sp2 Carbon Stable Radicals
Abstract
:1. Introduction
2. A Historical Excurse in the Virtual Spin Chemistry of Radicals
3. Grounds of UHF Computational Spin Chemistry of sp2 Nanocarbons
3.1. General Remarks
- Spin traits of the reactants that enter into reactions;
- Spin kind of the intermolecular interaction that controls such reactions;
- Spin nature of the reaction final products;
- Post-reaction storage of spin chemistry products.
- Ascertainment of the radical status of an open-shell molecule;
- Evaluation of the molecule chemical activity provided with spin density;
- Establishment of the spin density delocalization over the molecule atoms;
- Determination of the delocalization of the chemical activity over molecule, atoms thus presenting its “chemical portrait”;
- Detection of the spin-chemical topology caused by the multi-target character of the molecules.
3.2. Common Background for the sp2 Nanocarbons Features
- More than half of sp2C=C bonds of sp2 nanocarbons, including fullerenes, carbon nanotubes, and graphene molecules, are longer than critical interatomic distance , exceeding over which leads to the bond radicalization. The feature lays the foundation of the open-shell character of the species electron systems and radical character of the molecules.
- If bilength bond composition is characteristic for the {sp2C=C bonds} pool of fullerene C60, many-length composition is typical for more extended {sp2C=C bonds} networks starting from fullerene C70 and involving CNTs and graphene molecules.
- Application of UHF approach allows disclosing a collective response of {sp2C=C bonds} pool to each act of any chemical addition to the relevant species in all the cases, thus revealing delocalization of the bond distribution disturbance.
4. Spin Traits of Open-Shell Molecules of sp2 Nanocarbons
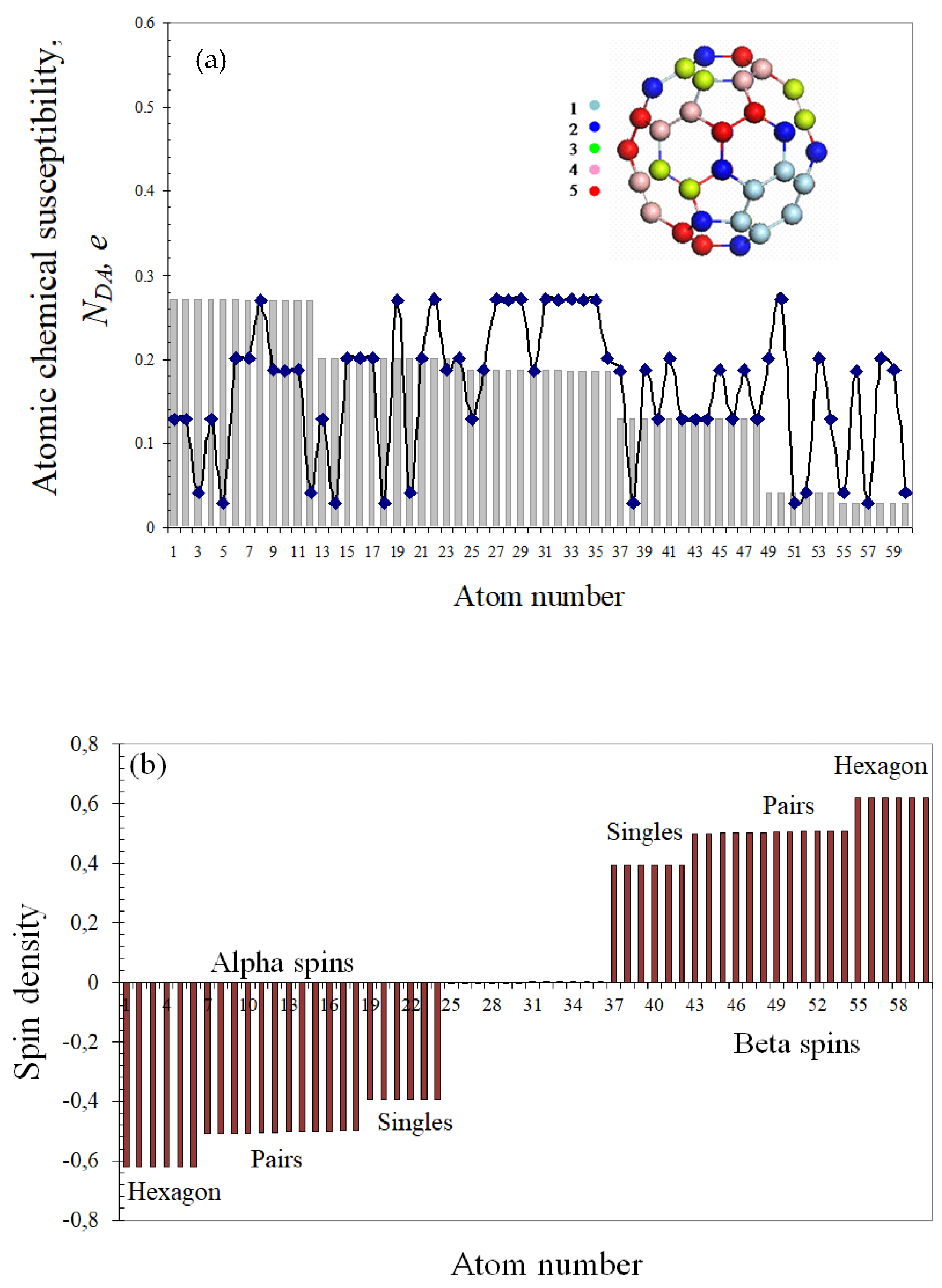
4.1. Fullerene C60
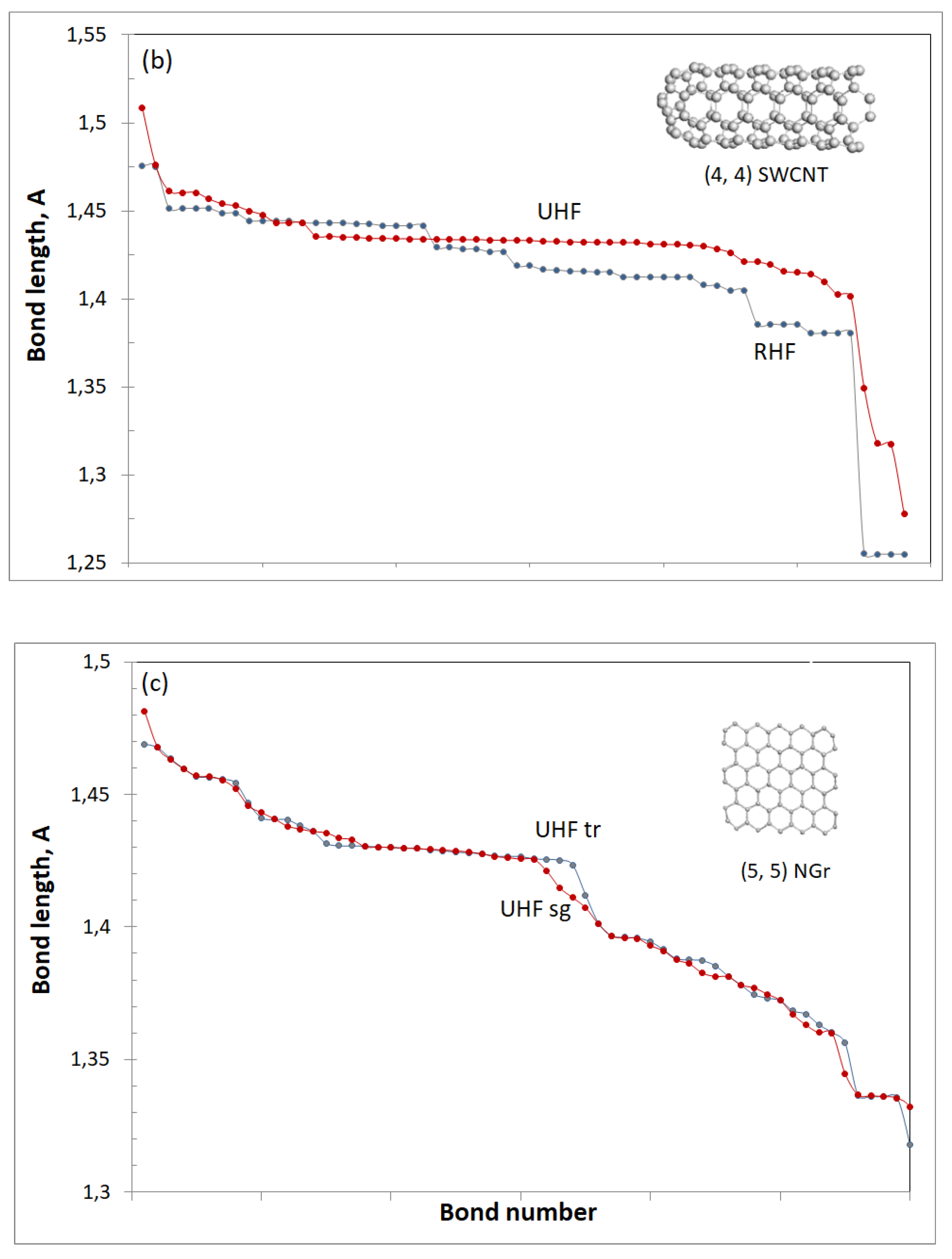
4.2. (4, 4) SWCNT Fragment
4.3. (5, 5) NGr Molecule
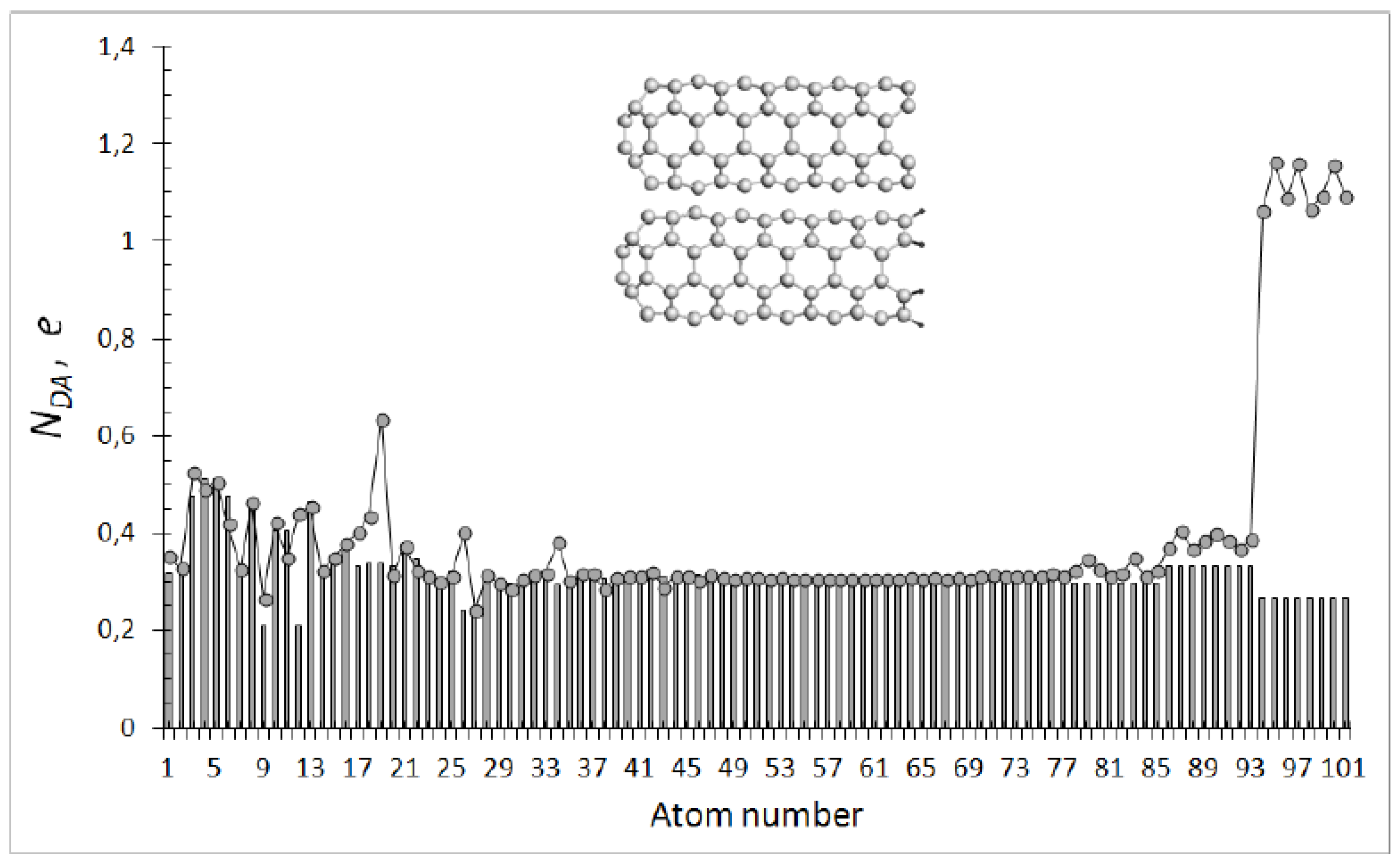
5. Electron Spins in Intermolecular Interaction
6. Spin Nature of the Reaction Final Products

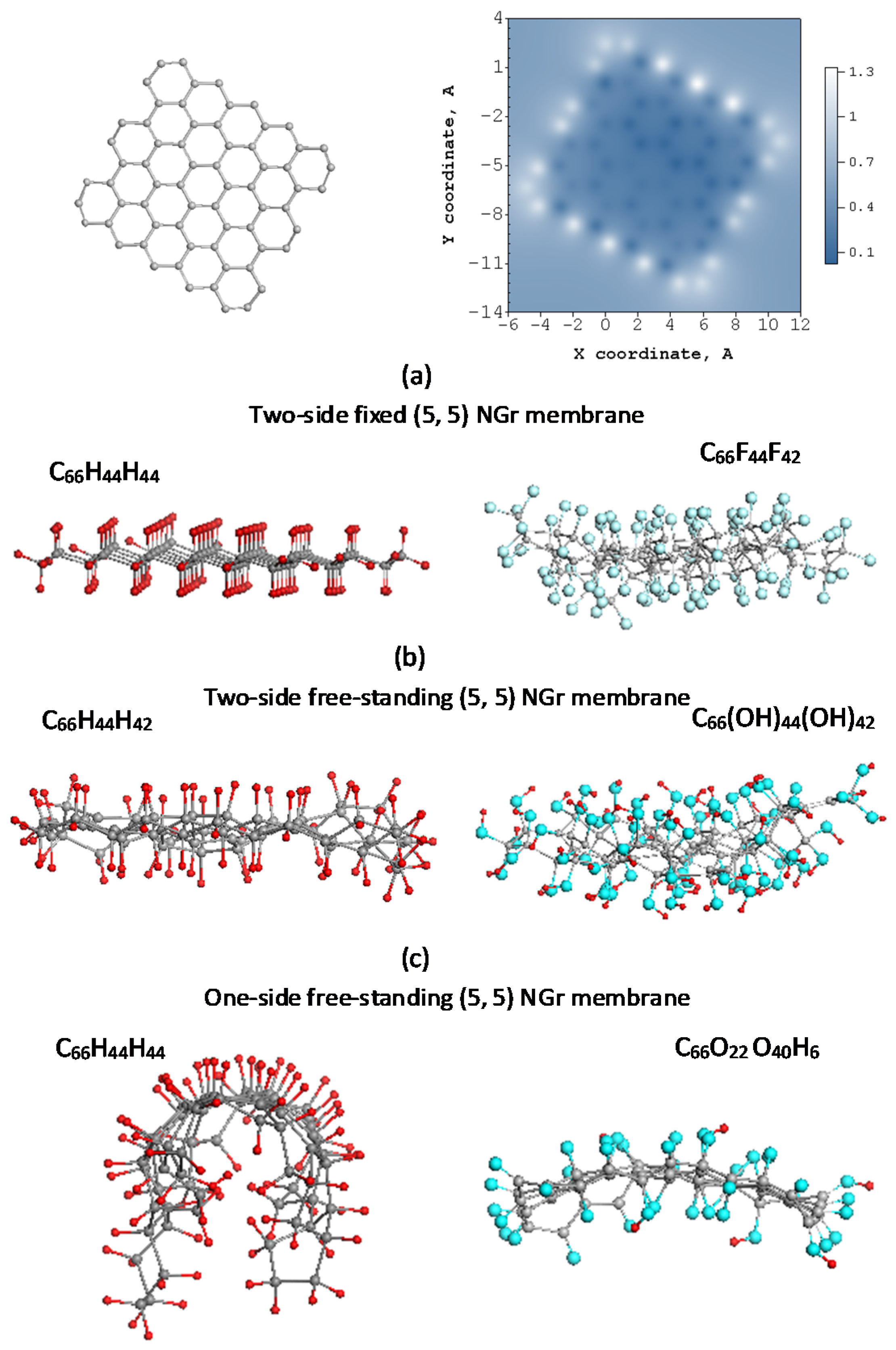
7. Post-Reaction Storage of the Spin Chemistry Products
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | atomic chemical susceptibility |
| BSS | broken spin symmetry |
| BSU | basic structural unit |
| BU | benzenoid unit |
| CI | configuration interaction |
| CNT | carbon nanotube |
| DBs | dangling bonds |
| DFT | density functional theory |
| DMRG | density matrix renormalization group |
| GO | graphene oxide |
| IMI | intermolecular interaction |
| MCS | molecular chemical susceptibility |
| NDA | effectively unpaired electrons fraction at atom A |
| ND | total number of effectively unpaired electrons |
| PAH | polyaromatic hydrocarbons |
| PH | photodynamics |
| RAS-SF | restricted active space spin–flip |
| rGO | reduced graphene oxide |
| RHF | restricted Hartree-Fock |
| SC | spin contamination |
| {sp2C=C bonds} | a complete set o C=C bonds |
| SpDA | spin density fraction at atom A |
| SpDtot | total spin density |
| SpM | spin multiplicity |
| SWCNT | single walled carbon nanotube |
| UDFT | unrestricted density functional theory |
| UHF | unrestricted Hartree-Fock |
References
- Pople, J.A.; Nesbet, R.K. Self-Consistent Orbitals for Radicals. J. Chem. Phys. 1954, 22, 571. [Google Scholar] [CrossRef]
- Löwdin, P.-O. Quantum Theory of Many-Particle Systems. III. Extension of the Hartree-Fock Scheme to Include Degenerate Systems and Correlation Effects. Phys. Rev. 1955, 97, 1509. [Google Scholar] [CrossRef]
