A Review of Seaweed Pre-Treatment Methods for Enhanced Biofuel Production by Anaerobic Digestion or Fermentation
Abstract
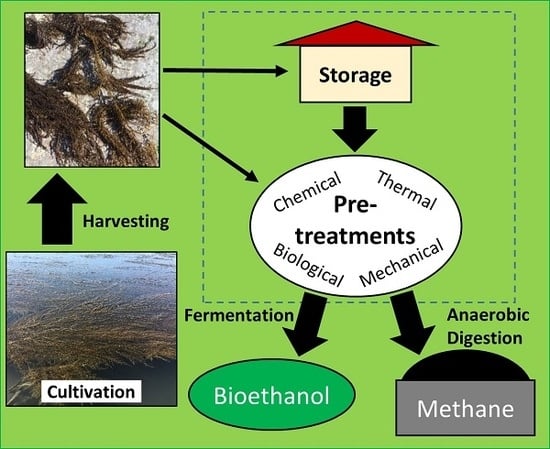
:1. Introduction
2. Structural and Chemical Composition
2.1. Moisture and Salt Content
2.2. Structural Composition
2.3. Polysaccharides
2.4. Chemical Composition Variability
3. Storage and Preservation
3.1. Drying for Storage
3.2. Ensilage for Storage and Preservation
4. Seaweed Hydrolysis Methods
4.1. Mechanical Treatment
4.1.1. Size Reduction
4.1.2. Beating
4.1.3. Washing
4.2. Thermal Treatment
4.2.1. Microwave
4.2.2. Steam Explosion
4.2.3. Other Thermal Pre-Treatment Methods
4.3. Chemical Treatment
4.3.1. Alkali or Acidic Treatment
4.3.2. Peroxide Treatment
4.4. Biological Treatment
4.5. Inhibitor Removal
4.5.1. Salts
4.5.2. Phenolics
4.5.3. Heavy Metals
4.5.4. Other
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- West, J.; Calumpong, H.P.; Martin, G. Chapter 14—Seaweeds. In The First Global Integrated Marine: Assessment World Ocean Assessment I; United Nations: Cambridge, United Kingdom, 2017; pp. 1–10. [Google Scholar]
- FishstatJ—Fishery and Aquaculture Statistics, version 3.04.9; Global Aquaculture Production 1950–2016; FAO Fisheries and Aquaculture Department: Rome, Italy, 2018.
- Milledge, J.J.; Heaven, S. Methods of energy extraction from microalgal biomass: A review. Rev. Environ. Sci. Bio/Technol. 2014, 13, 301–320. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, T.; Rong, J.; He, C.; Wang, Q. Microalgal biofuel revisited: An informatics-based analysis of developments to date and future prospects. Appl. Energy 2015, 155, 585–598. [Google Scholar] [CrossRef] [Green Version]
- Kerrison, P.D.; Stanley, M.S.; Edwards, M.D.; Black, K.D.; Hughes, A.D. The cultivation of European kelp for bioenergy: Site and species selection. Biomass Bioenergy 2015, 80, 229–242. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Potential process ‘hurdles’ in the use of macroalgae as feedstock for biofuel production in the British Isles. J. Chem. Technol. Biotechnol. 2016, 91, 2221–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, K.; McKinley, K.R. Use of macroalgae for marine biomass production and CO2 remediation: A review. J. Appl. Phycol. 1994, 6, 45–60. [Google Scholar] [CrossRef]
- Florentinus, A.; Harmelinck, C.; de Lint, S.; van Iersel, S. Worldwide Potential of Aquatic Biomass; Ecofys: Utrecht, The Netherlands, 2014; pp. 1–66. [Google Scholar]
- Pechsiri, J.S.; Thomas, J.-B.E.; Risén, E.; Ribeiro, M.S.; Malmström, M.E.; Nylund, G.M.; Jansson, A.; Welander, U.; Pavia, H.; Gröndahl, F. Energy performance and greenhouse gas emissions of kelp cultivation for biogas and fertilizer recovery in Sweden. Sci. Total Environ. 2016, 573, 347–355. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Available online: Https://ec.europa.eu/energy/en/topics/energy-strategy-and-energy-union/2030-energy-strategy (accessed on 24 September 2018).
- Murphy, J.D.; Drosg, B.; Allen, E.; Jerney, J.; Xia, A.; Herrmann, C. A Perspective on Algal Biogas; IEA Bioenergy: Paris, France, 2015; pp. 1–38. [Google Scholar]
- Horn, S.J. Bioenergy from brown seaweeds. Ph.D. Thesis, Norwegian University of Science and Technology NTNU, Trondheim, Norway, November 2000. [Google Scholar]
- Murphy, F.; Devlin, G.; Deverell, R.; McDonnell, K. Biofuel Production in Ireland—An Approach to 2020 Targets with a Focus on Algal Biomass. Energies 2013, 6, 6391–6412. [Google Scholar] [CrossRef] [Green Version]
- Milledge, J.J.; Smith, B.; Dyer, P.W.; Harvey, P. Macroalgae-derived biofuel: A review of methods of energy extraction from seaweed biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Saydah, B.; Eranki, P.; Colosi, L.M.; Greg Mitchell, B.; Rhodes, J.; Clarens, A.F. Pilot-scale data provide enhanced estimates of the life cycle energy and emissions profile of algae biofuels produced via hydrothermal liquefaction. Bioresour. Technol. 2013, 148, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Quarterman, J.; Jin, Y.-S.S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Wall, D.M.; Herrmann, C.; Xia, A.; Murphy, J.D. What is the gross energy yield of third generation gaseous biofuel sourced from seaweed? Energy 2015, 81, 352–360. [Google Scholar] [CrossRef]
- Murphy, J.; Braun, R.; Weiland, P.; Wellinger, A. Biogas from Crop Digestion Task 37—Energy from Biogas; IEA Bioenergy: Paris, France, 2011; pp. 1–23. [Google Scholar]
- Seghetta, M.; Østergård, H.; Bastianoni, S. Energy analysis of using macroalgae from eutrophic waters as a bioethanol feedstock. Ecol. Modell. 2014, 288, 25–37. [Google Scholar] [CrossRef]
- Taelman, S.E.; Champenois, J.; Edwards, M.D.; De Meester, S.; Dewulf, J. Comparative environmental life cycle assessment of two seaweed cultivation systems in North West Europe with a focus on quantifying sea surface occupation. Algal Res. 2015, 11, 173–183. [Google Scholar] [CrossRef]
- Alvarado-Morales, M.; Boldrin, A.; Karakashev, D.B.; Holdt, S.L.; Angelidaki, I.; Astrup, T. Life cycle assessment of biofuel production from brown seaweed in Nordic conditions. Bioresour. Technol. 2013, 129, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Aitken, D.; Bulboa, C.; Godoy-Faundez, A.; Turrion-Gomez, J.L.; Antizar-Ladislao, B. Life cycle assessment of macroalgae cultivation and processing for biofuel production. J. Clean. Prod. 2014, 75, 45–56. [Google Scholar] [CrossRef]
- Pastare, L.; Aleksandrovs, I.; Lauka, D.; Romagnoli, F. Mechanical Pre-treatment Effect on Biological Methane Potential from Marine Macro Algae: Results from Batch Tests of Fucus Vesiculosus. Energy Procedia 2016, 95, 351–357. [Google Scholar] [CrossRef]
- Hessami, M.J.; Phang, S.-M.; Salleh, A.; Rabiei, R. Evaluation of tropical seaweeds as feedstock for bioethanol production. Int. J. Environ. Sci. Technol. 2018, 15, 977–992. [Google Scholar] [CrossRef]
- Marquez, G.P.; Santiañez, W.J.; Trono, G.C.; Rose, S.; de la Rama, S.R.; Takeuchi, H.; Hasegawa, T. Seaweeds: A sustainable fuel source. In Seaweed Sustainability: Food and Non-Food Applications; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: Oxford, UK, 2015; pp. 421–458. ISBN 978-0-12-419958-3. [Google Scholar]
- Adams, J.M.M.; Toop, T.A.; Donnison, I.S.; Gallagher, J.A. Seasonal variation in Laminaria digitata and its impact on biochemical conversion routes to biofuels. Bioresour. Technol. 2011, 102, 9976–9984. [Google Scholar] [CrossRef] [PubMed]
- Borines, M.G.; de Leon, R.L.; Cuello, J.L. Bioethanol production from the macroalgae Sargassum spp. Bioresour. Technol. 2013, 138, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Golberg, A.; Vitkin, E.; Linshiz, G.; Khan, S.A.; Hillson, N.J.; Yakhini, Z.; Yarmush, M.L. Proposed design of distributed macroalgal biorefineries: Thermodynamics, bioconversion technology, and sustainability implications for developing economies. Biofuels Bioprod. Biorefining-Biofpr 2014, 8, 67–82. [Google Scholar] [CrossRef]
- Wang, D.; Yun, E.J.; Kim, S.; Kim, D.H.; Seo, N.; An, H.J.; Kim, J.-H.; Cheong, N.Y.; Kim, K.H. Efficacy of acidic pretreatment for the saccharification and fermentation of alginate from brown macroalgae. Bioprocess Biosyst. Eng. 2016, 39, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Nakamura, K.; Ariga, O.; Nakasaki, K. Production of high concentrations of bioethanol from seaweeds that contain easily hydrolyzable polysaccharides. Process Biochem. 2011, 46, 2111–2116. [Google Scholar] [CrossRef]
