Carbons Formed in Methane Thermal and Thermocatalytic Decomposition Processes: Properties and Applications
Abstract
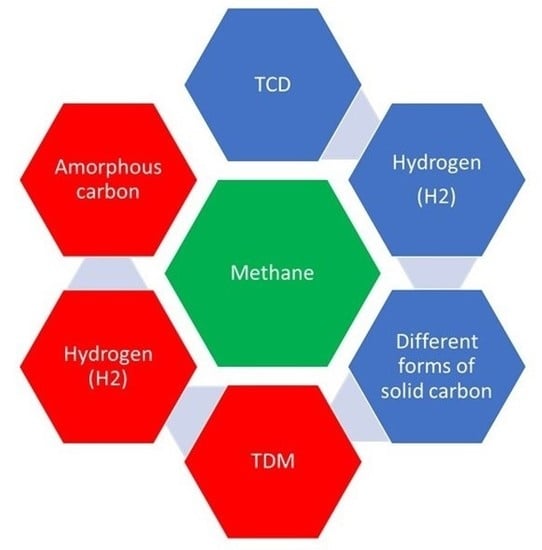
:1. Introduction
2. Production Methods for Hydrogen and Carbon from Methane
3. Carbon Morphologies
4. Process Parameters Affecting the Properties of Carbon in TDM and TCD Processes
4.1. Effect of Catalyst and Promoters on the Carbon Amount and Quality
4.2. Effect of Process Temperature on Carbon Amount and Quality
4.3. Effect of GHSV on Carbon Amount and Quality
4.4. Summary
5. Possible Applications for Produced Solid Carbon
5.1. Batteries
5.2. Supercapacitors
5.3. Water Treatment Application
5.4. Composite Materials
5.5. Cosmetics and Medical Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- European Comission. A Hydrogen Strategy for a Climate-Neutral Europe; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Yang, L.; Liu, F.; He, J. Natural Sand as a Non-Conventional Catalyst for Hydrogen Production by Methane Thermo-Catalytic Decomposition. Int. J. Hydrogen Energy 2019, 44, 11625–11633. [Google Scholar] [CrossRef]
- Gamal, A.; Eid, K.; El-Naas, M.H.; Kumar, D.; Kumar, A. Catalytic Methane Decomposition to Carbon Nanostructures and Cox-Free Hydrogen: A Mini-Review. Nanomaterials 2021, 11, 1226. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Abbas, H.F.; Wan Daud, W.M.A. Hydrogen Production by Methane Decomposition: A Review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Abdullah, B.; Abd Ghani, N.A.; Vo, D.V.N. Recent Advances in Dry Reforming of Methane over Ni-Based Catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef] [Green Version]
- Soltani, R.; Rosen, M.A.; Dincer, I. Assessment of CO2 Capture Options from Various Points in Steam Methane Reforming for Hydrogen Production. Int. J. Hydrogen Energy 2014, 39, 20266–20275. [Google Scholar] [CrossRef]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry Reforming of Methane: Influence of Process Parameters—A Review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef] [Green Version]
- Levalley, T.L.; Richard, A.R.; Fan, M. The Progress in Water Gas Shift and Steam Reforming Hydrogen Production Technologies—A Review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Prajapati, J.K. Hydrogen Production by Partial Oxidation of Methane: Modeling and Simulation. Int. J. Hydrogen Energy 2009, 34, 6655–6668. [Google Scholar] [CrossRef]
- De, C.; Roseno, K.T.; Schmal, M.; Brackmann, R.; Alves, R.M.B.; Giudici, R. Partial Oxidation of Methane on Neodymium and Lanthanium Chromate Based Perovskites for Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 8166–8177. [Google Scholar] [CrossRef]
- Larimi, A.S.; Alavi, S.M. Ceria-Zirconia Supported Ni Catalysts for Partial Oxidation of Methane to Synthesis Gas. Fuel 2012, 102, 366–371. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Hu, Y.H. Methane Partial Oxidation over NiO/MgO Solid Solution Catalysts. Appl. Catal. A Gen. 1999, 183, 85–92. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Al-Fatesh, A.S.; Khan, W.U.; Soliman, M.A.; Al Otaibi, R.L.; Fakeeha, A.H. Thermo-Catalytic Methane Decomposition: A Review of State of the Art of Catalysts. J. Chem. Soc. Pakistan 2015, 37, 1269–1297. [Google Scholar]
- Ashik, U.P.M.; Wan Daud, W.M.A.; Abbas, H.F. Production of Greenhouse Gas Free Hydrogen by Thermocatalytic Decomposition of Methane—A Review. Renew. Sustain. Energy Rev. 2015, 44, 221–256. [Google Scholar] [CrossRef] [Green Version]