- Löwdin, P.-O. Correlation Problem in Many-Electron Quantum Mechanics I. Review of Different Approaches and Discussion of Some Current Ideas. Adv. Chem. Phys. 1958, 2, 209. [Google Scholar] [CrossRef]
- Ray, S.S.; Manna, S.; Ghosh, A.; Chaudhuri, R.K.; Chattopadhyay, S. Multireference Perturbation Theory with Improved Virtual Orbitals for Radicals: More Degeneracies, More Problems. Int. J. Quant. Chem. 2018, 119, e25776. [Google Scholar] [CrossRef]
- Takatsuka, K.; Fueno, T.; Yamaguchi, K. Distribution of Odd Electrons in Ground-State Molecules. Theor. Chim. Acta 1978, 48, 175. [Google Scholar] [CrossRef]
- Staroverov, V.N.; Davidson, E.R. Distribution of Effectively Unpaired Electrons. Chem. Phys. Lett. 2000, 330, 161. [Google Scholar] [CrossRef]
- Anderson, P.W. More Is Different. Science 1972, 177, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laughlin, R.B. Nobel Lecture: Fractional Quantization. Rev. Mod. Phys. 1999, 71, 863. [Google Scholar] [CrossRef] [Green Version]
- Laughlin, R.B.; Pines, D. The Theory of Everything. Proc. Natl. Acad. Sci. USA 2000, 97, 28. [Google Scholar] [CrossRef] [Green Version]
- Yannouleas, C.; Landman, U. Symmetry Breaking and Quantum Correlations in Finite Systems: Studies of Quantum Dots and Ultracold Bose Gases and Related Nuclear and Chemical Methods. Rep. Prog. Phys. 2007, 70, 2067. [Google Scholar] [CrossRef] [Green Version]
- Pavarini, E.; Koch, E.; Schollwöck, U. (Eds.) Emergent Phenomena in Correlated Matter, Autumn School, Jülich, 23–27 September 2013; Forschungszentrum Jülich Zentralbibliothek: Jülich, Germany, 2013. [Google Scholar]
- Putz, M.V.; Ori, O.; Diudea, M.V.; Zefler, B.; Pop, R. Distance, Symmetry, and Topology in Carbon Nanomaterials; (Carbon Materials: Chemistry and Physics); Ashrafi, A.R., Diudea, M.V., Eds.; Springer: Berlin, Germany, 2016; Volume 9, p. 345. [Google Scholar]
- Sheka, E.F.; Popova, N.A.; Popova, V.A. Physics and Chemistry of Graphene. Emergentness, Magnetism, Mechanophysics and Mechanochemistry. Phys. Uspekhi 2018, 61, 645. [Google Scholar] [CrossRef]
- Fucutome, H. Unrestricted Hartree–Fock Theory and Its Applications to Molecules and Chemical Reactions. Int. J. Quant. Chem. 1981, 20, 955. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Saito, T.; Nakanishi, Y.; Kataoka, Y.; Matsui, T.; Kawakami, T.; Okumura, M.; Yamaguchi, K.J. Spin Contamination Error in Optimized Geometry of Singlet Carbene (1A1) by Broken-Symmetry Method. Phys. Chem. A 2009, 113, 15041. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Bulik, I.W.; Jiménez-Hoyos, C.A.; Henderson, T.M.; Scuseria, G.E. Proper and Improper Zero Energy Modes in Hartree-Fock Theory and Their Relevance for Symmetry Breaking and Restoration. J. Chem. Phys. 2013, 139, 154107. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Davidson, E.R.; Yang, W. Nature of Ground and Electronic Excited States of Higher Acenes. Proc. Natl. Acad. Sci. USA 2016, 113, E5098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachmann, J.; Dorando, J.J.; Aviles, M.; Chan, G.K.-L. The Radical Character of the Acenes: A Density Matrix Renormalization Group Study. J. Chem. Phys. 2007, 127, 134309. [Google Scholar] [CrossRef] [Green Version]
- Casanova, D.; Head-Gordon, M. Restricted Active Space Spin-Flip Configuration Interaction Approach: Theory, Implementation and Examples. Phys. Chem. Chem. Phys. 2009, 11, 9779. [Google Scholar] [CrossRef]
- Jacob, C.R.; Reiher, M. Spin in Density-Functional Theory. Int. J. Quantum Chem. 2012, 112, 3661. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, I.G. Problems in DFT with the Total Spin and Degenerate States. Int. J. Quant. Chem. 2007, 107, 2595. [Google Scholar] [CrossRef]