- Kawai, S.; Murata, K. Biofuel production based on carbohydrates from both brown and red macroalgae: Recent developments in key biotechnologies. Int. J. Mol. Sci. 2016, 17, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Gao, L.; Zhang, D.; Ye, N.; Chen, S.; Li, D. Enhanced hydrolysis of Macrocystis pyrifera by integrated hydroxyl radicals and hot water pretreatment. Bioresour. Technol. 2015, 179, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Barbot, Y.N.; Thomsen, C.; Thomsen, L.; Benz, R. Anaerobic digestion of laminaria japonica waste from industrial production residues in laboratory- and pilot-scale. Mar. Drugs 2015, 13, 5947–5975. [Google Scholar] [CrossRef] [PubMed]
- McKennedy, J.; Sherlock, O. Anaerobic digestion of marine macroalgae: A review. Renew. Sustain. Energy Rev. 2015, 52, 1781–1790. [Google Scholar] [CrossRef]
- Habig, C.; Ryther, J.H. Methane production from the anaerobic digestion of some marine macrophytes. Resour. Conserv. 1983, 8, 271–279. [Google Scholar] [CrossRef]
- Østgaard, K.; Indergaard, M.; Markussen, S.; Knutsen, S.H.; Jensen, A. Carbohydrate degradation and methane production during fermentation of Laminaria saccharina (Laminariales, Phaeophyceae). J. Appl. Phycol. 1993, 5, 333–342. [Google Scholar] [CrossRef]
- Hierholtzer, A.; Akunna, J.C. Modelling sodium inhibition on the anaerobic digestion process. Water Sci. Technol. 2012, 66, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Biomethane production from various segments of brown seaweed. Energy Convers. Manag. 2018, 174, 855–862. [Google Scholar] [CrossRef]
- Rodriguez, C.; Alaswad, A.; Mooney, J.; Prescott, T.; Olabi, A.G. Pre-treatment techniques used for anaerobic digestion of algae. Fuel Process. Technol. 2015, 138, 765–779. [Google Scholar] [CrossRef]
- Jung, K.A.; Lim, S.-R.R.; Kim, Y.; Park, J.M. Potentials of macroalgae as feedstocks for biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I. Experimental processing of seaweeds for biofuels. Wiley Interdiscip. Rev. Energy Environ. 2018, 7, 1–25. [Google Scholar] [CrossRef]
- Ross, A.B.; Jones, J.M.; Kubacki, M.L.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Vázquez, L.M.; Rojas-Pérez, A.; Fuentes-Caraballo, M.; Robles, I.V.; Jena, U.; Das, K.C. Demineralization of Sargassum spp. Macroalgae Biomass: Selective Hydrothermal Liquefaction Process for Bio-Oil Production. Front. Energy Res. 2015, 3. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Ensilage and anaerobic digestion of Sargassum muticum. J. Appl. Phycol. 2016, 28, 3021–3030. [Google Scholar] [CrossRef]
- Kloareg, B.; Quatrano, R.S. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr. Mar. Biol. An Annu. Rev. 1988, 26, 259–315. [Google Scholar]
- Darcy-Vrillon, B. Nutritional aspects of the developing use of marine macroalgae for the human food industry. Int. J. Food Sci. Nutr. 1993, 44, S23–S25. [Google Scholar]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; ISBN 0521145953. [Google Scholar]
- Synytsya, A.; Čopíková, J.; Kim, W.J.; Park, Y. Cell Wall Polysaccharides of Marine Algae. In Springer Handbook of Marine Biotechnology; Kim, S.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 543–590. ISBN 978-3-642-53971-8. [Google Scholar]
- Deniaud-Bouët, E.; Kervarec, N.; Michel, G.; Tonon, T.; Kloareg, B.; Hervé, C. Chemical and enzymatic fractionation of cell walls from Fucales: Insights into the structure of the extracellular matrix of brown algae. Ann. Bot. 2014, 114, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Yahmed, N.B.; Jmel, M.A.; Ben Alaya, M.; Bouallagui, H.; Marzouki, M.N.; Smaali, I. A biorefinery concept using the green macroalgae Chaetomorpha linum for the coproduction of bioethanol and biogas. Energy Convers. Manag. 2016, 119, 257–265. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and function properties of Ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Bobin-Dubigeon, C.; Lahaye, M.; Guillon, F.; Barry, J.L.; Gallant, D.J. Factors limiting the biodegradation of Ulva sp. cell-wall polysaccharides. J. Sci. Food Agric. 1997, 75, 341–351. [Google Scholar] [CrossRef]
- Vanegas, C.H.; Hernon, A.; Bartlett, J. Enzymatic and organic acid pretreatment of seaweed: Effect on reducing sugars production and on biogas inhibition. Int. J. Ambient Energy 2015, 36, 2–7. [Google Scholar] [CrossRef]
- McDermid, K.J.; Stuerckea, B.; Haleakala, O.J. Total dietary fiber content in Hawaiian marine algae. Bot. Mar. 2005, 48, 1–3. [Google Scholar] [CrossRef]
- Lahaye, M. Marine Algae as Sources of Fibers: Determination of Soluble and Insoluble Dietary Fiber Contents in Some ‘Sea Vegetables’. J. Sci. Food Agric. 1991, 54, 587–594. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.A.; Turner, L.B.; Adams, J.M.M.; Barrento, S.; Dyer, P.W.; Theodorou, M.K. Species variation in the effects of dewatering treatment on macroalgae. J. Appl. Phycol. 2018, 30, 2305–2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrita, A.R.J.J.; Maia, M.R.G.G.; Sousa-Pinto, I.; Fonseca, A.J.M.M. Ensilage of seaweeds from an integrated multi-trophic aquaculture system. Algal Res. Biofuels Bioprod. 2017, 24, 290–298. [Google Scholar] [CrossRef]
- Wei, F.; Ito, K.; Sakata, K.; Date, Y.; Kikuchi, J. Pretreatment and integrated analysis of spectral data reveal seaweed similarities based on chemical diversity. Anal. Chem. 2015, 87, 2819–2826. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Sakata, K.; Kikuchi, J. Chemical profiling of complex biochemical mixtures from various seaweeds. Polym. J. 2012, 44, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Chemical composition and antioxidant activity of sulphated polysaccharides extracted from Fucus vesiculosus using different hydrothermal processes. Chem. Pap. 2014, 68, 203–209. [Google Scholar] [CrossRef]
- Percival, E. The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Br. Phycol. J. 1979, 14, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Choi, I.-G.; Kim, K.H. Red macroalgae as a sustainable resource for bio-based products. Trends Biotechnol. 2015, 33, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Jang, J.-S.; Cho, Y.; Jeong, G.-T.; Kim, S.-K. Optimization of saccharification and ethanol production by simultaneous saccharification and fermentation (SSF) from seaweed, Saccharina japonica. Bioprocess Biosyst. Eng. 2012, 35, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Sousa, R.A.; Reis, R.L. A practical perspective on ulvan extracted from green algae. J. Appl. Phycol. 2013, 25, 407–424. [Google Scholar] [CrossRef] [Green Version]
- Robic, A.; Gaillard, C.; Sassi, J.-F.; Lerat, Y.; Lahaye, M. Ultrastructure of ulvan: A polysaccharide from green seaweeds. Biopolymers 2009, 91, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. The inhibition of anaerobic digestion by model phenolic compounds representative of those from Sargassum muticum. J. Appl. Phycol. 2018. [Google Scholar] [CrossRef]
