Variable Temperature Synthesis of Tunable Flame-Generated Carbon Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; Deangelo, B.J.; Flanner, M.G.; Ghan, S.; Kärcher, B.; Koch, D.; et al. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar]
- Ramanathan, V.; Carmichael, G. Global and regional climate changes due to black carbon. Nat. Geosci. 2008, 1, 221–227. [Google Scholar] [CrossRef]
- Kennedy, I.M. The health effects of combustion-generated aerosols. Proc. Combust. Inst. 2007, 31, 2757–2770. [Google Scholar] [CrossRef]
- Pedata, P.; Stoeger, T.; Zimmermann, R.; Peters, A.; Oberdörster, G.; D’Anna, A. Are we forgetting the smallest, sub 10 nm combustion generated particles? Part. Fibre Toxicol. 2015, 12, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Falco, G.; Terlizzi, M.; Sirignano, M.; Commodo, M.; D’Anna, A.; Aquino, R.P.; Pinto, A.; Sorrentino, R. Human peripheral blood mononuclear cells (PBMCs) from smokers release higher levels of IL-1-like cytokines after exposure to combustion-generated ultrafine particles. Sci. Rep. 2017, 7, 43016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodabakhshi, S.; Fulvio, P.F.; Andreoli, E. Carbon black reborn: Structure and chemistry for renewable energy harnessing. Carbon 2020, 162, 604–649. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ye, T.; Mao, C. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chem. Int. Ed. 2007, 46, 6473–6475. [Google Scholar] [CrossRef]
- Wei, Z.; Yan, K.; Chen, H.; Yi, Y.; Zhang, T.; Long, X.; Li, J.; Zhang, L.; Wang, J.; Yang, S. Cost-efficient clamping solar cells using candle soot for hole extraction from ambipolar perovskites. Energy Environ. Sci. 2014, 7, 3326–3333. [Google Scholar] [CrossRef]
- Bruno, A.; Commodo, M.; Haque, S.A.; Minutolo, P. Spectroscopic investigation of flame synthesized carbon nanoparticle/P3HT blends. Carbon 2015, 94, 955–961. [Google Scholar] [CrossRef]
- Kakunuri, M.; Sharma, C.S. Candle soot derived fractal-like carbon nanoparticles network as high-rate lithium ion battery anode material. Electrochim. Acta 2015, 180, 353–359. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, D.; Yu, B.; Zhou, F.; Liu, W. Candle soot as a supercapacitor electrode material. RSC Adv. 2014, 4, 2586–2589. [Google Scholar] [CrossRef]
- Li, R.; Mao, H.; Zhu, M.; Yang, Y.; Xiong, J.; Wang, W. Facile preparation of broadband absorbers based on patternable candle soot for applications of optical sensors. Sens. Actuators A 2019, 285, 111–117. [Google Scholar] [CrossRef]