- Kaplan, I.G. Symmetry Properties of the Electron Density and Following From It Limits on the KS-DFT Applications. Mol. Phys. 2018, 116, 658. [Google Scholar] [CrossRef]
- Shee, J.; Arthur, E.J.; Zhang, S.; Reichman, D.R.; Friesner, R.A.J. Singlet–Triplet Energy Gaps of Organic Biradicals and Polyacenes with Auxiliary-Field Quantum Monte Carlo. Chem. Theory Comput. 2019, 15, 4924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalakrishna, T.Y.; Zeng, W.; Lu, X.; Wu, J. From Open-Shell Singlet Diradicaloids to Polyradicaloids. Chem. Comm. 2018, 54, 2186. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Bonaccorso, F.; Fal’Ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.; Palermo, V.; Pugno, N.; et al. Science and Technology Roadmap for Graphene, Related Two-Dimensional Crystals, and Hybrid Systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Grapheme. Nature 2012, 490, 192. [Google Scholar] [CrossRef]
- Kauling, A.P.; Seefeldt, A.T.; Pisoni, D.P.; Pradeep, R.C.; Bentini, R.; Oliveira, R.V.B.; Novoselov, K.S.; Castro Neto, A.H. The Worldwide Graphene Flake Production. Adv. Mater. 2018, 30, 1803784. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Salinas, M.E.; Carreras, A.; Casanova, D. Triangular Graphene Nanofragments: Open-Shell Character And Doping. Phys. Chem. Chem. Phys. 2019, 21, 9069. [Google Scholar] [CrossRef] [PubMed]
- Sheka, E.F.; Chernozatonskii, L.A. Chemical Reactivity and Magnetism of Graphene. Int. J. Quant. Chem. 2010, 110, 1938. [Google Scholar] [CrossRef] [Green Version]
- Sheka, E.F. Nanocarbons through Computations: Fullerenes, Nanotubes, and Graphene. In Nanoscience and Nanotechnologies; Kharkin, V., Bai, C., Awadelkarim, O.O., Kapitaza, S., Eds.; Eolss Publishers: Abu Dhabi, UAE, 2011. [Google Scholar]
- Sheka, E.F. Computational Strategy for Graphene: Insight from Odd Electrons Correlation. Int. J. Quant. Chem. 2012, 112, 3076. [Google Scholar] [CrossRef] [Green Version]
- Sheka, E.F. Molecular Theory of Graphene. In Advances in Quantum Methods and Applications in Chemistry, Physics, and Biology; Hotokka, M., Brändas, E., Maruani, J., Eds.; Spinger: Berlin, Germany, 2013; p. 249. [Google Scholar]
- Sheka, E.F. The Uniqueness of Physical and Chemical Natures of Graphene: Their Coherence and Conflicts. Int. J. Quantum Chem. 2014, 114, 1079. [Google Scholar] [CrossRef] [Green Version]
- Sheka, E.F. Spin Effects of sp2 Nanocarbons in Light of Unrestricted Hartree-Fock Approach and Spin-Orbit Coupling Theory. In Quantum Systems in Physics, Chemistry, and Biology: Advances in Concepts and Applications; Tadjer, A., Pavlov, R., Maruani, J., Brändas, E., Delgado-Barrio, G., Eds.; Springer: Berlin, Germany, 2017; p. 39. [Google Scholar]
- Budyka, M.F.; Sheka, E.F.; Popova, N.A. Graphene Quantum Dots: Theory and Experiment. Rev. Adv. Mat. Sci. 2017, 51, 35. [Google Scholar]
- Sheka, E.F. Dirac Material Graphene. Rev. Adv. Mat. Sci. 2018, 53, 1. [Google Scholar] [CrossRef] [Green Version]
- Sheka, E.F. Spin Chemical Physics of Graphene; Pan Stanford: Singapore, 2018. [Google Scholar]
- Hoffmann, R. Small but Strong Lessons from Chemistry for Nanoscience. Ang. Chem. Int. Ed. 2013, 52, 93. [Google Scholar] [CrossRef] [PubMed]
- Sheka, E.F.; Razbirin, B.S.; Nelson, D.K. Continuous Symmetry of C60 Fullerene and Its Derivatives. J. Phys. Chem. A 2011, 115, 3480. [Google Scholar] [CrossRef] [Green Version]
- Sheka, E.F.; Popova, V.A. Virtual Spectrometer for Radicals Vibrations. 1. Polyacenes and Fullerenes. arXiv 2020, arXiv:2008.08645. [Google Scholar]
- Sheka, E.F. Stretching and Breaking of Chemical Bonds, Correlation of Electrons, and Radical Properties of Covalent Species. Adv. Quant. Chem. 2015, 70, 111. [Google Scholar]
