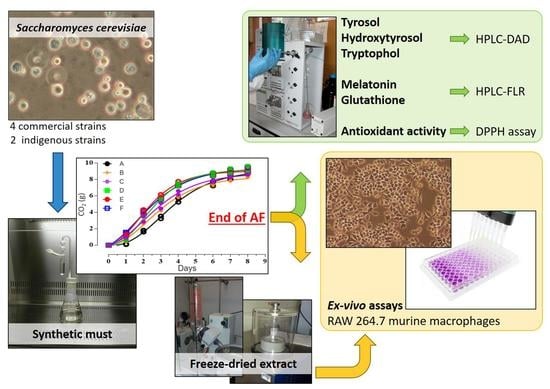
Impact of Saccharomyces cerevisiae Strains on Health-Promoting Compounds in Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Yeast Strains
2.3. Laboratory-Scale Fermentations
2.4. Chemical Analysis
2.4.1. Chemicals and Reagents
2.4.2. Analysis of Tyrosol, Hydroxytyrosol and Tryptophol
2.4.3. Analysis of Melatonin
2.4.4. Analysis of Reduced (GSH) and Oxidized Glutathione (GSSG)
2.4.5. DPPH Antioxidant Assay
2.5. Cell Cultures for Ex-Vivo Assays and Treatment
2.6. Cell Viability Assay
2.7. Measurement of Intracellular Reactive Oxygen Species (ROS)
2.8. IκB and iNOS Immunoblot Detection
2.9. Statistical Analysis
3. Results and Discussion
3.1. Cell Growth and Fermentation Kinetics of S. cerevisiae Strains
3.2. Ability of S. cerevisiae Strains to Produce Bioactive Compounds in Experimental Wines
3.3. Antioxidant Activity of Experimental Wines In Vitro Assay
3.4. Antioxidant and Anti-Inflammatory Activities of Samples from Experimental Wines in Ex-Vivo Assays
3.4.1. Antioxidant Activity in Cultured Cells
3.4.2. Anti-Inflammatory Activity of Experimental Samples
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Renaud, S.D.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Renaud, S.; Gueguen, R. The French paradox and wine drinking. Novartis Found. Symp. 1998, 216, 208–217. [Google Scholar] [PubMed]
- Ruf, J.C. Overview of epidemiological studies on wine, health and mortality. Drugs Exp. Clin. Res. 2003, 29, 173–179. [Google Scholar] [PubMed]
- Guilford, J.M.; Pezzuto, J.M. Wine and health: A review. Am. J. Enol. Vitic. 2011, 62, 471–486. [Google Scholar] [CrossRef]
- Poli, A.; Marangoni, F.; Avogaro, A.; Barba, G.; Bellentani, S.; Bucci, M.; Cambieri, R.; Catapano, A.L.; Costanzo, S.; Cricelli, C.; et al. Moderate alcohol use and health: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 487–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monagas, M.; Bartolome, B.; Gomez-Cordoves, C. Updated knowledge about the presence of phenolic compounds in wine. Crit. Rev. Food Sci. Nutr. 2005, 45, 85–118. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V. Fermented Foods in Health and Disease Prevention; Frías, J., Martínez-Villaluenga, C., Peñas, E., Eds.; Elsevier Inc.: New York, NY, USA, 2017; pp. 593–621. ISBN 978-0-12-802309-9. [Google Scholar]
- Ferreira, V.; Fernandes, F.; Pinto-Carnide, O.; Valentão, P.; Falco, V.; Martín, J.P.; Ortiz, J.M.; Arroyo-García, R.; Andrade, P.B.; Castro, I. Identification of Vitis vinifera L. grape berry skin color mutants and polyphenolic profile. Food Chem. 2016, 194, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ho, L.; Zhao, Z.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Pasinetti, G.M. Moderate consumption of Cabernet Sauvignon attenuated beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006, 20, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Zell, J.A.; McEligot, A.J.; Ziogas, A.; Holcombe, R.F.; Anton-Culver, H. Differential effects of wine consumption on colorectal cancer outcomes based on family history of the disease. Nutr. Cancer 2007, 59, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Pierini, R.; Gee, J.M.; Belshaw, N.J.; Johnson, I.T. Flavonoids and intestinal cancers. Br. J. Nutr. 2008, 99, ES53–ES59. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, E.K.; Xie, J.; Man, R.E.K.; Lim, L.L.; Flood, V.M.; Finger, R.P.; Wong, T.Y.; Lamoureux, E.L. Moderate consumption of white and fortified wine is associated with reduced odds of diabetic retinopathy. J. Diabetes Complic. 2015, 29, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Bassig, B.A.; Lan, Q.; Rothman, N.; Zhang, Y.; Zheng, T. Current understanding of lifestyle and environmental factors and risk of non-hodgkin lymphoma: An epidemiological update. J. Cancer Epidemiol. 2012, 2012, 978930. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, M.L.; Amsterdam, E.A. Alcohol, wine, and cardiovascular health. Clin. Cardiol. 2008, 31, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Phenolic Compounds. In Handbook of Enology, the Chemistry of Wine Stabilization and Treatments, 2nd ed.; Ribéreau-Gayon, P., Glories, Y., Maujean, A., Dubourdieu, D., Eds.; John Wiley and Sons Ltd.: Chichester, UK, 2000; Volume 2, pp. 141–201, ISBN-13 978-0-470-01037-2 (HB). [Google Scholar]
- El Darra, N.; Turk, M.F.; Ducasse, M.A.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Changes in polyphenol profiles and color composition of freshly fermented model wine due to pulsed electric field, enzymes and thermovinification pretreatments. Food Chem. 2016, 194, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.J.; Fugelsang, K.C. Red wine but not white: The importance of fully characterizing wines used in health studies. Am. J. Clin. Nutr. 1997, 66, 447. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Pyruvic acid and acetaldehyde production by different strains of Saccharomyces cerevisiae: Relationship with vitisin A and B formation in red wines. J. Agric. Food Chem. 2003, 51, 7402–7409. [Google Scholar] [CrossRef] [PubMed]
- Romboli, Y.; Mangani, S.; Buscioni, G.; Granchi, L.; Vincenzini, M. Effect of Saccharomyces cerevisiae and Candida zemplinina on quercetin, vitisin A and hydroxytyrosol contents in Sangiovese wines. World J. Microbiol. Biotechnol. 2015, 31, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Mas, A.; Guillamon, J.M.; Torija, M.J.; Beltran, G.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Bioactive Compounds Derived from the Yeast Metabolism of Aromatic Amino Acids during Alcoholic Fermentation. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Kritzinger, E.C. Winemaking Practices Affecting Glutathione Concentrations in White Wine. Ph.D. Thesis, Stellenbosch University, Matieland, South Africa, 2012. [Google Scholar]
- Mezzetti, F.; De Vero, L.; Giudici, P. Evolved Saccharomyces cerevisiae wine strains with enhanced glutathione production obtained by an evolution-based strategy. FEMS Yeast Res. 2014, 14, 977–987. [Google Scholar] [CrossRef] [PubMed]
- RESOLUTION OIV-OENO 370-2012 Guidelines for the Characterization of Wine Yeasts of the Genus Saccharomyces Isolated from Vitivinicultural Environment. Available online: http://www.oiv.int/public/medias/1429/oiv-oeno-370-2012-en.pdf (accessed on 22 June 2012).