- Pankaj, A.; Tewari, K.; Singh, S.; Singh, S.P. Waste candle soot derived nitrogen doped carbon dots based fluorescent sensor probe: An efficient and inexpensive route to determine Hg(II) and Fe(III) from water. J. Environ. Chem. Eng. 2018, 6, 5561–5569. [Google Scholar] [CrossRef]
- Liang, C.-J.; Liao, J.-D.; Li, A.-J.; Chen, C.; Lin, H.-Y.; Wang, X.-J.; Xu, Y.-H. Relationship between wettabilities and chemical compositions of candle soots. Fuel 2014, 128, 422–427. [Google Scholar] [CrossRef]
- Campbell, D.J.; Andrews, M.J.; Stevenson, K.J. New nanotech from an ancient material: Chemistry demonstrations involving carbon-based soot. J. Chem. Educ. 2012, 89, 1280–1287. [Google Scholar] [CrossRef]
- Commodo, M.; De Falco, G.; Larciprete, R.; D’Anna, A.; Minutolo, P. On the hydrophilic/hydrophobic character of carbonaceous nanoparticles formed in laminar premixed flames. Exp. Therm. Fluid Sci. 2016, 73, 56–63. [Google Scholar] [CrossRef]
- Cao, H.; Fu, J.; Liu, Y.; Chen, S. Facile design of superhydrophobic and superoleophilic copper mesh assisted by candle soot for oil water separation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 294–302. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Hussain, S.Z.; Subhani, T.; Hussain, I.; Rehman, H.-U. Mechanically robust superhydrophobic coating from sawdust particles and carbon soot for oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 391–398. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Fedchenko, Y.I.; Yankov, G.P.; Temelkov, K.A. Laser irradiation of super-nonwettable carbon soot coatings–physicochemical implications. Coatings 2021, 11, 58. [Google Scholar] [CrossRef]
- Mulay, M.R.; Chauhan, A.; Patel, S.; Balakrishnan, V.; Halder, A.; Vaish, R. Candle soot: Journey from a pollutant to a functional material. Carbon 2019, 144, 684–712. [Google Scholar] [CrossRef]
- Li, S.; Ren, Y.; Biswas, P.; Tse, S.D. Flame aerosol synthesis of nanostructured materials and functional devices: Processing, modeling, and diagnostics. Prog. Energy Combust. Sci. 2016, 55, 1–59. [Google Scholar] [CrossRef] [Green Version]
- Kumal, R.R.; Gharpure, A.; Viswanathan, V.; Mantri, A.; Skoptsov, G.; Wal, R.V. Microwave plasma formation of nanographene and graphitic carbon black. C. J. Carbon Res. 2020, 6, 70. [Google Scholar] [CrossRef]
- D’Anna, A. Combustion-formed nanoparticles. Proc. Combust. Inst. 2009, 32, 593–613. [Google Scholar] [CrossRef]
- Wang, H. Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 2011, 33, 41–67. [Google Scholar] [CrossRef]