- Zayets, V.A. CLUSTER-Z1: Quantum-Chemical Software for Calculations in the s,p-Basis; Institute of Surface Chemistry, National Academy of Sciences of Ukraine: Kiev, Ukraine, 1990. (In Russian) [Google Scholar]
- Zabrodsky, H.; Peleg, S.; Avnir, D.J. Continuous Symmetry Measures. Am. Chem. Soc. 1992, 114, 7843. [Google Scholar] [CrossRef]
- Sheka, E.F. Fullerenes: Nanochemistry, Nanomagnetism, Nanomedicine, Nanophotonics; T&F CRC Press: London, UK, 2011. [Google Scholar]
- Sheka, E.F.; Chernozatonsky, L.A. Broken Symmetry Approach and Chemical Susceptibility of Carbon Nanotubes. Int. J. Quant. Chem. 2010, 110, 1466. [Google Scholar] [CrossRef] [Green Version]
- Sheka, E.F.; Orlenko, E.V. Spin-Orbit Coupling of sp2 Nanocarbons and Magnetism of Fullerene C60 in View of Spin Peculiarities of Unrestricted Hartree-Fock Solution. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 289. [Google Scholar] [CrossRef]
- Sheka, E.F. “Chemical portrait” of Fullerene Molecules. J. Str. Chem. 2006, 47, 593. [Google Scholar] [CrossRef] [Green Version]
- Hauke, F.; Hirsch, A. Carbon Nanotubes and Related Structures Synthesis, Characterization, Functionalization, and Applications; Guldi, D.M., Martín, N., Eds.; Wiley-VCH: Weinheim, Germany, 2010; p. 135. [Google Scholar]
- Sheka, E.F. Topochemistry of Spatially Extended sp2 Nanocarbons. Fullerenes, Nanotubes, and Graphene. In Topological Modelling of Nanostructures and Extended Systems. Carbon Materials: Chemistry and Physics; Ashrafi, A.R., Cataldo, F., Iranmanesh, A., Ori, O., Eds.; Springer: Berlin, Germany, 2013; Volume 7, p. 137. [Google Scholar]
- Sheka, E.F. Intermolecular Interaction in C60-Based Electron Donor–Acceptor Complexes. Int. J. Quant. Chem. 2004, 100, 388. [Google Scholar] [CrossRef]
- Kasermann, F.; Kempf, C. Buckminsterfullerene and Photodynamic Inactivation of Viruses. Rev. Med. Virol. 1998, 8, 143. [Google Scholar] [CrossRef]
- Piotrovski, L.B. Carbon Nanotechnology; Dai, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; p. 235. [Google Scholar]
- Da Ros, T. Twenty Years of Promises: Fullerene in Medicinal Chemistry. In Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes; Cataldo, F., Da Ros, T., Eds.; Springer: Berlin, Germany, 2008; p. 1. [Google Scholar]
- Mroz, P.; Tegos, G.P.; Gali, H.; Wharton, T.; Sarna, T.; Hamblin, M.R. Fullerenes as Photosensitizers in Photodynamic Therapy. In Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes; Cataldo, F., Da Ros, T., Eds.; Springer: Berlin, Germany, 2008; p. 79. [Google Scholar]
- Sheka, E.F. Nanophotonics of Fullerene 1. Chemistry and Medicine. Nanosci. Nanothech. Let. 2011, 3, 28. [Google Scholar] [CrossRef]
- Sheka, E.F. Donor-Acceptor Interaction and Fullerene C60 Dimerization. Chem. Phys. Lett. 2007, 438, 119. [Google Scholar] [CrossRef]
- Sheka, E.F.; Razbirin, B.S.; Starukhin, A.N.; Nelson, D.K.; Degunov, M.Y.; Troshin, P.A.; Lyubovskaya, R.N. Fullerene-Cluster Amplifiers and Nanophotonics of Fullerene Solutions. J. Nanophot. 2009, 3, 033501. [Google Scholar] [CrossRef]
- Nath, S.; Pal, H.; Palit, D.K.; Sapre, A.V.; Mitta, J.P. Aggregation of Fullerene, C60, in Benzonitrile. J. Phys. Chem. B 1998, 102, 10158. [Google Scholar] [CrossRef]
- Samal, S.; Geckeler, K.E. Unexpected Solute Aggregation in Water on Dilution. J. Chem. Soc. Chem. Commun. 2001, 2224. [Google Scholar] [CrossRef] [PubMed]
- Razbirin, B.S.; Rozhkova, N.N.; Sheka, E.F.; Nelson, D.K.; Starukhin, A.N. Fractals of Graphene Quantum Dots in Photoluminescence of Shungite. J. Exp. Theor. Phys. 2014, 118, 735. [Google Scholar] [CrossRef] [Green Version]