- Keipi, T.; Tolvanen, K.E.S.; Tolvanen, H.; Konttinen, J. Thermo-Catalytic Decomposition of Methane: The Effect of Reaction Parameters on Process Design and the Utilization Possibilities of the Produced Carbon. Energy Convers. Manag. 2016, 126, 923–934. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Akmal, Z.S. Direct Decomposition of Methane over SBA-15 Supported Ni, Co and Fe Based Bimetallic Catalysts. Appl. Surf. Sci. 2015, 330, 418–430. [Google Scholar] [CrossRef]
- Liang, W.; Yan, H.; Chen, C.; Lin, D.; Tan, K.; Feng, X.; Liu, Y.; Chen, X.; Yang, C.; Shan, H. Revealing the Effect of Nickel Particle Size on Carbon Formation Type in the Methane Decomposition Reaction. Catalysts 2020, 10, 890. [Google Scholar] [CrossRef]
- Zhou, L.; Enakonda, L.R.; Harb, M.; Saih, Y.; Aguilar-Tapia, A.; Ould-Chikh, S.; Hazemann, J.-l.; Li, J.; Wei, N.; Gary, D.; et al. Fe Catalysts for Methane Decomposition to Produce Hydrogen and Carbon Nano Materials. Appl. Catal. B Environ. 2017, 208, 44–59. [Google Scholar] [CrossRef] [Green Version]
- Callister, W.D. Materials Science and Engineering: An Introduction, 7th ed.; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Noked, M.; Soffer, A.; Arubach, D. The Electrochemistry of Activated Carbonaceous Materials: Past, Present, and Future. J. Solid State Electrochem. 2011, 15, 1563–1578. [Google Scholar] [CrossRef]
- Qian, J.X.; Chen, T.W.; Enakonda, L.R.; Liu, D.B.; Basset, J.M.; Zhou, L. Methane Decomposition to Pure Hydrogen and Carbon Nano Materials: State-of-the-Art and Future Perspectives. Int. J. Hydrogen Energy 2020, 45, 15721–15743. [Google Scholar] [CrossRef]
- Gao, L.Z.; Kiwi-Minsker, L.; Renken, A. Growth of Carbon Nanotubes and Microfibers over Stainless Steel Mesh by Cracking of Methane. Surf. Coatings Technol. 2008, 202, 3029–3042. [Google Scholar] [CrossRef] [Green Version]
- Torres, D.; Pinilla, J.L.; Suelves, I. Cobalt Doping of α-Fe/Al2O3 Catalysts for the Production of Hydrogen and High-Quality Carbon Nanotubes by Thermal Decomposition of Methane. Int. J. Hydrogen Energy 2020, 45, 19313–19323. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, P.; He, J.; Liu, C.; Jiang, W. Catalytic Decomposition of Methane by Two-Step Cascade Catalytic Process: Simultaneous Production of Hydrogen and Carbon Nanotubes. Chem. Eng. Res. Des. 2020, 163, 96–106. [Google Scholar] [CrossRef]
- Soni, S.K.; Thomas, B.; Kar, V.R. A Comprehensive Review on CNTs and CNT-Reinforced Composites: Syntheses, Characteristics and Applications. Mater. Today Commun. 2020, 25, 101546. [Google Scholar] [CrossRef]
- Awadallah, A.E.; El-Desouki, D.S.; Aboul-Gheit, N.A.K.; Ibrahim, A.H.; Aboul-Gheit, A.K. Effect of Crystalline Structure and Pore Geometry of Silica Based Supported Materials on the Catalytic Behavior of Metallic Nickel Particles during Methane Decomposition to COx-Free Hydrogen and Carbon Nanomaterials. Int. J. Hydrogen Energy 2016, 41, 16890–16902. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Lázaro, M.J.; Moliner, R.; Suelves, I. Hydrogen and Multiwall Carbon Nanotubes Production by Catalytic Decomposition of Methane: Thermogravimetric Analysis and Scaling-up of Fe-Mo Catalysts. Int. J. Hydrogen Energy 2014, 39, 3698–3709. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.T. Hydrogen and Carbon Nanotubes from Pyrolysis-Catalysis of Waste Plastics: A Review. Waste Biomass Valorization 2020, 12, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Dagle, R.; Dagle, V.; Bearden, M.; Holladay, J.; Krause, T.; Ahmed, S. R & D Opportunities for Development of Natural Gas Conversion Technologies for Co-Production of Hydrogen and Value-Added Solid Carbon Products; U.S. Department of Energy Fuel Cell Technologies Office: Chicago, IL, USA, 2017.