- Michelsen, H.A. Probing soot formation, chemical and physical evolution, and oxidation: A review of in situ diagnostic techniques and needs. Proc. Combust. Inst. 2017, 36, 717–735. [Google Scholar] [CrossRef] [Green Version]
- Karataş, A.E.; Gülder, O.L. Soot formation in high pressure laminar diffusion flames. Prog. Energy Combust. Sci. 2012, 38, 818–845. [Google Scholar] [CrossRef]
- Zhao, B.; Yang, Z.; Wang, J.; Johnston, M.V.; Wang, H. Analysis of soot nanoparticles in a laminar premixed ethylene flame by scanning mobility particle sizer. Aerosol Sci. Technol. 2003, 37, 611–620. [Google Scholar] [CrossRef]
- Maricq, M.M. Size and charge of soot particles in rich premixed ethylene flames. Combust. Flame 2004, 137, 340–350. [Google Scholar] [CrossRef]
- Abid, A.D.; Camacho, J.; Sheen, D.A.; Wang, H. Quantitative measurement of soot particle size distribution in premixed flames—The burner-stabilized stagnation flame approach. Combust. Flame 2009, 156, 1862–1870. [Google Scholar] [CrossRef]
- Commodo, M.; De Falco, G.; Bruno, A.; Borriello, C.; Minutolo, P.; D’Anna, A. Physicochemical evolution of nascent soot particles in a laminar premixed flame: From nucleation to early growth. Combust. Flame 2015, 162, 3854–3863. [Google Scholar] [CrossRef]
- Carbone, F.; Attoui, M.; Gomez, A. Challenges of measuring nascent soot in flames as evidenced by high-resolution differential mobility analysis. Aerosol Sci. Technol. 2016, 50, 740–757. [Google Scholar] [CrossRef] [Green Version]
- Betrancourt, C.; Liu, F.; Desgroux, P.; Mercier, X.; Faccinetto, A.; Salamanca, M.; Ruwe, L.; Kohse-Höinghaus, K.; Emmrich, D.; Beyer, A.; et al. Investigation of the size of the incandescent incipient soot particles in premixed sooting and nucleation flames of n-butane using LII, HIM, and 1 nm-SMPS. Aerosol Sci. Technol. 2017, 51, 916–935. [Google Scholar] [CrossRef] [Green Version]
- De Falco, G.; Commodo, M.; Bonavolontà, C.; Pepe, G.P.; Minutolo, P.; D’Anna, A. Optical and electrical characterization of carbon nanoparticles produced in laminar premixed flames. Combust. Flame 2014, 161, 3201–3210. [Google Scholar] [CrossRef]
- De Falco, G.; Commodo, M.; Barra, M.; Chiarella, F.; D’Anna, A.; Aloisio, A.; Cassinese, A.; Minutolo, P. Electrical characterization of flame-soot nanoparticle thin films. Synth. Met. 2017, 229, 89–99. [Google Scholar] [CrossRef]
- Commodo, M.; De Falco, G.; Minutolo, P.; D’Anna, A. Structure and size of soot nanoparticles in laminar premixed flames at different equivalence ratios. Fuel 2018, 216, 456–462. [Google Scholar] [CrossRef]