- Granchi, L.; Carobbi, M.; Guerrini, S.; Vincenzini, M. Rapid enumeration of yeasts during wine fermentation by the combined use of Thoma chamber and epifluorescence microscopy. In Proceedings of the XXIXth World Congress of Vine and Wine, Logroño, Spain, 25–30 June 2006; pp. 1–5. [Google Scholar]
- Lefebvre, D.; Gabriel, V.; Vayssier, Y.; Fontagné-Faucher, C. Simultaneous HPLC Determination of Sugars, Organic Acids and Ethanol in Sourdough Process LWT. Food Sci. Technol. 2002, 35, 407–414. [Google Scholar] [CrossRef]
- Hernández, T.; Estrella, I.; Carlavilla, D.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V. Phenolic compounds in red wine subjected to industrial malolactic fermentation and ageing on lees. Anal. Chim. Acta 2006, 563, 116–125. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, I.M.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos-Villar, E.; Garcia-Parrilla, C.M. Melatonin is synthesised by yeast during alcoholic fermentation in wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos, E.; García-Parrilla, M.C. Melatonin: A new bioactive compound in wine. J. Food Compost. Anal. 2011, 24, 603–608. [Google Scholar] [CrossRef]
- Jones, D.P.; Carlson, J.L.; Samiec, P.S.; Sternberg, P., Jr.; Mody, V.C., Jr.; Reed, R.L.; Brown, L.A. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin. Chim. Acta 1998, 275, 175–184. [Google Scholar] [CrossRef]
- Tuberoroso, C.I.G.; Congiu, F.; Serrelli, G.; Mameli, S. Determination of dansylated amino acids and biogenic amines in Cannonau and Vermentino wines by HPLC-FLD. Food Chem. 2015, 175, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.M.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General Cytotoxicity Assessment by Means of the MTT Assay. Methods Mol. Biol. 2015, 1250, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, Z.; Cantos-Villar, E.; Palma, M.; Puertas, B. Direct liquid chromatography method for the simultaneous quantification of hydroxytyrosol and tyrosol in red wines. J. Agric. Food Chem. 2011, 59, 11683–11689. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Fernández, M.A.; Fernández-Cruz, E.; Cantos-Villar, E.; Troncoso, A.M.; García-Parrilla, M.C. Determination of hydroxytyrosol produced by winemaking yeasts during alcoholic fermentation using a validated UHPLC–HRMS method. Food Chem. 2018, 242, 345–351. [Google Scholar] [CrossRef] [PubMed]
- García-García, M.I.; Hernández-García, S.; Sánchez-Ferrer, A.; Garcia-Carmona, F. Kinetic study of hydroxytyrosol oxidation and its related compounds by Red Globe grape polyphenol oxidase. J. Agric. Food Chem. 2013, 61, 6050–6055. [Google Scholar] [CrossRef] [PubMed]
- RESOLUTION OIV-OENO 445-2015. Treatment of Must with Glutathione. Available online: http://www.oiv.int/public/medias/1686/oiv-oeno-445-2015-en.pdf (accessed on 10 July 2015).
- RESOLUTION OIV-OENO 446-2015. Treatment of Wine with Glutathione. Available online: http://www.oiv.int/public/medias/1687/oiv-oeno-446-2015-en.pdf (accessed on 10 July 2015).