- Gu, W.; Yushin, G. Review of Nanostructured Carbon Materials for Electrochemical Capacitor Applications: Advantages and Limitations of Activated Carbon, Carbide-Derived Carbon, Zeolite-Templated Carbon, Carbon Aerogels, Carbon Nanotubes, Onion-like Carbon, and Graphene. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 424–473. [Google Scholar] [CrossRef]
- Han, F.D.; Yao, B.; Bai, Y.J. Preparation of Carbon Nano-Onions and Their Application as Anode Materials for Rechargeable Lithium-Ion Batteries. J. Phys. Chem. C 2011, 115, 8923–8927. [Google Scholar] [CrossRef]
- Bai, Z.; Chen, H.; Li, B.; Li, W. Methane Decomposition over Ni Loaded Activated Carbon for Hydrogen Production and the Formation of Filamentous Carbon. Int. J. Hydrogen Energy 2007, 32, 32–37. [Google Scholar] [CrossRef]
- Lobo, L.S.; Carabineiro, S.A.C. Carbon Formation at High Temperatures. Catalysts 2020, 10, 465. [Google Scholar] [CrossRef]
- Guizani, C.; Escudero Sanz, F.J.; Salvador, S. The Nature of the Deposited Carbon at Methane Cracking over a Nickel Loaded Wood-Char. Comptes Rendus Chim. 2016, 19, 423–432. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Suelves, I. Co-, Cu-and Fe-Doped Ni/Al2o3 Catalysts for the Catalytic Decomposition of Methane into Hydrogen and Carbon Nanofibers. Catalysts 2018, 8, 300. [Google Scholar] [CrossRef] [Green Version]
- Upham, D.C.; Agarwal, V.; Khechfe, A.; Snodgrass, Z.R.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic Molten Metals for the Direct Conversion of Methane to Hydrogen and Separable Carbon. Science 2017, 358, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Saraswat, S.K.; Pant, K.K. Ni-Cu-Zn/MCM-22 Catalysts for Simultaneous Production of Hydrogen and Multiwall Carbon Nanotubes via Thermo-Catalytic Decomposition of Methane. Int. J. Hydrogen Energy 2011, 36, 13352–13360. [Google Scholar] [CrossRef]
- Christensen, K.O.; Chen, D.; Lødeng, R.; Holmen, A. Effect of Supports and Ni Crystal Size on Carbon Formation and Sintering during Steam Methane Reforming. Appl. Catal. A Gen. 2006, 314, 9–22. [Google Scholar] [CrossRef]
- Hasnan, N.S.N.; Timmiati, S.N.; Lim, K.L.; Yaakob, Z.; Kamaruddin, N.H.N.; Teh, L.P. Recent Developments in Methane Decomposition over Heterogeneous Catalysts: An Overview. Mater. Renew. Sustain. Energy 2020, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Takenaka, S.; Kobayashi, S.; Ogihara, H.; Otsuka, K. Ni/SiO2 Catalyst Effective for Methane Decomposition into Hydrogen and Carbon Nanofiber. J. Catal. 2003, 217, 79–87. [Google Scholar] [CrossRef]
- Marquardt, T.; Bode, A.; Kabelac, S. Hydrogen Production by Methane Decomposition: Analysis of Thermodynamic Carbon Properties and Process Evaluation. Energy Convers. Manag. 2020, 221. [Google Scholar] [CrossRef]
- Guil-Lopez, R.; Botas, J.A.; Fierro, J.L.G.; Serrano, D.P. Comparison of Metal and Carbon Catalysts for Hydrogen Production by Methane Decomposition. Appl. Catal. A Gen. 2011, 396, 40–51. [Google Scholar] [CrossRef]
- Muradov, N.; Smith, F.; T-Raissi, A. Catalytic Activity of Carbons for Methane Decomposition Reaction. Catal. Today 2005, 102–103, 225–233. [Google Scholar] [CrossRef]