- Sharma, S.; Neves, L.; Funderud, J.; Mydland, L.T.; Øverland, M.; Horn, S.J. Seasonal and depth variations in the chemical composition of cultivated Saccharina latissima. Algal Res. 2018, 32, 107–112. [Google Scholar] [CrossRef]
- Andrade, L.R.; Leal, R.N.; Noseda, M.; Duarte, M.E.R.; Pereira, M.S.; Mourão, P.A.S.; Farina, M.; Amado Filho, G.M. Brown algae overproduce cell wall polysaccharides as a protection mechanism against the heavy metal toxicity. Mar. Pollut. Bull. 2010, 60, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Plouguerne, E.; Le Lann, K.; Connan, S.; Jechoux, G.; Deslandes, E.; Stiger-Pouvreau, V. Spatial and seasonal variation in density, reproductive status, length and phenolic content of the invasive brown macroalga Sargassum muticum (Yendo) Fensholt along the coast of Western Brittany (France). Aquat. Bot. 2006, 85, 337–344. [Google Scholar] [CrossRef]
- Philippsen, A.; Wild, P.; Rowe, A. Energy input, carbon intensity and cost for ethanol produced from farmed seaweed. Renew. Sustain. Energy Rev. 2014, 38, 609–623. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Gerung, G.S.; Yasir, S.; Critchley, A.T. Cultivation of tropical red seaweeds in the BIMP-EAGA region. J. Appl. Phycol. 2014, 26, 707–718. [Google Scholar] [CrossRef]
- Gallagher, J.A.; Turner, L.B.; Adams, J.M.M.; Dyer, P.W.; Theodorou, M.K. Dewatering treatments to increase dry matter content of the brown seaweed, kelp (Laminaria digitata ((Hudson) JV Lamouroux)). Bioresour. Technol. 2017, 224, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Fitzgerald, J.; O’shea, R.; Xia, A.; O’kiely, P.; Murphy, J.D.; O’Shea, R.; Xia, A.; O’Kiely, P.; Murphy, J.D. Ensiling of seaweed for a seaweed biofuel industry. Bioresour. Technol. 2015, 196, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Sandbakken, I.S.; Sæther, M.; Funderud, J.; Aasen, I.M. Acid preservation of Saccharina latissima for application as a carbon source for fermentation to biofuels and chemicals. J. Appl. Phycol. 2018, 1–8. [Google Scholar] [CrossRef]
- Redden, H.; Milledge, J.J.; Greenwell, H.C.; Dyer, P.W.; Harvey, P.J. Changes in higher heating value and ash content of seaweed during ensiling. J. Appl. Phycol. 2017, 29, 1037–1046. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Sadek, M.S.; Harvey, P.J. Effect of Freshwater Washing Pretreatment on Sargassum muticum as a Feedstock for Biogas Production. Energies 2018, 11, 1771. [Google Scholar] [CrossRef]
- Charmley, E.; Savoie, P.; McRae, K.B.; Lu, X. Effect of maceration at mowing on silage conservation, voluntary intake, digestibility and growth rate of steers fed precision chopped or round bale silages. Can. J. Anim. Sci. 1999, 79, 195–202. [Google Scholar] [CrossRef]
- Muller, C.E. Long-stemmed vs. cut haylage in bales-Effects on fermentation, aerobic storage stability, equine eating behaviour and characteristics of equine faeces. Anim. Feed Sci. Technol. 2009, 152, 307–321. [Google Scholar] [CrossRef]
- Driehuis, F.; van Wikselaar, P.G. The occurrence and prevention of ethanol fermentation in high-dry-matter grass silage. J. Sci. Food Agric. 2000, 80, 711–718. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Bleathman, G.; Thomas, D.; Gallagher, J.A. The effect of mechanical pre-processing and different drying methodologies on bioethanol production using the brown macroalga Laminaria digitata (Hudson) JV Lamouroux. J. Appl. Phycol. 2017, 29, 2463–2469. [Google Scholar] [CrossRef]
- Raposo, F.; Fernández-Cegrí, V.; de la Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.C.; et al. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Vanegas, C.H.; Bartlett, J. Green energy from marine algae: Biogas production and composition from the anaerobic digestion of Irish seaweed species. Environ. Technol. 2013, 34, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Marrero Barroso, T.; Olabi, A.G. Optimization of mechanical pre-treatment of Laminariaceae spp. biomass-derived biogas. Renew. Energy 2014, 62, 527–534. [Google Scholar] [CrossRef]
- Hughes, A.D.; Kelly, M.S.; Black, K.D.; Stanley, M.S. Biogas from Macroalgae: Is it time to revisit the idea? Biotechnol. Biofuels 2012, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Comparison of pre-treatments to reduce salinity and enhance biomethane yields of Laminaria digitata harvested in different seasons. Energy 2017, 140, 546–551. [Google Scholar] [CrossRef]
- Schultz-Jensen, N.; Thygesen, A.; Leipold, F.; Thomsen, S.T.; Roslander, C.; Lilholt, H.; Bjerre, A.B. Pretreatment of the macroalgae Chaetomorpha linum for the production of bioethanol—Comparison of five pretreatment technologies. Bioresour. Technol. 2013, 140, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Korzen, L.; Pulidindi, I.N.; Israel, A.; Abelson, A.; Gedanken, A. Single step production of bioethanol from the seaweed Ulva rigida using sonication. RSC Adv. 2015, 5, 16223–16229. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Schmidt, A.; Gallagher, J.A. The impact of sample preparation of the macroalgae Laminaria digitata on the production of the biofuels bioethanol and biomethane. J. Appl. Phycol. 2015, 27, 985–991. [Google Scholar] [CrossRef]
- Amamou, S.; Sambusiti, C.; Monlau, F.; Dubreucq, E.; Barakat, A. Mechano-enzymatic deconstruction with a new enzymatic cocktail to enhance enzymatic hydrolysis and bioethanol fermentation of two macroalgae species. Molecules 2018, 23, 174. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.V.; Alves, M.M.; Costa, J.C. Design of experiments to assess pre-treatment and co-digestion strategies that optimize biogas production from macroalgae Gracilaria vermiculophylla. Bioresour. Technol. 2014, 162, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, H.B.; Heiske, S. Anaerobic digestion of macroalgae: Methane potentials, pre-treatment, inhibition and co-digestion. Water Sci. Technol. 2011, 64, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Montingelli, M.E.; Benyounis, K.Y.; Stokes, J.; Olabi, A.G. Pretreatment of macroalgal biomass for biogas production. Energy Convers. Manag. 2016, 108, 202–209. [Google Scholar] [CrossRef]
- Montingelli, M.E.; Benyounis, K.Y.; Quilty, B.; Stokes, J.; Olabi, A.G. Influence of mechanical pretreatment and organic concentration of Irish brown seaweed for methane production. Energy 2017, 118, 1079–1089. [Google Scholar] [CrossRef]
- Allen, E.; Browne, J.; Hynes, S.; Murphy, J.D. The potential of algae blooms to produce renewable gaseous fuel. Waste Manag. 2013, 33, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- Karray, R.; Hamza, M.; Sayadi, S. Evaluation of ultrasonic, acid, thermo-alkaline and enzymatic pre-treatments on anaerobic digestion of Ulva rigida for biogas production. Bioresour. Technol. 2015, 187, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Symons, G.E.; Buswell, A.M. The Methane Fermentation of Carbohydrates 1,2. J. Am. Chem. Soc. 1933, 55, 2028–2036. [Google Scholar] [CrossRef]
- Buswell, A.M.; Mueller, H.F. Mechanism of Methane Fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Angelidaki, I. Biogas production from ensiled meadow grass; effect of mechanical pretreatments and rapid determination of substrate biodegradability via physicochemical methods. Bioresour. Technol. 2015, 182, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Manns, D.; Andersen, S.K.; Saake, B.; Meyer, A.S. Brown seaweed processing: Enzymatic saccharification of Laminaria digitata requires no pre-treatment. J. Appl. Microbiol. 2016, 28, 1287–1294. [Google Scholar] [CrossRef] [Green Version]