- van der Lit, J.; Boneschanscher, M.P.; Vanmaekelbergh, D.; Ijäs, M.; Uppstu, A.; Ervasti, M.; Harju, A.; Liljeroth, P.; Swart, I. Suppression of Electron–Vibron Coupling in Graphene Nanoribbons Contacted via a Single Atom. Nat. Commun. 2013, 4, 2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, J.H.; Lin, Y.C.; He, K.; Koshino, M.; Suenaga, K. Atomic Level Spatial Variations of Energy States along Graphene Edges. Nano Lett. 2014, 14, 6155. [Google Scholar] [CrossRef]
- Larsson, K.J.A.; Elliott, S.D.; Greer, J.C.; Repp, J.; Meyer, G.; Allenspach, R. Orientation of Individual C60 Molecules Adsorbed on Cu(111): Low-Temperature Scanning Tunneling Microscopy and Density Functional Calculations. Phys. Rev. B 2008, 77, 115434. [Google Scholar] [CrossRef] [Green Version]
- Gross, L.; Mohn, F.; Moll, N.; Schuler, B.; Criado, A.; Guiti, E.; Peña, D.; Gourdon, A.; Meyer, G. Bond-Order Discrimination by Atomic Force Microscopy. Science 2012, 337, 1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, L.; Schuler, B.; Pavliček, N.; Fatayer, S.; Majzik, Z.; Moll, N.; Peña, D.; Meyer, G. Atomic Force Microscopy for Molecular Structure Elucidation. Angew. Chem. Int. Ed. 2018, 57, 3888. [Google Scholar] [CrossRef] [PubMed]
- Troshin, P.A.; Troshina, O.A.; Lyubovskaya, R.N.; Razumov, V.F. Functional Derivatives of Fullerenes. In Synthesis and Applications to Organic Electronics and Biomedicine; Ivanovo State University: Ivanovo, Russia, 2009. (In Russian) [Google Scholar]
- Sheka, E.F. Computational Synthesis of Hydrogenated Fullerenes from C60 to C60H60. J. Mol. Mod. 2011, 17, 1973. [Google Scholar] [CrossRef] [PubMed]
- Sheka, E.F. Step-Wise Computational Synthesis of Fullerene C60 derivatives. Fluorinated Fullerenes C60F2k. J. Exp. Theor. Phys. 2010, 111, 395. [Google Scholar]
- Sheka, E.F.; Popova, N.A. Odd-Electron Molecular Theory of Graphene Hydrogenation. J. Mol. Mod. 2012, 18, 3751. [Google Scholar] [CrossRef]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.G.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of Graphene’s Properties by Reversible Hydrogenation: Evidence for Graphane. Science 2009, 323, 610. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Ren, W.; Jalil, R.; Riaz, I.; Kravets, V.G.; Britnell, L.; Blake, P.; Schedin, F.; Mayorov, A.S.; Yuan, S.; et al. Fluorographene: A Two-Dimensional Counterpart of Teflon. Small 2010, 6, 2877. [Google Scholar] [CrossRef]
- Sheka, E.F.; Popova, N.A. Molecular Theory of Graphene Oxide. Phys. Chem. Chem. Phys. 2013, 15, 13304. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Liu, L.; Li, F. Graphene Oxide: Physics and Applications; Springer: Berlin, Germany, 2015. [Google Scholar]
- Eng, A.Y.C.; Chua, C.K.; Pumera, M. Refinements to the Structure of Graphite Oxide: Absolute Quantification of Functional Groups via Selective Labelling. Nanoscale 2015, 7, 20256. [Google Scholar] [CrossRef]
- Natkaniec, I.; Sheka, E.F.; Drużbicki, K.; Hołderna-Natkaniec, K.; Gubin, S.P.; Buslaeva, E.Y.; Tkachev, S.V. Computationally Supported Neutron Scattering Study of Parent and Chemically Reduced Graphene Oxide. J. Phys. Chem. C 2015, 119, 18650. [Google Scholar] [CrossRef]
- Sheka, E.F.; Golubev, E.A. Technical Graphene (Reduced Graphene Oxide) and Its Natural Analog (Shungite). Tech. Phys. 2016, 61, 1032. [Google Scholar] [CrossRef]
- Sheka, E.F.; Rozhkova, N.N. Shungite as the Natural Pantry of Nanoscale Reduced Graphene Oxide. Int. J. Smart. Nanomat. 2014, 5, 1. [Google Scholar] [CrossRef]
- Golubev, Y.A.; Isaenko, S.I.; Prikhodko, A.S.; Borgardt, N.I.; Suvorova, E.I. Raman Spectroscopic Study of Natural Nanostructured Carbon Materials: Shungite vs. Anthraxolite. Eur. J. Mineral. 2016, 28, 545. [Google Scholar] [CrossRef]