- Ahmed, S.; Aitani, A.; Rahman, F.; Al-Dawood, A.; Al-Muhaish, F. Decomposition of Hydrocarbons to Hydrogen and Carbon. Appl. Catal. A Gen. 2009, 359, 1–24. [Google Scholar] [CrossRef]
- Qian, J.X.; Chen, T.W.; Enakonda, L.R.; Liu, D.B.; Mignani, G.; Basset, J.M.; Zhou, L. Methane Decomposition to Produce COx-Free Hydrogen and Nano-Carbon over Metal Catalysts: A Review. Int. J. Hydrogen Energy 2020, 45, 7981–8001. [Google Scholar] [CrossRef]
- Saraswat, S.K.; Pant, K.K. Synthesis of Hydrogen and Carbon Nanotubes over Copper Promoted Ni/SiO2 Catalyst by Thermocatalytic Decomposition of Methane. J. Nat. Gas Sci. Eng. 2013, 13, 52–59. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Mazuki, M.Z.; Takriff, M.S.; Jahaya, S.S. One-Pot Sol-Gel Synthesis of MgO Nanoparticles Supported Nickel and Iron Catalysts for Undiluted Methane Decomposition into COx Free Hydrogen and Nanocarbon. Appl. Catal. B Environ. 2017, 218, 298–316. [Google Scholar] [CrossRef]
- Takenaka, S.; Shigeta, Y.; Tanabe, E.; Otsuka, K. Methane Decomposition into Hydrogen and Carbon Nanofibers over Supported Pd-Ni Catalysts. J. Catal. 2003, 220, 468–477. [Google Scholar] [CrossRef]
- Munera Parra, A.A.; Agar, D.W. Molten Metal Capillary Reactor for the High-Temperature Pyrolysis of Methane. Int. J. Hydrogen Energy 2017, 42, 13641–13648. [Google Scholar] [CrossRef]
- Guo, Z.; Zheng, J.E.; Liu, Y.; Chu, W. Insight into the Role of Metal/Oxide Interaction and Ni Availabilities on NiAl Mixed Metal Oxide Catalysts for Methane Decomposition. Appl. Catal. A Gen. 2018, 555, 1–11. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y. Ni/SiO2 and Fe/SiO2 Catalysts for Production of Hydrogen and Filamentous Carbon via Methane Decomposition. Catal. Today 2002, 77, 225–235. [Google Scholar] [CrossRef]
- Inaba, M.; Zhang, Z.; Matsuoka, K.; Soneda, Y. Optimization of the Reaction Conditions for Fe-Catalyzed Decomposition of Methane and Characterization of the Produced Nanocarbon Fibers. Catal. Today 2019, 332, 11–19. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Dahani, N.; Takriff, M.S.; Hassan, N.S.M. Production of COx Free Hydrogen and Nanocarbon via Methane Decomposition Over Unsupported Porous Nickel and Iron Catalysts. J. Clust. Sci. 2017, 28, 1579–1594. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Kadier, A.; Takriff, M.S.; Hassan, N.S.M. One-Pot Sol–Gel Synthesis of Ni/TiO2 Catalysts for Methane Decomposition into COx Free Hydrogen and Multiwalled Carbon Nanotubes. Int. J. Hydrogen Energy 2017, 42, 16495–16513. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Xie, X.; Liu, J.; Xu, Y.; Shen, W. Novel Ni Catalysts for Methane Decomposition to Hydrogen and Carbon Nanofibers. J. Catal. 2006, 238, 412–424. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Yang, Y.; Jiang, S. Ni-SiO2 and Ni-Fe-SiO2 Catalysts for Methane Decomposition to Prepare Hydrogen and Carbon Filaments. Int. J. Hydrogen Energy 2012, 37, 9058–9066. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Y.; Liu, G.; Li, Y. Production of Hydrogen and Nanocarbon from Catalytic Decomposition of Methane over a Ni-Fe/Al2O3 Catalyst. Energy Fuels 2013, 27, 4448–4456. [Google Scholar] [CrossRef]