- Tedesco, S.; Mac Lochlainn, D.; Olabi, A.G. Particle size reduction optimization of Laminaria spp. biomass for enhanced methane production. Energy 2014, 76, 857–862. [Google Scholar] [CrossRef]
- Rodriguez, C.; El-Hassan, Z.; Olabi, A.G.; Alaswad, A. Optimization of the anaerobic digestion process of mechanically pretreated algae. In 11th Conference on Sustainable Development of Energy, Water and Environment Systems, SDEWES; Faculty of Mechanical Engineering and Naval Architecture: Zagreb, Croatia, 2016; pp. 1–12. [Google Scholar]
- Wang, X.; Liu, X.; Wang, G. Two-stage hydrolysis of invasive algal feedstock for ethanol fermentation. J. Integr. Plant Biol. 2011, 53, 246–252. [Google Scholar] [CrossRef] [PubMed]
- González-López, N.; Moure, A.; Domínguez, H.; Gonzalez-Lopez, N.; Moure, A.; Dominguez, H. Hydrothermal fractionation of Sargassum muticum biomass. J. Appl. Phycol. 2012, 24, 1569–1578. [Google Scholar] [CrossRef]
- Choi, J.; Choi, J.W.; Suh, D.J.; Ha, J.M.; Hwang, J.W.; Jung, H.W.; Lee, K.Y.; Woo, H.C. Production of brown algae pyrolysis oils for liquid biofuels depending on the chemical pretreatment methods. Energy Convers. Manag. 2014, 86, 371–378. [Google Scholar] [CrossRef]
- Suutari, M.; Leskinen, E.; Fagerstedt, K.; Kuparinen, J.; Kuuppo, P.; Blomster, J. Macroalgae in biofuel production. Phycol. Res. 2015, 63, 1–18. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yoon, J.J.; Park, H.D.; Lim, D.J.; Kim, S.H. Anaerobic digestibility of algal bioethanol residue. Bioresour. Technol. 2012, 113, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. The effect of seasonal variation on biomethane production from seaweed and on application as a gaseous transport biofuel. Bioresour. Technol. 2016, 209, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Alam, M.A.; Kong, X.; Wang, Z.; Li, L.; Sun, Y.; Yuan, Z. Effect of salinity on the microbial community and performance on anaerobic digestion of marine macroalgae. J. Chem. Technol. Biotechnol. 2017, 92, 2392–2399. [Google Scholar] [CrossRef]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.J.; Zhang, Y.; Jiang, Y.; Heaven, S. Trace element requirements for stable food waste digestion at elevated ammonia concentrations. Bioresour. Technol. 2012, 104, 127–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdani, P.; Zamani, A.; Karimi, K.; Taherzadeh, M.J. Characterization of Nizimuddinia zanardini macroalgae biomass composition and its potential for biofuel production. Bioresour. Technol. 2015, 176, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Wi, S.G.; Jung, S.; Song, Y.; Bae, H.-J. Efficient approach for bioethanol production from red seaweed Gelidium amansii. Bioresour. Technol. 2015, 175, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Baek, G.; Kim, J.; Shin, S.G.; Lee, C. Mild-temperature thermochemical pretreatment of green macroalgal biomass: Effects on solubilization, methanation, and microbial community structure. Bioresour. Technol. 2016, 199, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Vivekanand, V.; Eijsink, V.G.H.; Horn, S.J. Biogas production from the brown seaweed Saccharina latissima: Thermal pretreatment and codigestion with wheat straw. J. Appl. Phycol. 2012, 24, 1295–1301. [Google Scholar] [CrossRef]
- Romagnoli, F.; Pastare, L.; Sabūnas, A.; Bāliņa, K.; Blumberga, D. Effects of pre-treatment on Biochemical Methane Potential (BMP) testing using Baltic Sea Fucus vesiculosus feedstock. Biomass Bioenergy 2017, 105, 23–31. [Google Scholar] [CrossRef]
- Vázquez-Delfín, E.; Robledo, D.; Freile-Pelegrín, Y. Microwave-assisted extraction of the Carrageenan from Hypnea musciformis (Cystocloniaceae, Rhodophyta). J. Appl. Phycol. 2014, 26, 901–907. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave Assisted Extraction—An Innovative and Promising Extraction Tool for Medicinal Plant Research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Kostas, E.T.; Beneroso, D.; Robinson, J.P. The application of microwave heating in bioenergy: A review on the microwave pre-treatment and upgrading technologies for biomass. Renew. Sustain. Energy Rev. 2017, 77, 12–27. [Google Scholar] [CrossRef] [Green Version]
- Sousa, A.M.M.; Alves, V.D.; Morais, S.; Delerue-Matos, C.; Gonçalves, M.P. Agar extraction from integrated multitrophic aquacultured Gracilaria vermiculophylla: Evaluation of a microwave-assisted process using response surface methodology. Bioresour. Technol. 2010, 101, 3258–3267. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Xu, X.; Jing, C.; Zou, P.; Zhang, C.; Li, Y. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Supercritical Carbon Dioxide Extraction of Fucoxanthin from Undaria pinnatifida. J. Agric. Food Chem. 2013, 61, 5792–5797. [Google Scholar] [CrossRef] [PubMed]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Microwave–hydrothermal extraction and degradation of fucoidan from supercritical carbon dioxide deoiled Undaria pinnatifida. Ind. Eng. Chem. Res. 2013, 52, 7940–7946. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Marhaeni, B.; Winanto, T.; Jeong, G.-T.; Khan, M.N.A.; Hong, Y.-K. Comparison of agarophytes (Gelidium, Gracilaria, and Gracilariopsis) as potential resources for bioethanol production. J. Appl. Phycol. 2013, 25, 1957–1961. [Google Scholar] [CrossRef]
- Park, J.-H.; Hong, J.-Y.; Jang, H.C.; Oh, S.G.; Kim, S.-H.; Yoon, J.-J.; Kim, Y.J. Use of Gelidium amansii as a promising resource for bioethanol: A practical approach for continuous dilute-acid hydrolysis and fermentation. Bioresour. Technol. 2012, 108, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, S.; Oono, K.; Onda, A.; Yanagisawa, K.; Azuma, J. Comparative decomposition kinetics of neutral monosaccharides by microwave and induction heating treatments. Carbohydr. Res. 2013, 375, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Macquarrie, D.J. Microwave Assisted Acid Hydrolysis of Brown Seaweed Ascophyllum nodosum for Bioethanol Production and Characterization of Alga Residue. ACS Sustain. Chem. Eng. 2015, 3, 1359–1365. [Google Scholar] [CrossRef]
- Teh, Y.Y.; Lee, K.T.; Chen, W.-H.; Lin, S.-C.; Sheen, H.-K.; Tan, I.S. Dilute sulfuric acid hydrolysis of red macroalgae Eucheuma denticulatum with microwave-assisted heating for biochar production and sugar recovery. Bioresour. Technol. 2017, 246, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.-P.P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.M.; Bertilsson, M.; Gorwa-Grauslund, M.F.; Gorsich, S.; Lidén, G. Metabolic effects of furaldehydes and impacts on biotechnological processes. Appl. Microbiol. Biotechnol. 2009, 82, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Kim, H.T.; Cho, K.M.; Yu, S.; Kim, S.; Choi, I.-G.; Kim, K.H. Pretreatment and saccharification of red macroalgae to produce fermentable sugars. Bioresour. Technol. 2016, 199, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Jensen, N.; Leipold, F.; Bindslev, H.; Thomsen, A.B. Plasma-Assisted Pretreatment of Wheat Straw. Appl. Biochem. Biotechnol. 2011, 163, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Heredia, J.; Torregrosa, J.; García, J.; Domínguez, J.R.; Tierno, J.C. Degradation of olive mill wastewater by the combination of Fenton’s reagent and ozonation processes with an aerobic biological treatment. Water Sci. Technol. 2001, 44, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, C.; Hernon, A.; Bartlett, J. Influence of Chemical, Mechanical, and Thermal Pretreatment on the Release of Macromolecules from Two Irish Seaweed Species. Sep. Sci. Technol. 2014, 49, 30–38. [Google Scholar] [CrossRef]