- Golubev, Y.A.; Rozhkova, N.N.; Kabachkov, E.N.; Shul’ga, Y.M.; Natkaniec-Hołderna, K.; Natkaniec, I.; Antonets, I.V.; Makeev, B.A.; Popova, N.A.; Popova, V.A.; et al. sp2 Amorphous Carbons in View of Multianalytical Consideration: Normal, Expected and New. J. Non-Cryst. Sol. 2019, 524, 119608. [Google Scholar] [CrossRef] [Green Version]
- Sheka, E.F.; Hołderna-Natkaniec, K.; Natkaniec, I.; Krawczyk, J.X.; Golubev, Y.A.; Rozhkova, N.N.; Kim, V.V.; Popova, N.A.; Popova, V.A. Computationally Supported Neutron Scattering Study of Natural and Synthetic Amorphous Carbons. J. Phys. Chem. C 2019, 123, 15841. [Google Scholar] [CrossRef]
- Sheka, E.F.; Natkaniec, I.; Ipatova, E.U.; Golubev, Y.A.; Kabachkov, E.N.; Popova, V.A. Heteroatom Necklaces of sp2 Amorphous Carbons. XPS Supported INS and DRIFT Spectroscopy. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 1010. [Google Scholar] [CrossRef]
- Sheka, E.F.; Golubev, Y.A.; Popova, N.A. Graphene Domain Signature of Raman Spectra of sp2 Amorphous Carbons. Nanomaterials 2020, 10, 2021. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Sung, Y.M.; Bao, N.; Tan, D.; Lee, R.; Zafra, J.L.; Lee, B.S.; Ishida, M.; Ding, J.; López Navarrete, J.T.; et al. Stable Tetrabenzo-Chichibabin’s Hydrocarbons: Tunable Ground State and Unusual Transition between Their Closed-Shell and Open-Shell Resonance Forms. Amer. Chem. Soc. 2012, 134, 14513. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Chi, C. Recent Highlights and Perspectives on Acene Based Molecules and Materials. Chem. Mater. 2014, 26, 4046–4056. [Google Scholar] [CrossRef]
- Trinquier, G.; David, G.; Malrieu, J.P. Qualitative Views on the Polyradical Character of Long Acenes. J. Phys. Chem. A 2018, 122, 6926. [Google Scholar] [CrossRef]
- Zhang, K.; Monteiro, M.; Jia, Z. Stable Organic Radical Polymers: Synthesis and Applications. Polym. Chem. 2016, 7, 5589. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Wu, Y.; Li, Y. A Review on the Origin of Synthetic Metal Radical: Singlet Open-Shell Radical Ground State? J. Phys. Chem. C 2017, 121, 8579. [Google Scholar] [CrossRef]
- Fisher, H. The Persistent Radical Effect: A Principle for Selective Radical Reactions and Living Radical Polymerizations. Chem. Rev. 2001, 101, 3581. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.G. What’s New in Stable Radical Chemistry? Org. Biomol. Chem. 2007, 5, 1321. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.G. (Ed.) Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Müllegger, S.; Rashidi, M.; Fattinger, M.; Koch, R.J. Interactions and Self-Assembly of Stable Hydrocarbon Radicals on a Metal Support. Phys. Chem. C 2012, 116, 22587. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhu, L.; Bai, R.; Lan, Y. Stabilization of Two Radicals with One Metal: A Stepwise Coupling Model for Copper-Catalyzed Radical-Radical Cross-Coupling. Sci. Rep. 2017, 7, 43579. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.X.; Li, Y.; Huang, F. Persistent and Stable Organic Radicals: Design, Synthesis, and Applications. Chem 2021, 7, 288. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, J. Open-Shell Graphene Fragments. Chem 2021, 7, 358. [Google Scholar] [CrossRef]
- Radovich, L.R. Chemistry and Physics of Carbon; Radovich, L.R., Ed.; Marcel Dekker: New York, NY, USA, 2001; Volume 27, p. 131. [Google Scholar]
- Serp, P.; Figueiredo, J.L. (Eds.) Carbon Materials for Catalysis; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Serp, P.; Machado, B. (Eds.) Nanostructured Carbon Materials for Catalysis; Royal Society of Chemistry: London, UK, 2015. [Google Scholar]