- Venugopal, A.; Naveen Kumar, S.; Ashok, J.; Hari Prasad, D.; Durga Kumari, V.; Prasad, K.B.S.; Subrahmanyam, M. Hydrogen Production by Catalytic Decomposition of Methane over Ni/SiO2. Int. J. Hydrogen Energy 2007, 32, 1782–1788. [Google Scholar] [CrossRef]
- Torres, D.; De Llobet, S.; Pinilla, J.L.; Lázaro, M.J.; Suelves, I.; Moliner, R. Hydrogen Production by Catalytic Decomposition of Methane Using a Fe-Based Catalyst in a Fluidized Bed Reactor. J. Nat. Gas Chem. 2012, 21, 367–373. [Google Scholar] [CrossRef]
- Sarada Prasad, J.; Dhand, V.; Himabindu, V.; Anjaneyulu, Y. Production of Hydrogen and Carbon Nanofibers through the Decomposition of Methane over Activated Carbon Supported Ni Catalysts. Int. J. Hydrogen Energy 2011, 36, 11702–11711. [Google Scholar] [CrossRef]
- Suelves, I.; Lázaro, M.J.; Moliner, R.; Pinilla, J.L.; Cubero, H. Hydrogen Production by Methane Decarbonization: Carbonaceous Catalysts. Int. J. Hydrogen Energy 2007, 32, 3320–3326. [Google Scholar] [CrossRef]
- Takenaka, S.; Serizawa, M.; Otsuka, K. Formation of Filamentous Carbons over Supported Fe Catalysts through Methane Decomposition. J. Catal. 2004, 222, 520–531. [Google Scholar] [CrossRef]
- Avdeeva, L.B.; Reshetenko, T.V.; Ismagilov, Z.R.; Likholobov, V.A. Iron-Containing Catalysts of Methane Decomposition: Accumulation of Filamentous Carbon. Appl. Catal. A Gen. 2002, 228, 53–63. [Google Scholar] [CrossRef]
- Fau, G.; Gascoin, N.; Gillard, P.; Steelant, J. Methane Pyrolysis: Literature Survey and Comparisons of Available Data for Use in Numerical Simulations. J. Anal. Appl. Pyrolysis 2013, 104, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rashed, A.O.; Merenda, A.; Kondo, T.; Lima, M.; Razal, J.; Kong, L.; Huynh, C.; Dumée, L.F. Carbon Nanotube Membranes—Strategies and Challenges towards Scalable Manufacturing and Practical Separation Applications. Sep. Purif. Technol. 2021, 257, 117929. [Google Scholar] [CrossRef]
- Ahmad, J.; Naeem, S.; Ahmad, M.; Usman, A.R.A.; Al-Wabel, M.I. A Critical Review on Organic Micropollutants Contamination in Wastewater and Removal through Carbon Nanotubes. J. Environ. Manag. 2019, 246, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Maziarka, P.; Sommersacher, P.; Wang, X.; Kienzl, N.; Retschitzegger, S.; Prins, W.; Hedin, N.; Ronsse, F. Tailoring of the Pore Structures of Wood Pyrolysis Chars for Potential Use in Energy Storage Applications. Appl. Energy 2021, 286, 116431. [Google Scholar] [CrossRef]
- Peng, X.; Li, Y.; Luan, Z.; Di, Z.; Wang, H.; Tian, B.; Jia, Z. Adsorption of 1,2-Dichlorobenzene from Water to Carbon Nanotubes. Chem. Phys. Lett. 2003, 376, 154–158. [Google Scholar] [CrossRef]
- Avcı, A.; İnci, İ.; Baylan, N. Adsorption of Ciprofloxacin Hydrochloride on Multiwall Carbon Nanotube. J. Mol. Struct. 2020, 1206, 127711. [Google Scholar] [CrossRef]