- Jmel, M.A.; Anders, N.; Yahmed, N.B.; Schmitz, C.; Marzouki, M.N.; Spiess, A.; Smaali, I. Variations in Physicochemical Properties and Bioconversion Efficiency of Ulva lactuca Polysaccharides after Different Biomass Pretreatment Techniques. Appl. Biochem. Biotechnol. 2018, 184, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Wi, S.G.; Kim, H.J.; Mahadevan, S.A.; Yang, D.-J.; Bae, H.-J. The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour. Technol. 2009, 100, 6658–6660. [Google Scholar] [CrossRef] [PubMed]
- Pezoa-Conte, R.; Leyton, A.; Anugwom, I.; von Schoultz, S.; Paranko, J.; Mäki-Arvela, P.; Willför, S.; Muszyński, M.; Nowicki, J.; Lienqueo, M.E.; et al. Deconstruction of the green alga Ulva rigida in ionic liquids: Closing the mass balance. Algal Res. 2015, 12, 262–273. [Google Scholar] [CrossRef]
- Mazumdar, S.; Lee, J.; Oh, M.-K. Microbial production of 2,3 butanediol from seaweed hydrolysate using metabolically engineered Escherichia coli. Bioresour. Technol. 2013, 136, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Yahmed, N.B.; Carrere, H.; Marzouki, M.N.; Smaali, I. Enhancement of biogas production from Ulva sp. by using solid-state fermentation as biological pretreatment. Algal Res. 2017, 27, 206–214. [Google Scholar] [CrossRef]
- Schiener, P.; Stanley, M.S.; Black, K.D.; Green, D.H. Assessment of saccharification and fermentation of brown seaweeds to identify the seasonal effect on bioethanol production. J. Appl. Phycol. 2016, 28, 3009–3020. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Falk, H.M.; Benz, R. Thermo-acidic pretreatment of marine brown algae Fucus vesiculosus to increase methane production—a disposal principle for macroalgae waste from beaches. J. Appl. Phycol. 2015, 27, 601–609. [Google Scholar] [CrossRef]
- Jard, G.; Dumas, C.; Delgenes, J.P.; Marfaing, H.; Sialve, B.; Steyer, J.P.; Carrère, H. Effect of thermochemical pretreatment on the solubilization and anaerobic biodegradability of the red macroalga Palmaria palmata. Biochem. Eng. J. 2013, 79, 253–258. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Thomsen, L.; Benz, R. Thermo-acidic pretreatment of beach macroalgae from rügen to optimize biomethane production-double benefit with simultaneous bioenergy production and improvement of local beach and waste management. Mar. Drugs 2015, 13, 5681–5705. [Google Scholar] [CrossRef] [PubMed]
- Mutripah, S.; Meinita, M.D.N.; Kang, J.-Y.; Jeong, G.-T.; Susanto, A.B.; Prabowo, R.E.; Hong, Y.-K.; Dyah, M.; Meinita, N.; Kang, J.-Y.; et al. Bioethanol production from the hydrolysate of Palmaria palmata using sulfuric acid and fermentation with brewer’s yeast. J. Appl. Phycol. 2014, 26, 687–693. [Google Scholar] [CrossRef]
- Ye Lee, J.; Li, P.; Lee, J.; Ryu, H.J.; Oh, K.K. Ethanol production from Saccharina japonica using an optimized extremely low acid pretreatment followed by simultaneous saccharification and fermentation. Bioresour. Technol. 2013, 127, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Takatori, M.; Hayashi, T.; Mori, D.; Takashima, O.; Yoshida, S.; Sato, K.; Kawamoto, H.; Tamura, J.; Izawa, H.; et al. Depolymerization of sulfated polysaccharides under hydrothermal conditions. Carbohydr. Res. 2014, 384, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Holme, H.K.; Lindmo, K.; Kristiansen, A.; Smidsrød, O. Thermal depolymerization of alginate in the solid state. Carbohydr. Polym. 2003, 54, 431–438. [Google Scholar] [CrossRef]
- Chen, S.-T.; Tsai, Y.-P.; Ciou, J.-H.; Huang, Z.-Y.; Lin, W.-C.; Shiu, H. Study on saccharification techniques of alga waste harvested from a eutrophic water body for the transformation of ethanol. Renew. Energy 2017, 101, 311–315. [Google Scholar] [CrossRef]
- Jeong, G.-T.; Park, D.-H. Production of Sugars and Levulinic Acid from Marine Biomass Gelidium amansii. Appl. Biochem. Biotechnol. 2010, 161, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Dandikas, V.; Heuwinkel, H.; Lichti, F.; Drewes, J.E.; Koch, K. Correlation between biogas yield and chemical composition of energy crops. Bioresour. Technol. 2014, 174, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Dandikas, V.; Heuwinkel, H.; Lichti, F.; Eckl, T.; Drewes, J.E.; Koch, K. Correlation between hydrolysis rate constant and chemical composition of energy crops. Renew. Energy 2018, 118, 34–42. [Google Scholar] [CrossRef]
- Gao, L.; Li, D.; Gao, F.; Liu, Z.; Hou, Y.; Chen, S.; Zhang, D. Hydroxyl radical-aided thermal pretreatment of algal biomass for enhanced biodegradability. Biotechnol Biofuels 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, J.; Zhang, G.; Liu, Z.; Guan, H.; Hwang, H.; Aker, W.G.; Wang, P. Optimization study on the hydrogen peroxide pretreatment and production of bioethanol from seaweed Ulva prolifera biomass. Bioresour. Technol. 2016, 214, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.M.T.; Pajares, I.G.; Alcantara, V.A.; Simbahan, J.F. Bacterial laminarinase for application in ethanol production from brown algae Sargassum sp. using halotolerant yeast. Biofuel Res. J. 2018, 17, 792–797. [Google Scholar] [CrossRef]
- Hou, X.; Hansen, J.H.; Bjerre, A.-B. Integrated bioethanol and protein production from brown seaweed Laminaria digitata. Bioresour. Technol. 2015, 197, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ra, C.H.; Kim, S.-K.; Hun Ra, C.; Kim, S.-K.; Ra, C.H.; Kim, S.-K.; Hun Ra, C.; Kim, S.-K. Optimization of Pretreatment Conditions and Use of a Two-stage Fermentation Process for the Production of Ethanol from Seaweed, Saccharina japonica. Biotechnol. Bioprocess Eng. 2013, 18, 715–720. [Google Scholar] [CrossRef]
- Karray, R.; Hamza, M.; Sayadi, S. Production and characterization of enzymatic cocktail produced by Aspergillus niger using green macroalgae as nitrogen source and its application in the pre-treatment for biogas production from Ulva rigida. Bioresour. Technol. 2016, 216, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Karray, R.; Karray, F.; Loukil, S.; Mhiri, N.; Sayadi, S. Anaerobic co-digestion of Tunisian green macroalgae Ulva rigida with sugar industry wastewater for biogas and methane production enhancement. Waste Manag. 2017, 61, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Um, Y.; Yoon, H.H. Pretreatment of macroalgae for volatile fatty acid production. Bioresour. Technol. 2013, 146, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Owens, L.; Busico-Salcedo, N. Vibrio harveyi: Pretty Problems in Paradise. In The Biology of Vibrios; Thompson, F.L., Austin, B., Swings, J., Eds.; American Society of Microbiology: Washington, DC, USA, 2006; pp. 266–280. ISBN 9781555815714. [Google Scholar]