- Radovich, L.R. Physicochemical Properties of Carbon Materials: A Brief Overview. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; Wiley: Hoboken, NJ, USA, 2009; p. 1. [Google Scholar]
- Bansal, R.C.; Donet, J.-B. Carbon Black. Science and Technology; Donet, J.-B., Bansal, R.C., Eds.; Marcel Dekker: New York, NY, USA, 1993; p. 175. [Google Scholar]
- Villa, A.; Dimitratos, N. (Eds.) Metal-Free Functionalized Carbons in Catalysis: Synthesis, Characterization and Applications; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Bandosz, T.J. Surface Chemistry of Carbon Materials. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; Wiley: Hoboken, NJ, USA, 2009; p. 45. [Google Scholar]
- Bel’skaya, R.I. Shungites—New Carbon Raw Materials; Karelian Science Center: Apatity, Russia, 1984. (In Russian) [Google Scholar]
- Grigoyeva, E.N.; Rozhkova, N.N. Behavior of Shungite Carbon in Reactions Simulating Thermal Transformations of Coal. Russ. J. Appl. Chem. 2000, 73, 600. (In Russian) [Google Scholar]
- Rozhkova, N.N. Perspectives of Fullerene Nanotechnology; Osawa, E., Ed.; Springer: Berlin, Germany, 2002; p. 237. [Google Scholar]
- Rozhkova, N.N.; Gorlenko, L.E.; Yemel’yanova, G.I.; Jankowska, A.; Korobov, M.V.; Lunin, V.V.; Osawa, E. Effect of Ozone on the Structure and Physicochemical Properties of Ultradisperse Diamond and Shungite Nanocarbon Elements. Pure Appl. Chem. 2009, 81, 2093. [Google Scholar] [CrossRef]
- Hu, F.; Patel, M.; Luo, F.; Flach, C.; Mendelsohn, R.; Garfunkel, E.; He, H.; Szostak, M.J. Graphene-Catalyzed Direct Friedel-Crafts Alkylation Reactions: Mechanism, Selectivity, and Synthetic Utility. Am. Chem. Soc. 2015, 137, 14473. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Dai, L. Carbon-Based Metal-Free Catalysts for Electrocatalysis beyond the ORR. Ang. Chem. Int. Ed. 2016, 55, 11736. [Google Scholar] [CrossRef] [PubMed]
- Sheka, E.F. Graphene Oxyhydride Catalysts in View of Spin Radical Chemistry. Materials 2020, 13, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapta, P.; Bartl, A.; Gromov, A.; Stasoeko, A.; Dunsch, L. Efficient and Accurate Approximations to the Molecular Spin-Orbit Coupling Operator and Their Use in Molecular G-Tensor Calculations. Chem. Phys. Chem. 2002, 3, 351. [Google Scholar] [CrossRef]
- Kosaka, M.; Ebbesen, T.W.; Hiura, H.; Tanigaki, K. Annealing Effect on Carbon Nanotubes. An ESR Study. Chem. Phys. Lett. 1995, 233, 47. [Google Scholar] [CrossRef]
- Trenikhin, M.V.; Ivashchenko, O.V.; Eliseev, V.S.; Tolochko, B.P.; Arbuzova, A.B.; Muromtsev, I.V.; Kryazhev, Y.; Drozdov, G.; Sazhina, E.; Likholobov, V.A. Electron Microscopy Investigation of Structural Transformation of Carbon Black under Influence of High-Energy Electron Beam. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 801. [Google Scholar] [CrossRef]
- Krasnovyd, S.V.; Konchits, A.A.; Shanina, B.D.; Valakh, M.Y.; Yanchuk, I.B.; Yukhymchuk, V.O.; Yefanov, A.V.; Skoryk, M.A. Local Structure and Paramagnetic Properties of the Nanostructured Carbonaceous Material Shungite. Nanoscale Res. Lett. 2015, 10, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silaev, V.I.; Ilchenko, V.O.; Lyutoev, V.P.; Filippov, V.N.; Golubev, Y.A.; Kovaleva, O.V. Problemy geologii i mineralogii (Problems in Geology and Mineralogy); Yushkin, N.P., Ed.; Geoprint: Syktyvkar, Russia, 2006; p. 283. (In Russian) [Google Scholar]
- Razumovskii, S.D.; Gorshenev, V.N.; Kovarskii, A.L.; Kuznetsov, A.M.; Shchegolikhin, A.N. Carbon Nanostructure Reactivity: Reactions of Graphite Powders with Ozone. Fuller. Nanotub. Carbon Nanostruct. 2007, 15, 53. [Google Scholar] [CrossRef]