- Liu, Y.; Song, C.; Lv, G.; Fan, C.; Zhang, X.; Qiao, Y. Relationships between the electrical properties and nanostructure of soot particles in a laminar inverse diffusion flame. Proc. Combust. Inst. 2019, 37, 1185–1192. [Google Scholar] [CrossRef]
- De Falco, G.; Mattiello, G.; Commodo, M.; Minutolo, P.; Shi, X.; D’Anna, A.; Wang, H. Electronic band gap of flame-formed carbon nanoparticles by scanning tunneling spectroscopy. Proc. Combust. Inst. 2021, 38, 1805–1812. [Google Scholar] [CrossRef]
- Liu, Y.; Song, C.; Lv, G.; Zhang, W.; Chen, H. Evaluation of the oxidative reactivity and electrical properties of soot particles. Carbon 2021, 178, 37–47. [Google Scholar] [CrossRef]
- Öktem, B.; Tolocka, M.P.; Zhao, B.; Wang, H.; Johnston, M.V. Chemical species associated with the early stage of soot growth in a laminar premixed ethylene–oxygen–argon flame. Combust. Flame 2005, 142, 364–373. [Google Scholar] [CrossRef]
- Cain, J.P.; Camacho, J.; Phares, D.J.; Wang, H.; Laskin, A. Evidence of aliphatics in nascent soot particles in premixed ethylene flames. Proc. Combust. Inst. 2011, 33, 533–540. [Google Scholar] [CrossRef]
- Miller, J.H.; Herdman, J.D.; Green, C.D.O.; Webster, E.M. Experimental and computational determinations of optical band gaps for PAH and soot in a N2-diluted, ethylene/air non-premixed flame. Proc. Combust. Inst. 2013, 34, 3669–3675. [Google Scholar] [CrossRef]
- Schulz, F.; Commodo, M.; Kaiser, K.; De Falco, G.; Minutolo, P.; Meyer, G.; D’Anna, A.; Gross, L. Insights into incipient soot formation by atomic force microscopy. Proc. Combust. Inst. 2019, 37, 885–892. [Google Scholar] [CrossRef]
- Commodo, M.; Kaiser, K.; De Falco, G.; Minutolo, P.; Schulz, F.; D’Anna, A.; Gross, L. On the early stages of soot formation: Molecular structure elucidation by high-resolution atomic force microscopy. Combust. Flame 2019, 205, 154–164. [Google Scholar] [CrossRef]
- Jacobson, R.S.; Korte, A.R.; Vertes, A.; Miller, J.H. The molecular composition of soot. Angew. Chem. Int. Ed. 2020, 59, 4484–4490. [Google Scholar] [CrossRef] [PubMed]
- Dasappa, S.; Camacho, J. Evolution in size and structural order for incipient soot formed at flame temperatures greater than 2100 K. Fuel 2021, 291, 120196. [Google Scholar] [CrossRef]
- Bonpua, J.; Yagües, Y.; Aleshin, A.; Dasappa, S.; Camacho, J. Flame temperature effect on sp2 bonds on nascent carbon nanoparticles formed in premixed flames (Tf, max & gt; 2100 K): A Raman spectroscopy and particle mobility sizing study. Proc. Combust. Inst. 2019, 37, 943–951. [Google Scholar]