- Tapia-Tussell, R.; Avila-Arias, J.; Domínguez Maldonado, J.; Valero, D.; Olguin-Maciel, E.; Pérez-Brito, D.; Alzate-Gaviria, L. Biological Pretreatment of Mexican Caribbean Macroalgae Consortiums Using Bm-2 Strain (Trametes hirsuta) and Its Enzymatic Broth to Improve Biomethane Potential. Energies 2018, 11, 494. [Google Scholar] [CrossRef]
- Qin, H.-M.; Xu, P.; Guo, Q.; Cheng, X.; Gao, D.; Sun, D.; Zhu, Z.; Lu, F. Biochemical characterization of a novel ulvan lyase from Pseudoalteromonas sp. strain PLSV. RSC Adv. 2018, 8, 2610–2615. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A. Carrageenans and carrageenases: Versatile polysaccharides and promising marine enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- Williams, A.G.; Withers, S.; Sutherland, A.D. The potential of bacteria isolated from ruminal contents of seaweed-eating North Ronaldsay sheep to hydrolyse seaweed components and produce methane by anaerobic digestion in vitro. Microb. Biotechnol. 2013, 6, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Moen, E.; Horn, S.; Østgaard, K. Biological degradation of Ascophyllum nodosum. J. Appl. Phycol. 1997, 9, 347–357. [Google Scholar] [CrossRef]
- Moen, E.; Horn, S.; Østgaard, K. Alginate degradation during anaerobic digestion of Laminaria hyperborea stipes. J. Appl. Phycol. 1997, 9, 157–166. [Google Scholar] [CrossRef]
- Marquez, G.P.B.; Takeuchi, H.; Hasegawa, T. Biogas Production of Biologically and Chemically-pretreated Seaweed, Ulva spp., under Different Conditions: Freshwater and Thalassic. J. Japan Inst. Energy 2015, 94, 1066–1073. [Google Scholar] [CrossRef]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Han, S.K.; Sung, S. Sodium inhibition of thermophilic methanogens. J. Environ. Eng. 2003, 129, 506–512. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Kumaraswamy, S.; Mallick, K.; Adhya, T.K.; Rao, V.R.; Sethunathan, N. Effect of various anionic species on net methane production in flooded rice soils. World J. Microbiol. Biotechnol. 1998, 14, 743–749. [Google Scholar] [CrossRef]
- El-Dessouky, H.T.; Ettouney, H.M. Fundamentals of Salt Water Desalination; Elsevier: Amsterdam, The Netherlands, 2002; ISBN 978-0-444-50810-2. [Google Scholar]
- Roberts, K.P.; Heaven, S.; Banks, C.J. Quantification of methane losses from the acclimatisation of anaerobic digestion to marine salt concentrations. Renew. Energy 2016, 86, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Savithramma, N.; Linga Rao, M.; Venkateswarlu, P. Isolation and Identification of Phenolic Compounds from Boswellia ovalifoliolata Bal. and Henry and Their Free Radical Scavenger Activity. Int. J. Drug Deliv. Technol. 2014, 4, 14–21. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Le Lann, K.; Jegou, C.; Stiger-Pouvreau, V. Effect of different conditioning treatments on total phenolic content and antioxidant activities in two Sargassacean species: Comparison of the frondose Sargassum muticum (Yendo) Fensholt and the cylindrical Bifurcaria bifurcata R. Ross. Phycol. Res. 2008, 56, 238–245. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial Derivatives of Marine Algae: An Overview of Pharmacological Mechanisms and Applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Tanniou, A.; Vandanjon, L.; Incera, M.; Leon, E.S.; Husa, V.; Le Grand, J.; Nicolas, J.L.; Poupart, N.; Kervarec, N.; Engelen, A.; et al. Assessment of the spatial variability of phenolic contents and associated bioactivities in the invasive alga Sargassum muticum sampled along its European range from Norway to Portugal. J. Appl. Phycol. 2014, 26, 1215–1230. [Google Scholar] [CrossRef]
- Hierholtzer, A.; Chatellard, L.; Kierans, M.; Akunna, J.C.C.; Collier, P.J.J. The impact and mode of action of phenolic compounds extracted from brown seaweed on mixed anaerobic microbial cultures. J. Appl. Microbiol. 2013, 114, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.; Stewart, D.; Allwood, J.W.; McDougall, G.J. Extracts from the edible seaweed, Ascophyllum nodosum, inhibit lipase activity in vitro: Contributions of phenolic and polysaccharide components. Food Funct. 2018, 9, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Seasonal variation of chemical composition and biomethane production from the brown seaweed Ascophyllum nodosum. Bioresour. Technol. 2016, 216, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.S.; Smyth, T.J.; Rai, D.K.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Enrichment of polyphenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chem. 2013, 139, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, C.; Beaulieu, L.; Bonnet, C.; Pelletier, É. Assessment of the Antioxidant and Antibacterial Activities of Three Species of Edible Seaweeds. J. Food Biochem. 2015, 39, 377–387. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Stokes, J. Valorisation to biogas of macroalgal waste streams: A circular approach to bioproducts and bioenergy in Ireland. Chem. Pap. 2017, 71, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.R.; Salgado, L.T.; Farina, M.; Pereira, M.S.; Mourão, P.A.S.; Amado Filho, G.M. Ultrastructure of acidic polysaccharides from the cell walls of brown algae. J. Struct. Biol. 2004, 145, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Strezov, A.; Nonova, T. Environmental Monitoring of Heavy Metals in Bulgarian Black Sea Green Algae. Environ. Monit. Assess. 2005, 105, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Akcali, I.; Kucuksezgin, F. A biomonitoring study: Heavy metals in macroalgae from eastern Aegean coastal areas. Mar. Pollut. Bull. 2011, 62, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Ortiz, R.; Steele, T.W.J.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Nkemka, V.N.; Murto, M. Evaluation of biogas production from seaweed in batch tests and in UASB reactors combined with the removal of heavy metals. J. Environ. Manage. 2010, 91, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Nkemka, V.N.; Murto, M. Exploring strategies for seaweed hydrolysis: Effect on methane potential and heavy metal mobilisation. Process Biochem. 2012, 47, 2523–2526. [Google Scholar] [CrossRef]
- Shobana, S.; Kumar, G.; Bakonyi, P.; Saratale, G.D.; Al-Muhtaseb, A.H.; Nemestóthy, N.; Bélafi-Bakó, K.; Xia, A. A review on the biomass pretreatment and inhibitor removal methods as key-steps towards efficient macroalgae-based biohydrogen production. Bioresour. Technol. 2017, 244, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.; Andersson, J.; Gorton, L.; Larsson, S.; Nilvebrant, N.O.; Jönsson, L.J. Effect of different forms of alkali treatment on specific fermentation inhibitors and on the fermentability of lignocellulose hydrolysates for production of fuel ethanol. J. Agric. Food Chem. 2002, 50, 5318–5325. [Google Scholar] [CrossRef] [PubMed]



| Seaweed Type | Polysaccharides | Sugars | Ref. |
|---|---|---|---|
| Red | Agar 1, carrageenan 1, agaropectin, cellulose, xylans, mannans | d-galactose, d-fructose, 3,6-anhydro-d-galactose, glucose | [45,60,64] |
| Green | Ulvan 1, starch, xylopyranose, glucopyranose, xyloglucan, glucuronan, cellulose, hemicellulose | Glucose, xylose, uronic acids, rhamnose, galactose | [52,60] |
| Brown | Fucoidan 1, laminaran, alginates 1, cellulose | Mannitol, glucose, guluronate, mannuronate, glucuronate, sulphated fucose | [60,65,66] |