- Du Toit, W.J.; Lisjak, K.; Stander, M.; Prevoo, D. Using LC-MSMS to asses glutathione levels in South African white grape juices and wines made with different levels of oxygen. J. Agric. Food Chem. 2007, 55, 2765–2769. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, V.; Pons, A.; Dubourdieu, D. Assay of glutathione in must and wines using capillary electrophoresis and laser-induced fluorescence detection—Changes in concentration in dry white wines during alcoholic fermentation and aging. J. Chromatogr. 2007, 1139, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Fracassetti, D. Investigation on Cysteinyl Thiol Compounds from Yeast Affecting Wine Properties. Ph.D. Thesis, Universita’ degli Studi di Milano, Milano, Itlay, 2010. [Google Scholar]
- Mercolini, L.; Addolorata Saracino, M.; Bugamelli, F.; Ferranti, A.; Malaguti, M.; Hrelia, S.; Raggi, M.A. HPLC-F analysis of melatonin and resveratrol isomers in wine using an SPE procedure. J. Sep. Sci. 2008, 31, 1007–1114. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Park, M.Y.; Cho, Y.J.; Lee, J.H.; Yoo, C.G.; Lee, C.T.; Lee, S.M. Anti-inflammatory Effect of Erdosteine in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflammation 2016, 39, 1573–1581. [Google Scholar] [CrossRef] [PubMed]




| Gompertz Parameters | |||||
|---|---|---|---|---|---|
| S. cerevisiae Strain | Growth Parameters | Fermentation Parameters | |||
| µmax | C | Lag | CO2Rmax | CO2 max | |
| R6 | 2.43 ± 0.07 a | 1.75 ± 0.00 b | 0.36 ± 0.04 c | 2.26 ± 0.03 ab | 9.40 ± 0.30 |
| P8 | 2.27 ± 0.15 a | 1.70 ± 0.02 b | 0.57 ± 0.08 bc | 2.06 ± 0.11 bc | 8.84 ± 0.15 |
| BM45 | 2.49 ± 0.03 a | 1.83 ± 0.01 a | 0.51 ± 0.04 bc | 2.57 ± 0.10 a | 9.18 ± 0.28 |
| EC1118 | 2.08 ± 0.11 ab | 1.70 ± 0.01 b | 0.74 ± 0.01 b | 2.53 ± 0.04 a | 9.40 ± 0.60 |
| Zinfandel | 1.51 ± 0.09 b | 1.70 ± 0.04 b | 1.19 ± 0.06 a | 1.88 ± 0.23 bc | 9.55 ± 0.24 |
| VL1 | 2.41 ± 0.37 a | 1.72 ± 0.01 b | 0.64 ± 0.09 b | 1.79 ± 0.00 c | 8.86 ± 0.14 |
| S. cerevisiae Strain | Tyrosol (mg/L) | Tryptophol (mg/L) | Glutathione (mg/L) | Melatonin (ng/mL) |
|---|---|---|---|---|
| R6 | 6.17 ± 0.18 b | 4.70 ± 0.83 ab | 7.29 ± 0.48 bc | 6.23 ± 0.87 b |
| P8 | 4.97 ± 0.25 c | 3.47± 1.22 abc | 5.39 ± 0.03 c | 11.74 ± 1.71 ab |
| BM45 | 7.10 ± 0.11 a | 5.46 ± 0.28 a | 16.28 ± 1.85 a | 12.98 ± 3.34 a |
| EC1118 | 4.11 ± 0.07 d | 2.11 ± 0.18 c | 5.84 ± 0.45 c | 11.01 ± 0.74 ab |
| Zinfandel | 4.06± 0.15 d | 1.73 ± 0.33 c | 6.85 ± 0.48 c | 9.24 ± 0.98 ab |
| VL1 | 4.63 ± 0.00 cd | 2.72 ± 0.30 bc | 10.51 ± 0.76 b | 5.34 ± 0.53 b |
| S. cerevisiae Strains | Tyrosol (µg/µL) | Tryptophol (µg/µL) | Glutathione (µg/µL) | Melatonin (ng/µL) |
|---|---|---|---|---|
| R6 | 0.2 | 0.16 | 0.24 | 0.2 |
| P8 | 0.166 | 0.116 | 0.18 | 0.4 |
| BM45 | 0.24 | 0.18 | 0.76 | 0.42 |
| EC1118 | 0.14 | 0.07 | 0.2 | 0.36 |
| ZINFANDEL | 014 | 0.06 | 0.22 | 0.3 |
| VL1 | 0.154 | 0.08 | 0.34 | 0.18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrini, S.; Mangani, S.; Romboli, Y.; Luti, S.; Pazzagli, L.; Granchi, L. Impact of Saccharomyces cerevisiae Strains on Health-Promoting Compounds in Wine. Fermentation 2018, 4, 26. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation4020026
Guerrini S, Mangani S, Romboli Y, Luti S, Pazzagli L, Granchi L. Impact of Saccharomyces cerevisiae Strains on Health-Promoting Compounds in Wine. Fermentation. 2018; 4(2):26. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation4020026
Chicago/Turabian StyleGuerrini, Simona, Silvia Mangani, Yuri Romboli, Simone Luti, Luigia Pazzagli, and Lisa Granchi. 2018. "Impact of Saccharomyces cerevisiae Strains on Health-Promoting Compounds in Wine" Fermentation 4, no. 2: 26. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation4020026