- De Falco, G.; Moggia, G.; Sirignano, M.; Commodo, M.; Minutolo, P.; D’Anna, A. Exploring soot particle concentration and emissivity by transient thermocouples measurements in laminar partially premixed coflow flames. Energies 2017, 10, 232. [Google Scholar] [CrossRef]
- Abid, A.D.; Heinz, N.; Tolmachoff, E.D.; Phares, D.J.; Campbell, C.S.; Wang, H. On evolution of particle size distribution functions of incipient soot in premixed ethylene–oxygen–argon flames. Combust. Flame 2008, 154, 775–788. [Google Scholar] [CrossRef]
- Gu, C.; Lin, H.; Camacho, J.; Lin, B.; Shao, C.; Li, R.; Gu, H.; Guan, B.; Huang, Z.; Wang, H. Particle size distribution of nascent soot in lightly and heavily sooting premixed ethylene flames. Combust. Flame 2016, 165, 177–187. [Google Scholar] [CrossRef]
- Ferreira, E.H.M.; Moutinho, M.V.O.; Stavale, F.; Lucchese, M.M.; Capaz, R.B.; Achete, C.A.; Jorio, A. Evolution of the Raman spectra from single-, few-, and many-layer graphene with increasing disorder. Phys. Rev. B Condens. 2010, 82, 125429. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlen, A.; Buijnsters, J.G.; Pardanaud, C. A guide to and review of the use of multiwavelength raman spectroscopy for characterizing defective aromatic carbon solids: From graphene to amorphous carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Lapuerta, M.; Oliva, F.; Agudelo, J.R.; Stitt, J.P. Optimization of Raman spectroscopy parameters for characterizing soot from different diesel fuels. Combust. Sci. Technol. 2011, 183, 1203–1220. [Google Scholar] [CrossRef]
- Herdman, J.D.; Connelly, B.C.; Smooke, M.D.; Long, M.B.; Miller, J.H. A comparison of Raman signatures and laser-induced incandescence with direct numerical simulation of soot growth in non-premixed ethylene/air flames. Carbon 2011, 49, 5298–5311. [Google Scholar] [CrossRef]
- Seong, H.J.; Boehman, A.L. Evaluation of Raman parameters using visible Raman microscopy for soot oxidative reactivity. Energy Fuels 2013, 27, 1613–1624. [Google Scholar] [CrossRef]
- Minutolo, P.; Commodo, M.; Santamaria, A.; De Falco, G.; D’Anna, A. Characterization of flame-generated 2-D carbon nano-disks. Carbon 2014, 68, 138–148. [Google Scholar] [CrossRef]
- Le, K.C.; Lefumeux, C.; Bengtsson, P.-E.; Pino, T. Direct observation of aliphatic structures in soot particles produced in low-pressure premixed ethylene flames via online Raman spectroscopy. Proc. Combust. Inst. 2019, 37, 869–876. [Google Scholar] [CrossRef]