| Algae (Harvest Time) | Pre-treatment | Fermentation | Ethanol Yield (mg g−1 DW) | % Change | BI (%) | ∆BI * (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Size reduction | |||||||
| Gelidium sesquipedale (unknown time) | Freshwater washed and air-dried. Test: Cutting milled then centrifugally milled, 12,000 rpm. Control: Cutting milled: <2 mm. | Simultaneous saccharification and fermentation (SSF): 5% seaweed loading fermented with Haliatase enzyme (β-glucanase, carragenase, agarase), and S. cerevisiae, stirred (37 °C, 72 h (h)) | 351 | +80 | 69 | +79.8 1 | [92] |
| Ulva lactuca (unknown time) | 527 | −4.4 | 64 | −4.3 1 | |||
| Chaetomorpha linum (September 2010) | Freshwater washed, dried (40 °C, 48 h) Test: 25 g ball milled (25 balls), 18 h, 180 rpm to <2 mm size. Control: untreated biomass | SSF: 10% pre-treated seaweed pre-hydrolysed, inoculated: cellulase enzymes, and S. cerevisiae, 32 °C, 200 h | 180 | +63.6 | 77 | +41.9 2 | [89] |
| Washing | |||||||
| L. digitata (July 2009) | Test: Freshwater washed (W) Oven dried (OD) (70 °C, 72 h); frozen (−20 °C) and OD (FOD). Control: Unwashed, OD or FOD. All milled to <1 mm. | SSF: 5% seaweed with laminarinase and yeast Pichia angophorae stirred at 24 °C, 88 h | W + OD: 12.3 µL g−1 DW | −9.6 | 19 | +49.2 3 | [91] |
| W + FOD: 11.1 µL g−1 DW | −26 | 15 | −8.1 3 | ||||
| Sonication | |||||||
| U. rigida (unknown time) | Dried (70 °C, 48 h), ground: ≤1 mm particle size. SSF: 4% (w/v) seaweed, amyloglucosidase, α-amylase, cellulase enzymes, buffer, and S. cerevisiae. Test: Incubated in sonicator bath, 40 kHz, 120W, 37 °C, 3 h. Control: conventional incubator, 150 rpm, 37 °C, 48 h | 64.7 4 | +58.8 4 | 65 | −2.9 5 | [90] | |
| Algae (Harvest Time) | Pre-treatment | I/S Ratio; Source | BMP Method | CH4 Yield (mL g−1 VS) | % Change | BI (%) | ∆BI (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Size reduction | ||||||||
| G. vermiculophylla (March 2012) | Test: Unwashed and macerated (UM); washed and macerated (WM); washed, dried (37 °C) and macerated (WDM). Control: without maceration. | 4:1; brewery wastewater treatment plant (WWT) | Glass vials with rubber stopper, aluminium crimp, 37 °C, 28 days. | UM: 338 | +14.6 | - | - | [93] |
| WM: 481 | +11.9 | - | - | |||||
| WDM: 349 | +7.7 | - | - | |||||
| G. vermiculophylla | Frozen at harvest (unknown time of harvest). Test: Fresh water washed, macerated (M). Control: Fresh water washed and chopped (2 × 2 cm) | 6:1 in 500 mL; lab reactor using cattle manure | Bottles with rubber stoppers and aluminium crimp, 53 °C, 34 days | 147 | +11.4 | - | - | [94] |
| C. linum | 195 | +17.5 | - | - | ||||
| U. lactuca | 255 | +67.7 | - | - | ||||
| S. latissima | 333 | −2.1 | - | - | ||||
| Laminaria spp. (November 2013) | Test: Ball milled (20 balls) unwashed seaweed, dried at 80 °C for 24 h Particle size: 1–2 mm. Control: cut, unwashed. | 1:1.33 in 400 mL; WWT | Bottle sealed with adaptor attached to gas measuring device (GMD), 38 °C, 25 days, manually shaken | 1 mm: 241 | −26.5 | - | - | [95] |
| 2mm: 260 | −20.7 | - | - | |||||
| F. vesiculosus (Autumn 2014) | Frozen until treatment Test: Washing (W) and chopped (C) (<5 mm); unwashed (UW) and chopped; washed and not chopped (NC). Control: not washed or chopped | 5:1 in 60 mL; WWT | Serum bottles, gas measured with syringe, 37 °C, 30 days. | W + C: 81.1 | +574.3 | 25 1 | - | [23] |
| UW + C: 67.3 | +493.6 | 21 1 | - | |||||
| W + NC: 73.1 | +527.5 | 23 1 | - | |||||
| Beating | ||||||||
| Laminaria spp. (November 2013) | Test: Cut without washing and beaten (Hollander beater), 76 µm gap, 10 min (min). Control: only cut, unwashed. | 1:1.33 in 400 mL; WWT | Bottle with adaptor attached to GMD, 38 °C, 25 days, shaken manually | 335 | +2.1 | - | - | [95] |
| Laminaria spp. (May 2014) | Test: Cut without washing and beaten (Hollander beater), 76 µm gap, 15 min. Control: only cut, unwashed. | 1.2:1 (Laminaria spp.), 3:1 (A. nodosum) in 400 mL; WWT | Bottle attached to GMD, 38 °C, 14 days, shaken manually | 240 | +8.6 | - | - | [96] |
| A. nodosum (August 2014) | 169 | +30 | - | - | ||||
| Washing | ||||||||
| U. lactuca (June 2011) | Test: Washed and dried (room temperature) (24 h). Control: Unwashed Both frozen (−20 °C), grinded: 10–15 mm. | 3:1 in 400 mL; reactor using grass, dairy slurry and seaweed. | Bioprocess AMPTS II system, 37 °C, 30 days. | 221 | +33.9 | 55 1 | +53.2 1 | [97] |
| U. lactuca (April) | Test: Washed 2% (w/v) seaweed in water, 24 h, chopped (2 × 2 cm) (C) or macerated (M). Control: Unwashed (C or M) | 8:1 in 500 mL; reactor using cattle manure. | Bottles with rubber stoppers, aluminium crimp, 52 °C, 42 days. | W + C: 171 | −1.7 | - | - | [98] |
| W + M: 200 | −26.2 | - | - | |||||
| L. digitata (July 2009) | Milled to <1 mm particle size. Test: Freshwater washed (W), oven dried (70 °C, 72 h) (OD) or frozen (−20 °C) and OD (FOD). Control: Unwashed, OD or FOD. | 6:1 in 500 mL; Unknown | Bottle with rubber stoppers, aluminium caps, shaken, 35 °C, 35 days. | W + OD: 202.9 | −13.8 | - | - | [91] |
| W + FOD: 248.1 | +29.4 | - | - | |||||
| S. muticum (June 2017) | Test: Freshwater washed Control: unwashed Both frozen (−20 °C), then blended. | 9:1 in 400 mL; paper WWT | Automated CJC system, 37 °C, 28 days. | 177 | −21.3 | 48 1,2 | −21.3 1,2 | [79] |
| L. digitata (March (M) and September (S)) | Test: Washed in cold water (CO) (15 °C); hot water (H) (40 °C), 3 min, cut (4 cm) Control: unwashed, cut to 4 cm. | 2:1 in 400 mL; reactor using grass, dairy slurry and seaweed. | Bioprocess AMPTS II system, 37 °C, 30 days. | M,CO: 258 | +5.3 | 59 | +13.4 | [88] |
| M,H: 283 | +15.5 | 60 | +15.4 | |||||
| S,CO: 303 | +8.2 | 67 | +8.0 | |||||
| S,H: 326 | +16.4 | 76 | +22.6 | |||||
| Sonication | ||||||||
| U. rigida (July–September 2013) | Test: 30 mL blended seaweed (80% (w/v) in water), sonicated (5 min, 40 kHz, 120 W) Control: 80% w/v, (assumed) blended | 1:1 in 500 mL; Unknown | Bottles with rubber stoppers, gas measured by syringe plunger, 37 °C 48 days. | - | +10.2 | 57 3 | +6.6 3 | [99] |
| Algae (Harvest Time) | Treatment | I/S Ratio; Source | BMP Method | CH4 Yield (mL g−1 VS) | % Change | Ref. |
|---|---|---|---|---|---|---|
| Ulva spp. (Korea, Spring 2014) | Freshwater rinsed, blended into slurry Thermal: no chemical, 90 °C HCl: 0.1 M, 90 °C NaOH: 0.1 M, 90 °C All magnetically stirred (10 mins), oven (6 h), shaken 1 min every half hour. Control: untreated slurry | unknown (35 mL slurry and 70 mL inoculum); sewage sludge digester | Bottles with rubber stopper and aluminium cap, 35 °C, 30 days. Shaken manually intermittently. | Thermal: 293.0 | +15.8 | [118] |
| 0.1 M HCl: 284.8 | +12.7 | |||||
| 0.1 M NaOH: 251.3 | −0.7 | |||||
| S. latissima (August 2010) | Defrosted, shredded into slurry. Test: steam exploded 130 °C or 160 °C, 10 mins. Control: untreated slurry | 7:1 in 700 g; sewage treatment plant. | Bottles with rubber stopper, aluminium screw caps, shaker (90 rpm, 37 °C). Re-fed day 67, biogas shown: day 119. | 130 °C: 268 | +20.2 | [119] |
| 160 °C: 260 | +16.6 | |||||
