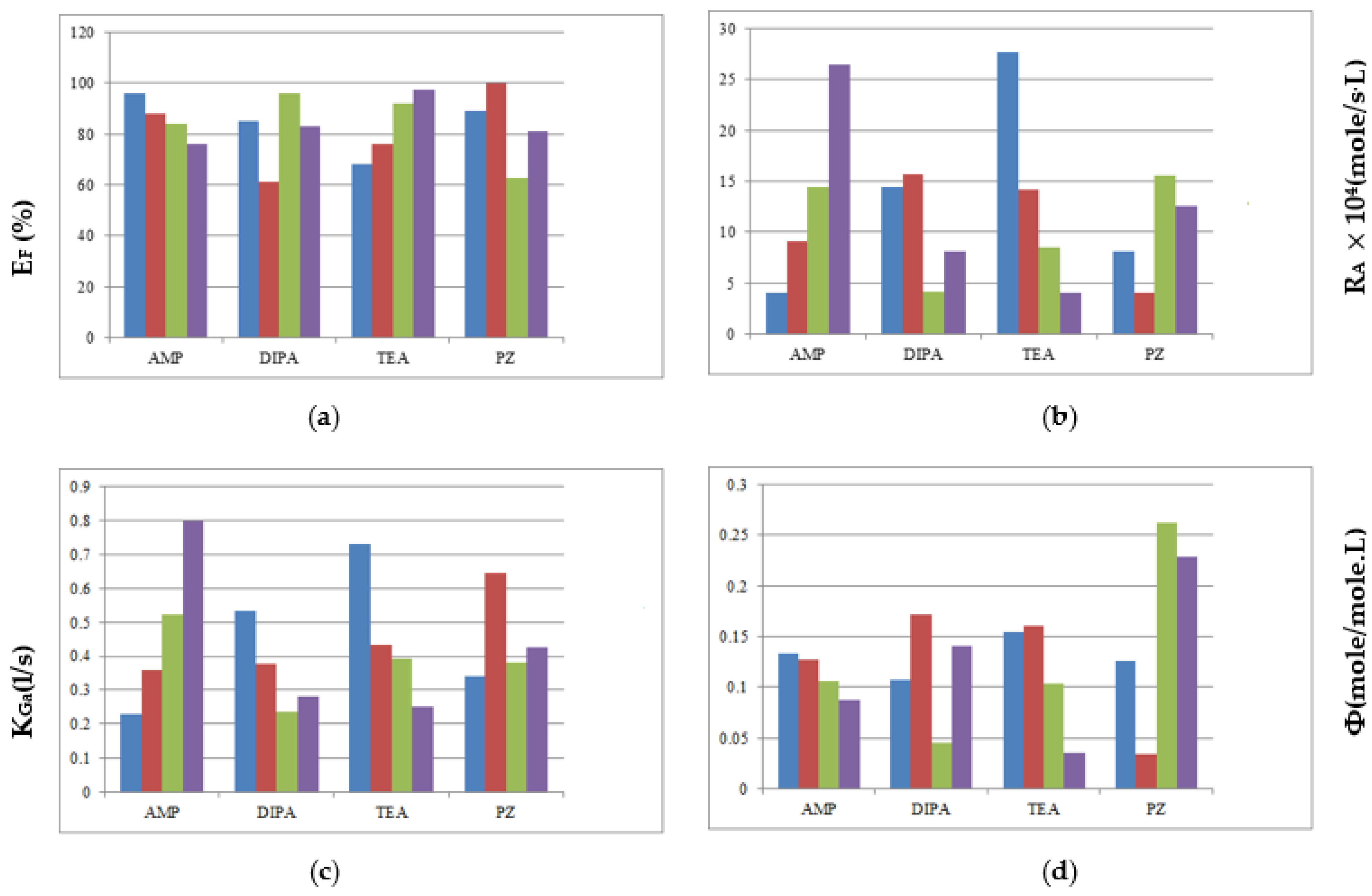
| Mixed Amines | AMP | DIPA | TEA | PZ |
|---|---|---|---|---|
| EF | ◎ | × | ○ | △ |
| RA | ○ | △ | ◎ | × |
| KGa | ◎ | × | ○ | △ |
| △ | ○ | △ | ◎ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-C.; Cho, H.-H.; Jhuang, J.-H.; Ku, C.-H. Selection of Mixed Amines in the CO2 Capture Process. C 2021, 7, 25. https://0-doi-org.brum.beds.ac.uk/10.3390/c7010025
Chen P-C, Cho H-H, Jhuang J-H, Ku C-H. Selection of Mixed Amines in the CO2 Capture Process. C. 2021; 7(1):25. https://0-doi-org.brum.beds.ac.uk/10.3390/c7010025
Chicago/Turabian StyleChen, Pao-Chi, Hsun-Huang Cho, Jyun-Hong Jhuang, and Cheng-Hao Ku. 2021. "Selection of Mixed Amines in the CO2 Capture Process" C 7, no. 1: 25. https://0-doi-org.brum.beds.ac.uk/10.3390/c7010025