- Ess, M.N.; Ferry, D.; Kireeva, E.D.; Niessner, R.; Ouf, F.-X.; Ivleva, N.P. In situ Raman microspectroscopic analysis of soot samples with different organic carbon content: Structural changes during heating. Carbon 2016, 105, 572–585. [Google Scholar] [CrossRef]
- Casiraghi, C.; Piazza, F.; Ferrari, A.C.; Grambole, D.; Robertson, J. Bonding in hydrogenated diamond-like carbon by Raman spectroscopy. Diam. Relat. Mater. 2005, 14, 1098–1102. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Gago, R.; Jiḿnez, I.; Camero, M.; Agulló-Rueda, F.; Gómez-Aleixandre, C. Hydrogen quantification in hydrogenated amorphous carbon films by infrared, Raman, and x-ray absorption near edge spectroscopies. J. Appl. Phys. 2009, 105, 093510. [Google Scholar] [CrossRef] [Green Version]
- Schuepfer, D.B.; Badaczewski, F.; Guerra-Castro, J.M.; Hofmann, D.M.; Heiliger, C.; Smarsly, B.; Klar, P.J. Assessing the structural properties of graphitic and non-graphitic carbons by Raman spectroscopy. Carbon 2020, 161, 359–372. [Google Scholar] [CrossRef]



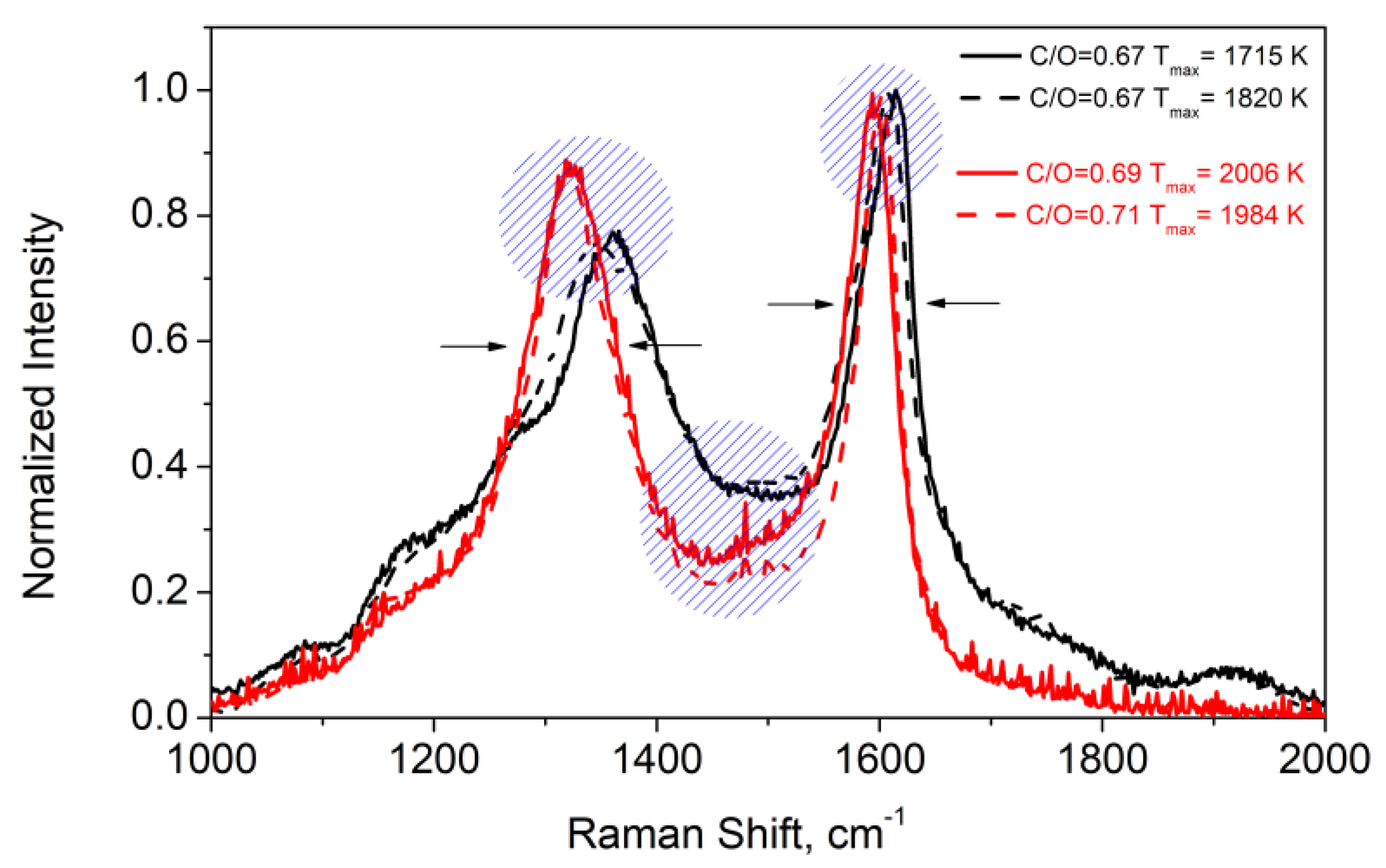
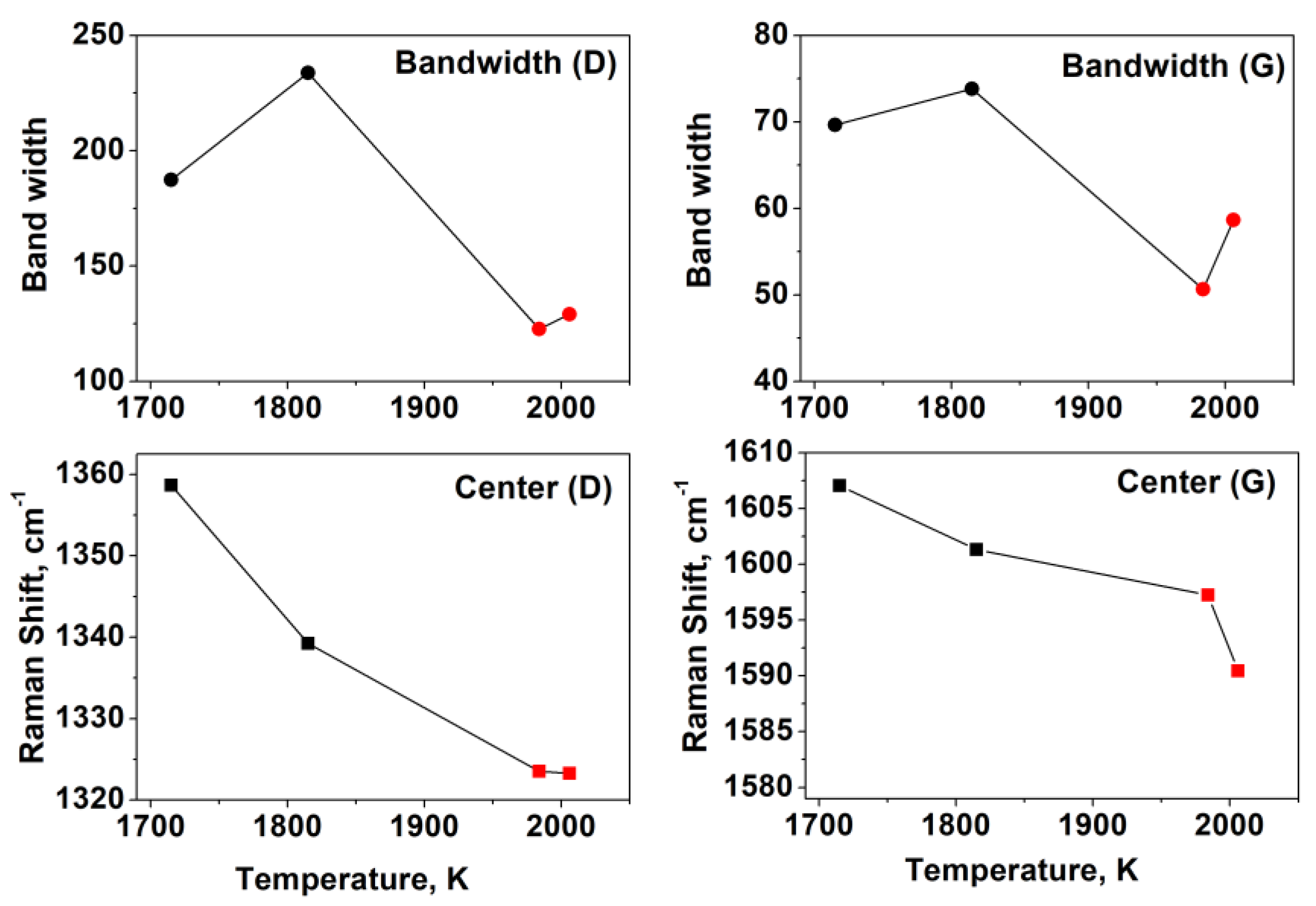
| Flame | Feed Gas | C/O | Gas Velocity, cm/s | Max Temperature, K |
|---|---|---|---|---|
| A | Ethylene/air | 0.67 | 9.8 cm/s | 1715 |
| B | Ethylene/air | 0.67 | 9.8 cm/s | 1820 |
| C | Ethylene/air | 0.69 | 20 cm/s | 2006 |
| D | Ethylene/air | 0.71 | 20 cm/s | 1984 |
| Flame | C/O Tmax HAB | <Dp> | I(D)/I(G) | La (nm) | m/I(G) | H [%] |
|---|---|---|---|---|---|---|
| A | C/O = 0.67 1715 K 8 mm | 3.34 nm | 0.76 | 1.10 | 3.2 | 30.0 |
| B | C/O = 0.67 1820 K 7 mm | 3.80 nm | 0.75 | 1.09 | 6.6 | 35.3 |
| C | C/O = 0.69 2006 K 9 mm | 3.30 nm | 0.88 | 1.18 | 0.3 | 13.5 |
| D | C/O = 0.71 1984 K 8 mm | 4.06 nm | 0.89 | 1.19 | 0.5 | 17.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picca, F.; Pietro, A.D.; Commodo, M.; Minutolo, P.; D’Anna, A. Variable Temperature Synthesis of Tunable Flame-Generated Carbon Nanoparticles. C 2021, 7, 44. https://0-doi-org.brum.beds.ac.uk/10.3390/c7020044
Picca F, Pietro AD, Commodo M, Minutolo P, D’Anna A. Variable Temperature Synthesis of Tunable Flame-Generated Carbon Nanoparticles. C. 2021; 7(2):44. https://0-doi-org.brum.beds.ac.uk/10.3390/c7020044
Chicago/Turabian StylePicca, Francesca, Angela Di Pietro, Mario Commodo, Patrizia Minutolo, and Andrea D’Anna. 2021. "Variable Temperature Synthesis of Tunable Flame-Generated Carbon Nanoparticles" C 7, no. 2: 44. https://0-doi-org.brum.beds.ac.uk/10.3390/c7020044