- Peng, J.; He, Y.; Zhou, C.; Su, S.; Lai, B. The Carbon Nanotubes-Based Materials and Their Applications for Organic Pollutant Removal: A Critical Review. Chin. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon Nanotubes: Evaluation of Toxicity at Biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef]
- Keipi, T.; Hankalin, V.; Nummelin, J.; Raiko, R. Techno-Economic Analysis of Four Concepts for Thermal Decomposition of Methane: Reduction of CO2 Emissions in Natural Gas Combustion. Energy Convers. Manag. 2016, 110, 1–12. [Google Scholar] [CrossRef]
- Dhineshbabu, N.R.; Mahadevi, N.; Assein, D. Electronic Applications of Multi-Walled Carbon Nanotubes in Polymers: A Short Review. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Wang, X.; Yang, B.; Liu, J.; Zhu, Y.; Yang, C.; He, Q. A Flexible Triboelectric-Piezoelectric Hybrid Nanogenerator Based on P(VDF-TrFE) Nanofibers and PDMS/MWCNT for Wearable Devices. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Zahir, H.; Wojkiewicz, J.; Alexander, P.; Kone, L.; Belkacem, B.; Bergheul, S.; Lasri, T. Design Fabrication and Characterisation of Polyaniline and Multiwall Carbon Nanotubes Composites-based Patch Antenna. IET Microw. Antennas Propag. 2016, 10, 88–93. [Google Scholar] [CrossRef]
- Turkani, V.S.; Maddipatla, D.; Narakathu, B.B.; Saeed, T.S.; Obare, S.O.; Bazuin, B.J.; Atashbar, M.Z. A Highly Sensitive Printed Humidity Sensor Based on a Functionalized MWCNT/HEC Composite for Flexible Electronics Application. Nanoscale Adv. 2019, 1, 2311–2322. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Dudem, B.; Yu, J.S. High-Performance Flexible Piezoelectric-Assisted Triboelectric Hybrid Nanogenerator via Polydimethylsiloxane-Encapsulated Nanoflower-like ZnO Composite Films for Scavenging Energy from Daily Human Activities. ACS Sustain. Chem. Eng. 2018, 6, 8525–8535. [Google Scholar] [CrossRef]
- Kar, E.; Bose, N.; Dutta, B.; Mukherjee, N.; Mukherjee, S. MWCNT@SiO2 Heterogeneous Nanofiller-Based Polymer Composites: A Single Key to the High-Performance Piezoelectric Nanogenerator and X-Band Microwave Shield. ACS Appl. Nano Mater. 2018, 1, 4005–4018. [Google Scholar] [CrossRef]
- Rincon, A.M. Chapter 6—Presence of nanomaterials on consumer products: Food, cosmetics, and drugs. In Exposure to Engineered Nanomaterials in the Environment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–181. ISBN 9780128148365. [Google Scholar]
- Viana, Í.E.L.; Weiss, G.S.; Sakae, L.O.; Niemeyer, S.H.; Borges, A.B.; Scaramucci, T. Activated Charcoal Toothpastes Do Not Increase Erosive Tooth Wear. J. Dent. 2021, 109, 103677. [Google Scholar] [CrossRef]
- Sanchez, N.; Fayne, R.; Burroway, B. Charcoal: An Ancient Material with a New Face. Clin. Dermatol. 2020, 38, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Nafisi, S.; Maibach, H.I. Nanotechnology in cosmetics. In Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 337–361. ISBN 9780128020548. [Google Scholar]




| Production Method | Chemical Equation | Reaction Enthalpies | Equation Number |
|---|---|---|---|
| Steam methane reforming (SMR) | CH4 + H2O → CO + 3H2 | ΔH298K = 206 kJ/mol | (1) |
| Water gas shift reaction (WGS) | CO + H2O → CO2 + H2 | ΔH298K = −41 kJ/mol | (2) |