- Cataldo, F.; Putz, M.V.; Ursini, O.; Angelini, G.; Garcia-Hernandez, D.A.; Manchado, A. A New Route to Graphene Starting From Heavily Ozonized Fullerenes: Part 3—An Electron Spin Resonance Study. Fuller. Nanotub. Carbon Nanostruct. 2016, 24, 195. [Google Scholar] [CrossRef]
- Johansson, K.O.; Head-Gordon, M.P.; Schrader, P.E.; Wilson, K.R.; Michelsen, H.A. Resonance-Stabilized Hydrocarbon-Radical Chain Reactions May Explain Soot Inception And Growth. Science 2018, 361, 997. [Google Scholar] [CrossRef] [Green Version]
- Gronert, S.; Keeffe, J.R.; More O’Ferrall, R.A. Contemporary Carbene Chemistry; Moss, R.A., Doyle, M.P., Eds.; Wiley: Hoboken, NJ, USA, 2014; p. 3. [Google Scholar]
- Piccini, G.M.; Mendels, D.; Parrinello, M.J. Metadynamics with Discriminants: A Tool for Understanding Chemistry. Chem. Theory Comput. 2018, 14, 5040. [Google Scholar] [CrossRef] [Green Version]
- Bettinger, H.F. Electronic Structure of Higher Acenes and Polyacene: The Perspective Developed by Theoretical Analyses. Pure Appl. Chem. 2010, 82, 905. [Google Scholar] [CrossRef]
- Plasser, F.; Pašalić, H.; Gerzabek, M.H.; Libisch, F.; Reiter, R.; Burgdörfer, J.; Müller, T.; Shepard, R.; Lischka, H. The Multiradical Character of One- and Two-Dimensional Graphene Nanoribbons. Ang. Chem. Int. Ed. 2013, 52, 2581. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-Y.; Narita, A.; Müllen, K. Precision Synthesis Versus Bulk-Scale Fabrication of Graphenes. Nat. Rev. Chem. 2017, 2, 0100. [Google Scholar] [CrossRef]
- Krutous, V.A. Medicinal Properties of Shungite; Shungite: Karelia, Russia, 2016. (In Russian) [Google Scholar]
- Goryunov, A.S.; Borisova, A.G.; Rozhkov, S.P. Raman Scattering of Bioconjugates of Serum Albumin and Shungite Nanocarbon. Proc. Karelian Res. Center RAS Exp. Biol. Ser. 2012, 2, 154. (In Russian) [Google Scholar]
- Kamanina, N.V.; Serov, S.V.; Shurpo, N.A.; Rozhkova, N.N. Photoinduced Changes in Refractive Index of Nanostructured Shungite-Containing Polyimide Systems. Tech. Phys. Lett. 2011, 37, 949. [Google Scholar] [CrossRef]
- Usol’tseva, N.V.; Smirnova, M.V.; Kazak, A.V.; Smirnova, A.I.; Bumbina, N.V.; Il’in, S.O.; Rozhkova, N.N. Effects of Mechanical Strength, Working Temperature and Wax Lubricant on Tribological Behavior of Polystyrene. J. Frict. Wear 2015, 36, 380. [Google Scholar] [CrossRef]
- Politi, S.; Carcione, R.; Tamburri, E.; Matassa, R.; Lavecchia, T.; Angjellari, M.; Terranova, M.L. Graphene Platelets from Shungite Rock Modulate Electropolymerization and Charge Storage Mechanisms of Soft-Template Synthetized Polypyrrole-Based Nanocomposites. Sci. Rep. 2018, 8, 17045. [Google Scholar] [CrossRef] [PubMed]
- Sadovnichii, R.V.; Mikhaylina, A.A.; Rozhkova, N.N.; Inina, I.S. The morphological and Structural Features of Quartz of Shungite Rocks of Maksovo Deposit. Trans. KarRC RAS 2016, 2, 73. (In Russian) [Google Scholar]





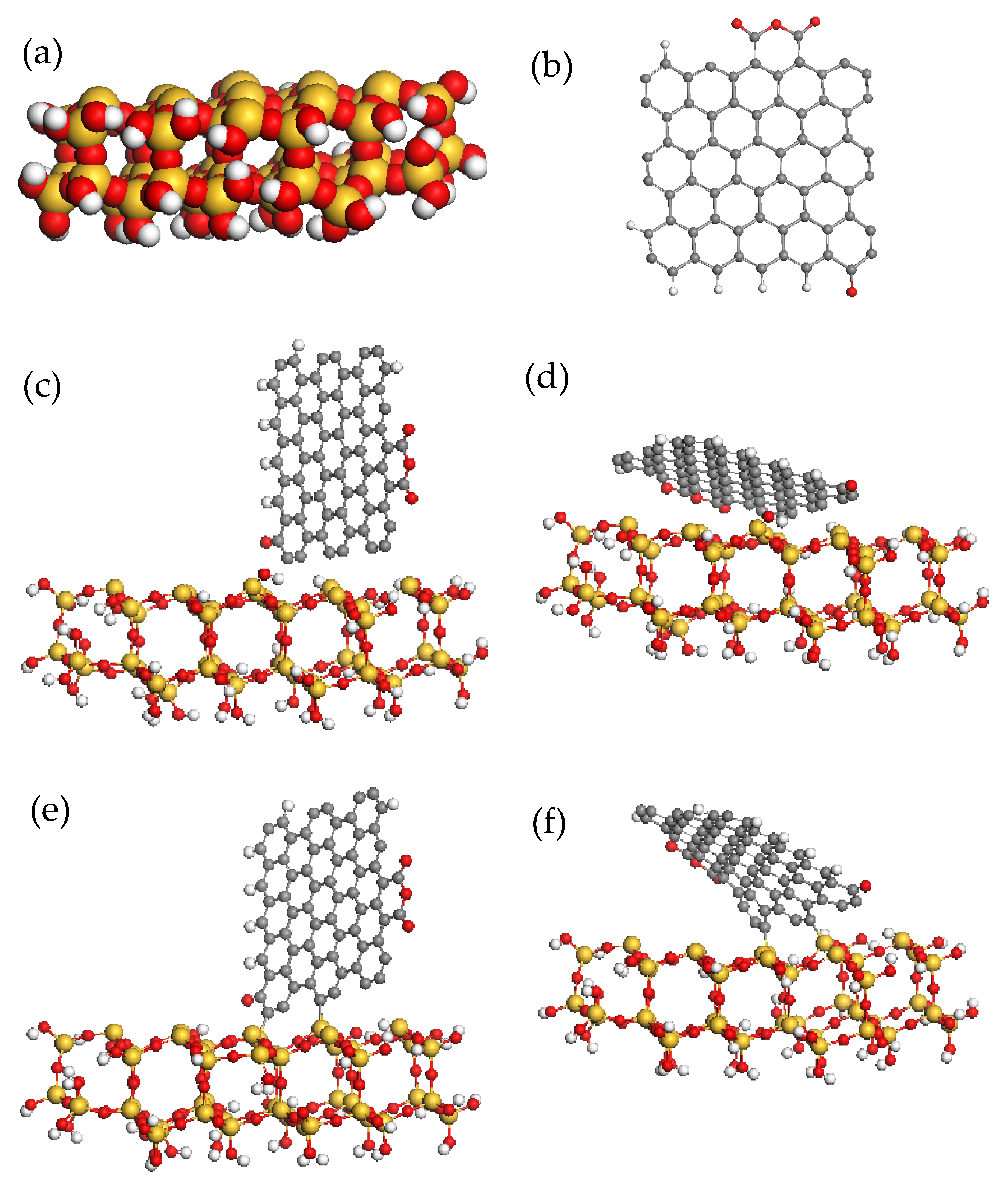
| Molecules | ||||||||
|---|---|---|---|---|---|---|---|---|
| C60 | 970.180 | 955.362 | 14.818 | 982.917 | 27.555 | 4.92 | 0 | 9.84 |
| (4, 4) SWCNT | 1819.509 | 1648.338 | 171.171 | 1627.308 | −21.030 | 19.758 sg | 0 | 39.515 sg |
| 20.325 tr | 2 | 38.651 tr | ||||||
| (5, 5) NGr | 1802.381 | 1454.492 | 347.889 | 1448.396 | −6.096 | 16.930 sg | 0 | 33.860 sg |
| 18.121 tr | 2 | 34.242 tr |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheka, E.F. sp2 Carbon Stable Radicals. C 2021, 7, 31. https://0-doi-org.brum.beds.ac.uk/10.3390/c7020031
Sheka EF. sp2 Carbon Stable Radicals. C. 2021; 7(2):31. https://0-doi-org.brum.beds.ac.uk/10.3390/c7020031
Chicago/Turabian StyleSheka, Elena F. 2021. "sp2 Carbon Stable Radicals" C 7, no. 2: 31. https://0-doi-org.brum.beds.ac.uk/10.3390/c7020031