| N. zanardini (July) | Washed, dried (40 °C, 24 h); hammer milled to <1 mm. Test: 5% seaweed, 121 °C, 0.5 h. Control: untreated. | unknown; WWT | Bottles closed with rubber stopper, aluminium caps, 37 °C, 40 days. | 143 | +22 | [116] |
| Laminaria spp. (November 2013) | Test: Cut seaweed, Freshwater immersed, microwaved (560 W) till water boiled, held for 30 s. Control: cut unwashed seaweed. | 1:1.33 in 400 mL; WWT | Bottles sealed with adaptor attached to GMD, 38 °C, 25 days, shaken daily. | 244 | −25.6 | [95] |
| F. vesiculosus (October 2014) | Cut and grounded (mortar and pestle) Test: microwaved (700 W), 3 mins Control: not microwaved | 1:3; WWT | Bottles with rubber stopper and metal cap, 37 °C, 22 days. Shaken daily. | 146.9 | +92.3 | [120] |
| Algae (Harvest Time) | Treatment | Fermentation | Ethanol Yield (mg g−1 DW) | % change | BI 1 (%) | ΔBI 1 (%) | Ref. |
|---|---|---|---|---|---|---|---|
| C. linum (July 2009) | Washed, dried (40 °C) and milled. Thermal: 4% (w/v) seaweed, autoclaved (200 °C), 10 mins, 1 bar. Wet oxidation (WO): same as thermal, 12 bars O2 Steam explosion: 1.2 kg (35% DW, 1.9 MPa), 200 °C, 5 mins. Plasma assisted: 2.5 g, 1% O3, 1 h. Control: untreated biomass | SSF: 10% pre-treated seaweed pre-hydrolysed, inoculated with cellulase enzymes and S. cerevisiae (32 °C 200 h). | Thermal: 150 | +36.4 | 68.4 | +25.7 | [89] |
| WO: 170 | +54.5 | 77.2 | +41.9 | ||||
| Steam: 130 | +18.2 | 66.7 | +22.6 | ||||
| Plasma: 150 | +36.4 | 71.9 | +32.2 |
| Algae (Harvest Time) | Pre-treatment | I/S Ratio; Source | BMP Method | CH4 Yield (mL g−1 VS) | % Change | Ref. |
|---|---|---|---|---|---|---|
| G. vermiculophylla (March 2012) | Algae washed, macerated Test: 0.1, 0.3, 0.5 g NaOH g−1 seaweed (20 °C, 55 °C, 90 °C, 1 bar, 3.5 bar, 6 bar, 60 and 90 mins) Control: Untreated | 4:1; brewery WWT | Glass vials with rubber stopper and aluminium crimp, 37 °C, 24 days | 353–380 | −21 to −26.6 | [93] |
| P. palmata (March 2010) | Dried (40 °C), chopped (2 × 2 cm) Test: 0.04 g NaOH g−1 TS (50 g L−1): 20–70 °C, 24 h; 160 °C, 0.5 h. 0.02 g HCl g−1 TS, 160 °C, 0.5 h. Control: untreated | 2:1 in 400 mL; sugar WWT | Glass vials with rubber stopper and aluminium crimp, 35 °C, 60 days. | 20–70 °C: 362–365 | +17.5 to 18.5 | [147] |
| 160 °C NaOH: 282 | −8.4 | |||||
| 160 °C HCl: 268 | −13 | |||||
| Seaweed mixture: Spermothamnion family (80-90%), Chaetophorales family (5–15%), eelgrass (2–5%) (August, 2011) | Dried (54 °C), shredded Test: Only H2O; flue gas condensate (FGC) (pH 1.2); 0.05 M HCl (80 °C, 2 h); 0.2 M HCl (80 °C, 1.5 h) Control: Untreated | 2:1 in 2 L; Mixture: WWT, maize silage and cattle manure, seaweed adapted sludge. | Fermenter tanks with CO2 absorbing unit, gas drying unit, and gas volume sensor, 37 °C, 22 days. | H2O: 80 | −8.0 | [148] |
| FGC: 108 | +24.1 | |||||
| 0.05 M HCl: 66 | −24.1 | |||||
| 0.2 M HCl: 121 | +39.1 | |||||
| Ulva spp. (March 2015) | Washed, sun dried (1–2 weeks) Test: 0.04 g NaOH g−1 TS (20 °C, 24 h); 0.04 g HCl g−1 TS (150 °C, 0.5 h) Control: untreated | 2:1 in 400 mL; sugar wastewater industry | Glass vials with rubber stopper, aluminium crimp, 35 °C until no gas production. | NaOH: 148 | +12.1 | [144] |
| HCl: 77 | −41.7 | |||||
| Ulva spp. (Spring 2014) | Fresh water rinsed, blended to slurry. Test: 500 mL slurry, no chemical; 0.01 M HCl; 0.1 M NaOH. All 90 °C, 6 h, manual shaking every 0.5 h, 1 min. Control: untreated | Sewage sludge digester | Bottles with rubber stopper, aluminium cap, 35 °C, 30 days, shaken manually intermittently. | Only thermal: 293.0 | +15.8 | [118] |
| 0.1M HCl: 284.8 | +12.7 | |||||
| 0.1 M NaOH: 251.3 | −0.7 | |||||
| L. digitata (Unknown time) | Fresh Water rinsed, dried (75 °C, 24 h), milled. Test: 20% solids loading, 2.5% citric acid (CA); 6% citric acid; 1% lactic acid (LA), autoclaved (120 °C, 1 h, 1 atm) Control: untreated | 2:1 in 30 mL; bovine slurry adapted to seaweed. | Serum bottles (pH 7.3–7.5) with rubber stopper and aluminium crimp, 35 °C, 32 days. | 2.5% CA: 237 | +3.9 | [53] |
| 6% CA: 69 | −69.7 | |||||
| LA: 161 | −29.4 | |||||
| F. vesiculosus (Unknown time) | Dried, crushed, homogenised Test: 0.2M HCl (80 °C, 12 h); FGC (pH 2.2, 0.13 M, 80 °C, 24 h) Control: untreated | 2:1 in 2 L; mixture: WWT, corn silage, seaweed adapted sludge. | Fermenter tanks, CO2 absorbing unit and gas volume sensor, 37 °C, 20 days. | HCl: 116 | +147 | [146] |
| FGC: 65 | +38.3 |
| Algae (Harvest Time) | Pre-treatment | Inoculum | BMP Method | CH4 Yield (ml g−1 VS) | % Change | BI (%) | ΔBI (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ulva rigida (July–September 2013) | Test: 7.5 mL A. niger filtrate to 50 mL blended seaweed (80% (w/v) in water), 50 °C, 100 rpm, 2 h. Repeated with β-glucosidase. Control: untreated seaweed. | I/S ratio: 1:1 in 500 mL Source: (unknown) treatment plant. | Bottles with rubber stoppers, 37 °C. Gas measured using syringe plunger. | - | A. niger: +33 1 | 63 2 | +17.1 2 | [99] |
| - | β-glu.: +28 1 | 58 2 | +7.8 2 | |||||
| Ulva spp. (March 2015) | Test: washed, sun dried (1–2 weeks), grounded. 35% seaweed in Mandels’ salt solution, autoclaved (120 °C, 20 mins); inoculated: A. fumigatus SL1 conidia suspension; incubated (50 °C, eight days). Control: untreated. | I/S ratio: 2:1 in 400 mL with buffer and nutrients Source: sugar wastewater industry. | Vials with rubber stopper and aluminium crimp, 35 °C till gas production halts. | 153 | +15.9 | 57 3 | +16.3 3 | [144] |
| Ulva spp. (June 2013) | Test: 100 g washed or unwashed seaweed (macerated <5 mm) in 100 g freshwater or thalassic hydrolytic inoculum; 3 days, 37 °C. Control: untreated with hydrolytic inoculum | Source: Freshwater: washed seaweed-adapted, original slurry: food waste. Thalassic: Unwashed seaweed-adapted, original slurry: seawater, mud, sand | 100 g of respective methanogenic inoculum, 37 °C, 6 days. | 77.7 | Freshwater: +42.8 | 27 | +42.4 3 | [172] |
| 180.9 | Thalassic: +72 | 63 | +71.8 3 | |||||
| L. digitata (unknown time) | Freshwater rinsed, dried (75 °C, 24 h), milled. 20% (w/v) seaweed in water with: Cellulase (C): 37 °C; Alginate lyase (AL): 37 °C; or Celluclast® 1.5L (C1.5): 40 °C. All incubated: 300 rpm, 24 h Control: water (room temp., 24 h) | I/S ratio: 2:1 in 30 mL (pH 7.3–7.5) Source: bovine slurry (seaweed-adapted). | Bottles with rubber stopper and aluminium crimp, 35 °C, 32 days. | C: 232 | +1.8 | - | - | [53] |
| AL: 225 | −1.3 | - | - | |||||
| C1.5: 72 | −68.4 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maneein, S.; Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. A Review of Seaweed Pre-Treatment Methods for Enhanced Biofuel Production by Anaerobic Digestion or Fermentation. Fermentation 2018, 4, 100. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation4040100
Maneein S, Milledge JJ, Nielsen BV, Harvey PJ. A Review of Seaweed Pre-Treatment Methods for Enhanced Biofuel Production by Anaerobic Digestion or Fermentation. Fermentation. 2018; 4(4):100. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation4040100
Chicago/Turabian StyleManeein, Supattra, John J. Milledge, Birthe V. Nielsen, and Patricia J. Harvey. 2018. "A Review of Seaweed Pre-Treatment Methods for Enhanced Biofuel Production by Anaerobic Digestion or Fermentation" Fermentation 4, no. 4: 100. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation4040100