| Dry reforming of methane (DRM) | CH4 + CO2 → 2CO + 2H2 | ΔH298K = 247 kJ/mol | (3) |
| Partial oxidation (PO) | CH4 + 0.5O2 → CO + 2H2 | ΔH298K = −23 kJ/mol | (4) |
| Thermal decomposition of methane (TDM) Thermocatalytic decomposition of methane (TCD) | CH4 → C + 2H2 | ΔH298K = 75 kJ/mol | (5) |
| Catalyst | Support | Operating Temperature [K] | GHSV [mL/gcat h] | gC/gCH4 Fed | Carbon Yield % | Source |
|---|---|---|---|---|---|---|
| 50 wt% Ni | SiO2 | 1023 | 1800 | 0.504 | 600% | [47] |
| 50 wt% Ni/5 wt% Cu | SiO2 | 1023 | 1800 | 0.531 | 610% | |
| 50 wt% Ni/10 wt% Cu | SiO2 | 1023 | 1800 | 0.619 | 710% | |
| 50 wt% Ni/15 wt% Cu | SiO2 | 1023 | 1800 | 0.467 | 550% | |
| 50 wt% Ni/20 wt% Cu | SiO2 | 1023 | 1800 | 0.374 | 500% |
| Catalyst | Support | Operating Temperature [K] | GHSV [mL/gcat h] | gC/gCat | Carbon Yield % | Source |
|---|---|---|---|---|---|---|
| Ni0.5Al (Molar ratio) | MMOs | 973 | 60,000 * | 0.11 | [51] | |
| Ni1Al | MMOs | 973 | 60,000 * | 0.49 | ||
| Ni2Al | MMOs | 973 | 60,000 * | 2.02 | ||
| Ni3Al | MMOs | 973 | 60,000 * | 4.55 | ||
| Ni0.5 | Al2O3 | 973 | 60,000 * | 0.67 | ||
| Ni1 | Al2O3 | 973 | 60,000 * | 1.15 | ||
| Ni2 | Al2O3 | 973 | 60,000 * | 1.36 | ||
| Ni3 | Al2O3 | 973 | 60,000 * | 1.29 | ||
| 85wt% Fe | ZrO2 | 973 | 8000 | 13.5 | [52] | |
| 85wt% Fe | Al2O3 | 973 | 8000 | 14 | ||
| 85wt% Fe | TiO2 | 973 | 8000 | 17.4 | ||
| 85wt% Fe | SiO2 | 973 | 8000 | 45 | ||
| 85wt% Fe | – | 973 | 8000 | 16.5 | ||
| 40 wt% Ni | Z-25 | 973 | 6000 * | 176% | [27] | |
| 40 wt% Ni | Z-400 | 973 | 6000 * | 372% | ||
| 40 wt% Ni | Amorphous silica | 973 | 6000 * | 9% |
| Catalyst | Support | Operating Temperature [K] | GHSV [mL/gcat h] | Carbon Yield % | Source |
|---|---|---|---|---|---|
| Ni | MgO | 1173 | 9000 * | 927% | [48] |
| Ni | MgO | 1073 | 9000 * | 863% | |
| Ni | MgO | 973 | 9000 * | 608% | |
| Fe | MgO | 1173 | 9000 * | 1205% | |
| Fe | MgO | 1073 | 9000 * | 1055% | |
| Fe | MgO | 973 | 9000 * | 810% | |
| NiO | 1173 | 9000 * | 815% | [54] | |
| NiO | 1073 | 9000 * | 691% | ||
| NiO | 973 | 9000 * | 341% | ||
| Fe2O3 | 1173 | 9000 * | 663% | ||
| Fe2O3 | 1073 | 9000 * | 419% | ||
| Fe2O3 | 973 | 9000 * | 196% |
| Catalyst | Support | Operating Temperature [K] | GHSV [mL/gcath] | Carbon Deposits [gC/gcat] | Carbon Morphology | Source |
|---|---|---|---|---|---|---|
| Fe (porous) | 1173 | 9000 | 6.62 | Layered graphene sheets | [55] | |
| Ni | 773 | 9000 | 354–398 | CNT | [56] | |
| 40 wt% Ni | SiO2 | 773 | 90,000 * | 491 | Fish-bone carbon nanofibers | [41] |
| 40 wt% Ni | SiO2 | 973 | 90,000 * | MWCNT | ||
| 75 wt% Ni | SiO2 | 823 | 30,000 * | Longer and thicker than at 923 K | [57] | |
| 35 wt% Ni/40 wt% Fe | SiO2 | 823 | 30,000 * | Thinner and shorter than without Fe | ||
| 65 wt% NI/10 wt% Fe | SiO2 | 823 | 30,000 * | |||
| 75 wt% Ni | SiO2 | 923 | 18,000 * | MWCNT | ||
| 35 wt% Ni/40 wt% Fe | SiO2 | 923 | 18,000 * | bamboo shaped | ||
| 65 wt% NI/10 wt% Fe | SiO2 | 923 | 18,000 * | |||
| 50 wt% Ni/25 wt% Fe | Al2O3 | 973 | 4200 * | 562 | CNT | [58] |
| 50 wt% Ni | MCM-22 | 1023 | 1800 | 3.63 | CNT | [38] |
| 50 wt% Ni/5 wt% Cu | MCM-22 | 1023 | 1800 | 4.26 | CNT | |
| 50 wt% Ni/5 wt% Cu/5 wt% Zn | MCM-22 | 1023 | 1800 | 5.68 | CNT | |
| 50 wt% Ni/10 wt% Cu | MCM-22 | 1023 | 1800 | 5.5 | CNT | |
| 50 wt% Ni/10 wt% Zn | MCM-22 | 1023 | 1800 | 5.45 | CNT | |
| 5 wt% Ni | SiO2 | 873 | 2440 | 27.16 ** | [59] | |
| 10 wt% Ni | SiO2 | 873 | 2440 | 39.73 ** | ||
| 20 wt% Ni | SiO2 | 873 | 2440 | 57.94 ** | ||
| 30 wt% Ni | SiO2 | 873 | 2440 | 62.05 ** | CNT | |
| 40 wt% Ni | SiO2 | 873 | 2440 | 35.99 ** | ||
| 50 wt% Ni | SiO2 | 873 | 2440 | 23.91 ** | ||
| 60 wt% Ni | SiO2 | 873 | 2440 | 16.54 ** | ||
| 70 wt% Ni | SiO2 | 873 | 2440 | 9.01 ** | ||
| 90 wt% Ni | SiO2 | 873 | 2440 | 1.00 ** | CNT | |
| 69 wt% Fe | Al2O3 | 973 | 6000 | 2.28 | [60] | |
| 69 wt% Fe | Al2O3 | 1073 | 6000 | 4.25 | ||
| 69 wt% Fe | Al2O3 | 1123 | 6000 | 2.3 | ||
| 69 wt% Fe | Al2O3 | 1173 | 6000 | 3.02 | ||
| 69 wt% Fe | Al2O3 | 1073 | 3000 | 3.6 | ||
| 69 wt% Fe | Al2O3 | 1073 | 8000 | 4.8 | ||
| 12.3Fe/1Mo/6.15Al2O3(molar ratio) | Al2O3 | 1023 | 1500 | 1.92 | bamboo shaped | [28] |
| 50 wt% 12.3Fe/1Mo/6.15MgO(molar ratio) | MgO | 1023 | 1500 | 8.26 | tubular | |
| 8.6 wt% Ni | AC | 1123 | 1620 | 7.01 | [61] | |
| 16.6 wt% Ni | AC | 1123 | 1620 | 9.23 | ||
| 23.3 wt% Ni | AC | 1123 | 1620 | 7.92 | ||
| 30 wt% Ni | AC | 1123 | 1620 | 6.10 | ||
| Graphitized carbon black (Carbopack C) | 1123 | 3800 | 0.08 | [62] | ||
| Graphitized carbon black (Carbopack B) | 1123 | 3800 | 0.12 | |||
| Carbon black (Fluke 05120) | 1123 | 3800 | 0.65 | |||
| Carbon black (Fluka 05120) | 1123 | 9500 | 0.69 | |||
| Carbon black (Fluka 03866) | 1123 | 3800 | 0.212 | |||
| Carbon black (Black pearls 2000) | 1123 | 3800 | 0.22 | |||
| Industrial carbon black (HS-50) | 1123 | 3800 | 0.28 | |||
| Commercial activated carbon (CGNorit) | 1123 | 3800 | 0.45 | |||
| Commercial activated carbon (CGNorit) | 1123 | 9500 | 0.6 | |||
| Commercial activated carbon (CGNorit) | 1123 | 19,000 | 0.66 | |||
| 26.5 wt% Fe | SiO2 | 1073 | 105,000 | 7.5 | CNT | [63] |
| 26.5 wt% Fe | SiO2 | 1073 | 105,000 | 22.5 | CNT | |
| 90 wt% Fe | Al2O3 | 898 | 45,000 | 5.5 | [64] | |
| 85 wt% Fe/5 wt% Co | Al2O3 | 823 | 45,000 | 16 | ||
| 50 wt% Fe | Al2O3 | 823 | 45,000 | 26.5 | ||
| 50 wt% Fe/6 wt% Co | Al2O3 | 823 | 45,000 | 52.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Välimäki, E.; Yli-Varo, L.; Romar, H.; Lassi, U. Carbons Formed in Methane Thermal and Thermocatalytic Decomposition Processes: Properties and Applications. C 2021, 7, 50. https://0-doi-org.brum.beds.ac.uk/10.3390/c7030050
Välimäki E, Yli-Varo L, Romar H, Lassi U. Carbons Formed in Methane Thermal and Thermocatalytic Decomposition Processes: Properties and Applications. C. 2021; 7(3):50. https://0-doi-org.brum.beds.ac.uk/10.3390/c7030050
Chicago/Turabian StyleVälimäki, Emmi, Lasse Yli-Varo, Henrik Romar, and Ulla Lassi. 2021. "Carbons Formed in Methane Thermal and Thermocatalytic Decomposition Processes: Properties and Applications" C 7, no. 3: 50. https://0-doi-org.brum.beds.ac.uk/10.3390/c7030050